Abstract
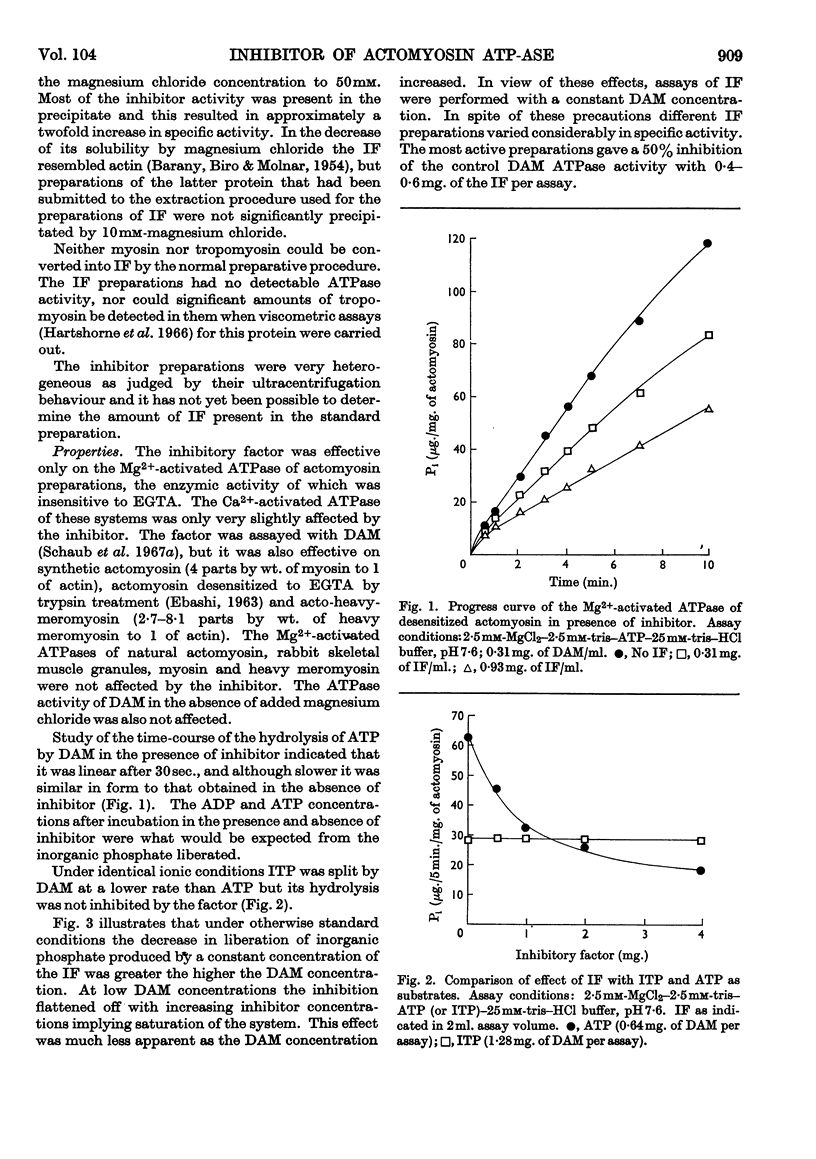
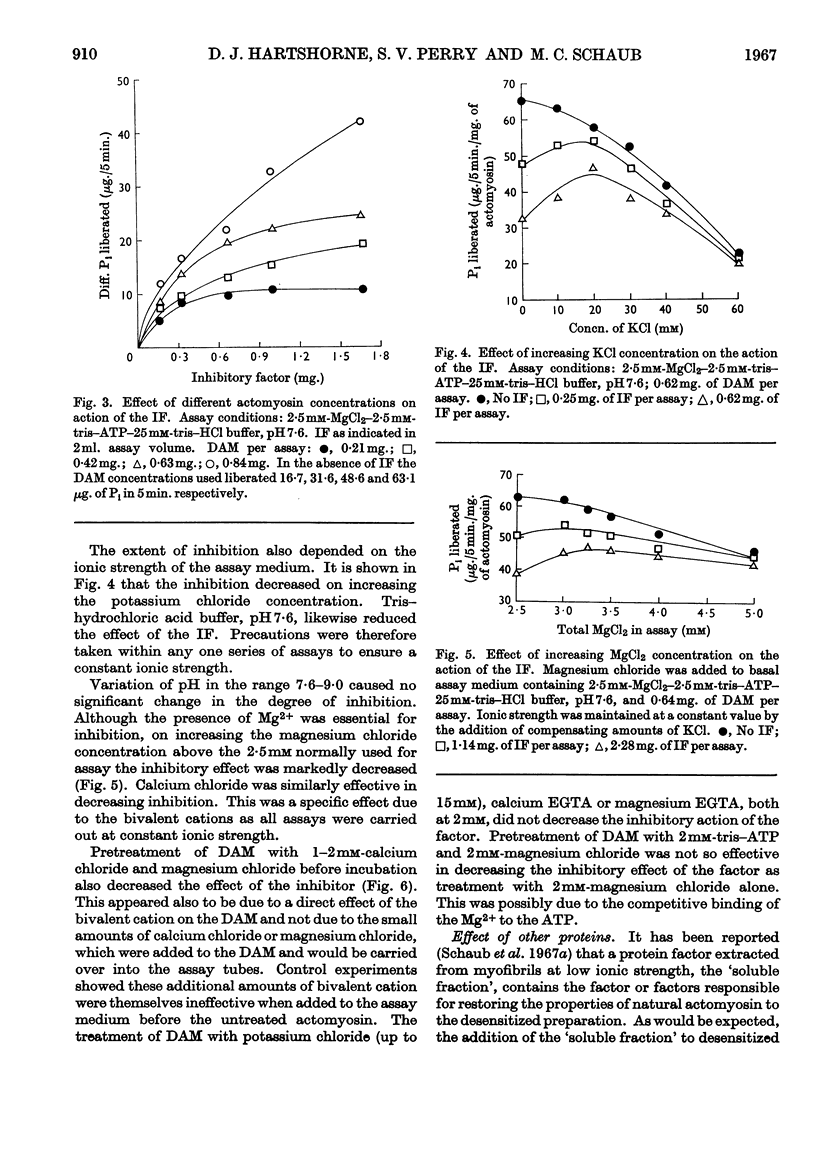
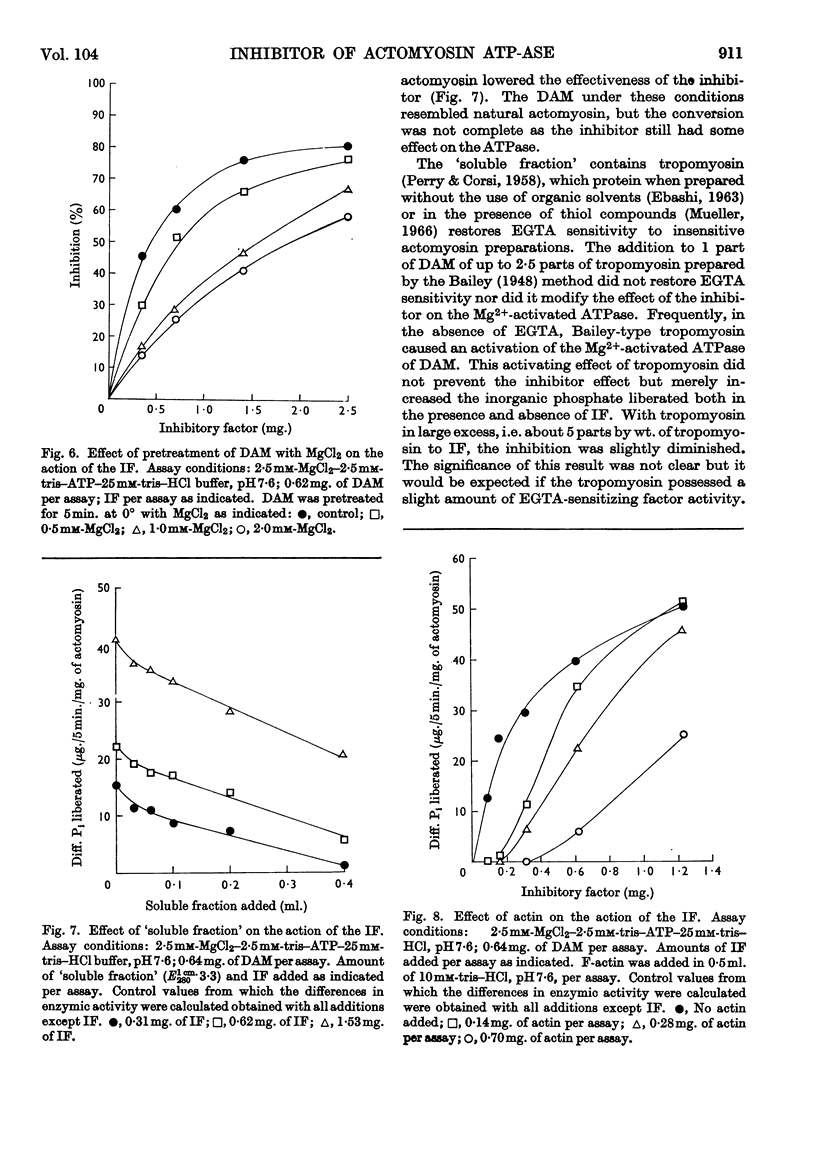
1. The preparation and properties of a myofibrillar protein factor which inhibits the Mg2+-activated adenosine triphosphatase of desensitized actomyosin is described. 2. This factor had negligible effect on the Mg2+-activated adenosine triphosphatase of natural actomyosin and on the Ca2+-activated adenosine triphosphatases of desensitized actomyosin and myosin. 3. The Mg2+-activated inosine triphosphatase activity of desensitized actomyosin was not affected by the factor. 4. The inhibitory effect was sensitive to ionic strength. In addition to their ionic effects Mg2+ and Ca2+ appeared to have a specific action in reducing the effect of the inhibitor. 5. F-actin reduced the inhibition whereas Bailey-type tropo-myosin had little effect. 