Abstract
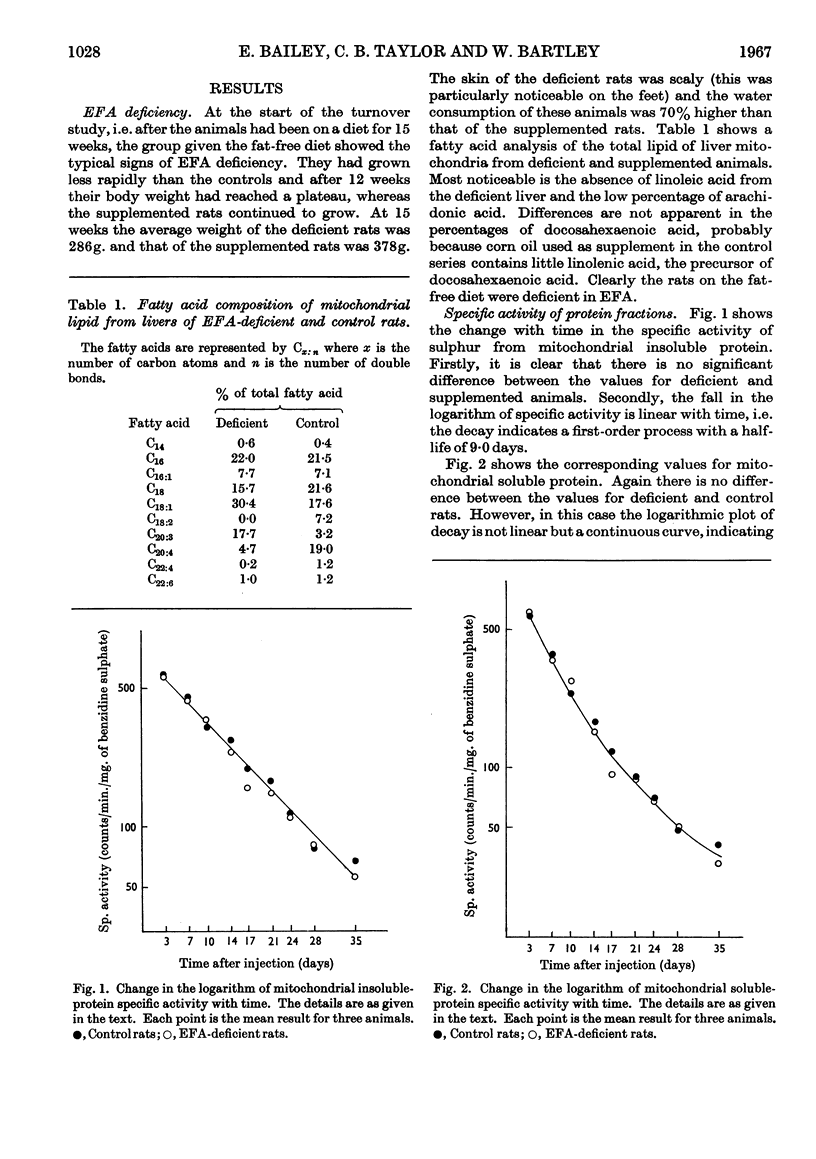
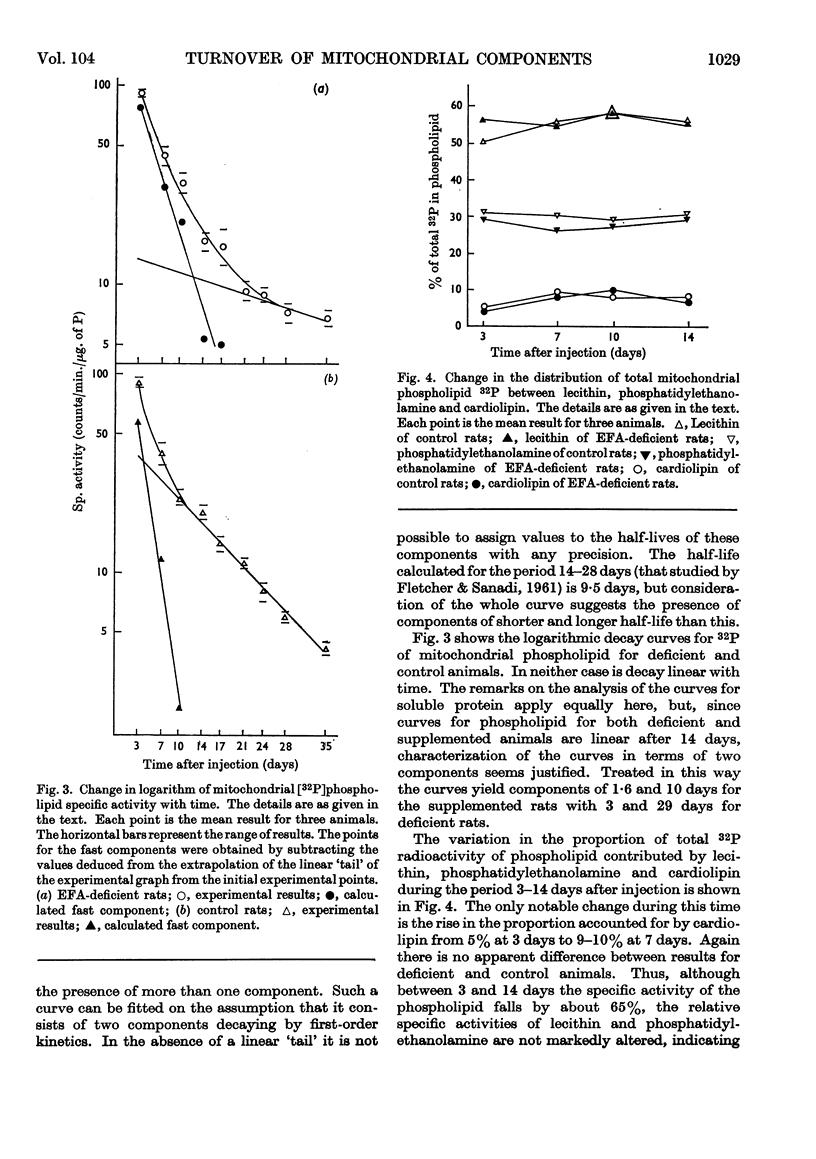
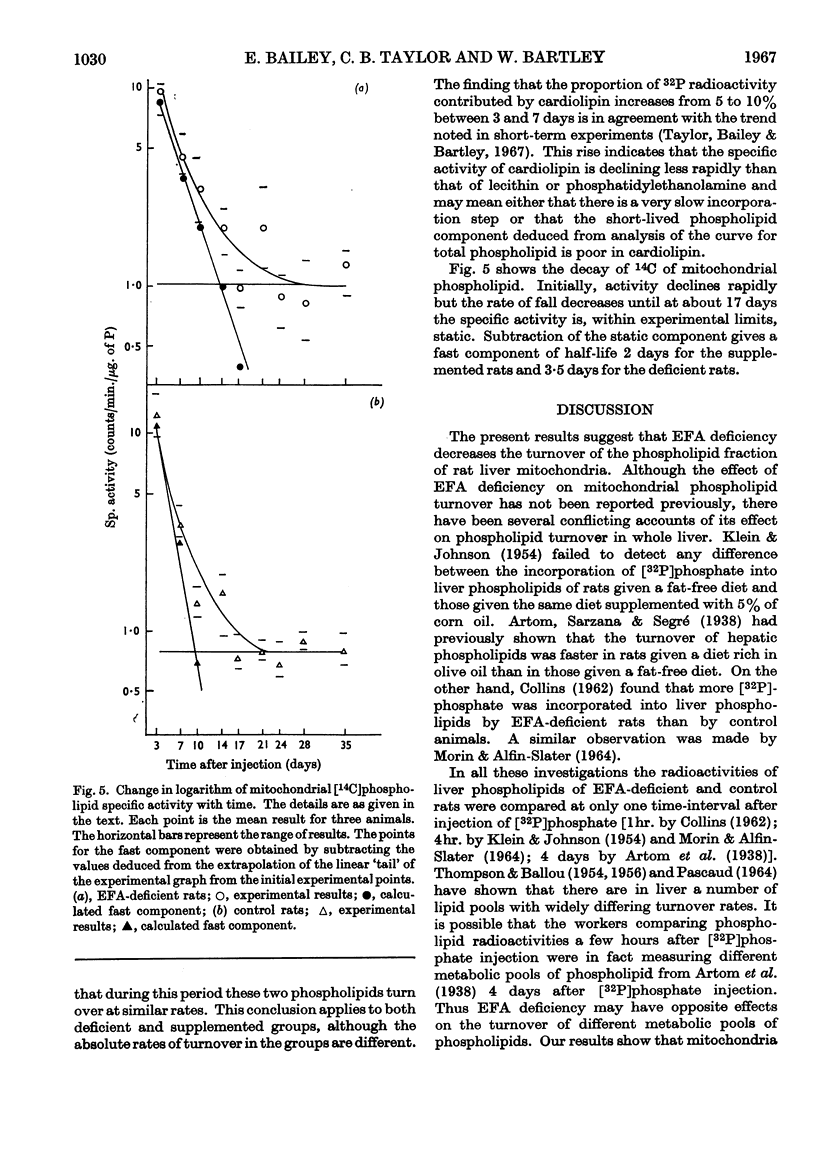
1. Essential fatty acid (EFA)-deficient and control rats were injected intraperitoneally with [32P]phosphate, l-[35S]methionine and [2-14C]acetate. The animals were killed at various time-intervals after injection and their liver mitochondria fractionated into soluble protein, insoluble protein, and lipid. 2. The 35S was assayed in the protein fractions and 32P and 14C were assayed in the lipid fraction. Curves of log (specific activity) plotted against time were prepared for the different fractions. 3. There was no significant difference between the insoluble protein results for control and EFA-deficient animals, both sets of results indicating the presence of a single component of half-life 9 days. 4. There was no significant difference between the soluble protein results for the two sets of animals and both sets of results indicated the presence of at least two components. 5. The [32P]-phospholipid results indicate that in the control animals the liver mitochondrial phospholipids contain components of half-life 1·6 and 10 days whereas the mitochondrial phospholipids of the EFA-deficient animals contain components of half-life 3 and 29 days. 6. The specific activity of mitochondrial [14C]phospholipid initially fell rapidly in both groups of animals, but after 17 days there was no further significant decrease. A fast component with maximum half-life 2–4 days was clearly demonstrated for both groups of animals. Whether or not these results also indicate the presence of a very long-lived mitochondrial phospholipid is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLOU J. E., THOMPSON R. C. Studies of metabolic turnover with tritium as a tracer. V. The predominantly non-dynamic state of body constituents in the rat. J Biol Chem. 1956 Dec;223(2):795–809. [PubMed] [Google Scholar]
- BARTLEY W. Efficiency of oxidative phosphorylation during the oxidation of pyruvate. Biochem J. 1953 Jul;54(4):677–682. doi: 10.1042/bj0540677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. S., Basford R. E., Koritz S. B. Studies on the biosynthesis of mitochondrial protein components. Biochemistry. 1966 Mar;5(3):926–930. doi: 10.1021/bi00867a018. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS F. D. Phospholipid metabolism in essential fatty acid deficient rats. Biochem Biophys Res Commun. 1962 Oct 31;9:289–292. doi: 10.1016/0006-291x(62)90041-4. [DOI] [PubMed] [Google Scholar]
- De Pury G. G., Collins F. D. A raised level of free fatty acids in serum of rats deficient in essential fatty acids as a contributing cause of their fatty livers. Biochim Biophys Acta. 1965 Jul 7;106(1):213–214. doi: 10.1016/0005-2760(65)90113-x. [DOI] [PubMed] [Google Scholar]