6. As far as can be judged from the reported experiments this factor is different from any of the previously described myofibrillar components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S., Eisenberg E., Moos C. Actin-myosin interaction: inhibition of the myosin adenosine triphosphatase by actin. Science. 1966 Mar 25;151(3717):1541–1542. doi: 10.1126/science.151.3717.1541. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Perry S. V., Davies V. A factor inhibiting the adenosine triphosphatase activity and the superprecipitation of actomyosin. Nature. 1966 Mar 26;209(5030):1352–1353. doi: 10.1038/2091352a0. [DOI] [PubMed] [Google Scholar]
- LEADBEATER L., PERRY S. V. The effect of actin on the magnesium-activated adenosine triphosphatase of heavy meromyosin. Biochem J. 1963 May;87:233–239. doi: 10.1042/bj0870233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K. Effect of actinins on the adenosinetriphosphatase activity of actomyosin at low ionic strength. J Biochem. 1966 Apr;59(4):422–424. doi: 10.1093/oxfordjournals.jbchem.a128319. [DOI] [PubMed] [Google Scholar]
- Mueller H. Effect of Bailey's tropomyosin purification on EGTA-sensitizing activity. Nature. 1966 Mar 12;209(5028):1128–1129. doi: 10.1038/2091128a0. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cotterill J. The action of thiol inhibitors on the interaction of F-actin and heavy meromyosin. Biochem J. 1964 Sep;92(3):603–608. doi: 10.1042/bj0920603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Davies V., Hayter D. Natural tropomyosin and the factor sensitizing actomyosin adenosine triphosphatase to ethylenedioxybis(ethyleneamino)tetra-acetic acid. Biochem J. 1966 Apr;99(1):1C–2C. doi: 10.1042/bj0990001c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. The adeonosine-triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Jul;104(1):263–269. doi: 10.1042/bj1040263. [DOI] [PMC free article] [PubMed] [Google Scholar]