- FLETCHER M. J., SANADI D. R. Turnover of rat-liver mitochondria. Biochim Biophys Acta. 1961 Aug 5;51:356–360. doi: 10.1016/0006-3002(61)90177-9. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GAITONDE M. K., RICHTER D. The uptake of 35S into tissues after injection of (35S) methionine. Biochem J. 1955 Apr;59(4):690–696. doi: 10.1042/bj0590690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr M. I., Prottey C., Hawthorne J. N. The phospholipids of liver-cell fractions. II. Incorporation of [32P]orthophosphate in vivo in normal and regenerating rat liver. Biochim Biophys Acta. 1965 Oct 4;106(2):357–370. [PubMed] [Google Scholar]
- HAYASHIDA T., PORTMAN O. W. CHANGES IN SUCCINIC DEHYDROGENASE ACTIVITY AND FATTY ACID COMPOSITION OF RAT LIVER MITOCHONDRIA IN ESSENTIAL FATTY ACID DEFICIENCY. J Nutr. 1963 Oct;81:103–109. doi: 10.1093/jn/81.2.103. [DOI] [PubMed] [Google Scholar]
- Haldar D., Freeman K., Work T. S. Biogenesis ommitochondria. Nature. 1966 Jul 2;211(5044):9–12. doi: 10.1038/211009a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. M. ADENOSINE TRIPHOSPHATASE AND ATP-PI EXCHANGE IN MITOCHONDRIA OF ESSENTIAL FATTY ACID-DEFICIENT RATS. J Nutr. 1963 Dec;81:411–414. doi: 10.1093/jn/81.4.411. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. M. SWELLING STUDIES ON LIVER MITOCHONDRIA FROM ESSENTIAL FATTY ACID DEFICIENT RATS. Exp Cell Res. 1963 Oct;32:118–129. doi: 10.1016/0014-4827(63)90073-9. [DOI] [PubMed] [Google Scholar]
- KHAN A. A., WILSON J. E. STUDIES OF TURNOVER IN MAMMALIAN SUBCELLULAR PARTICLES: BRAIN NUCLEI, MITOCHONDRIA AND MICROSOMES. J Neurochem. 1965 Feb;12:81–86. doi: 10.1111/j.1471-4159.1965.tb11942.x. [DOI] [PubMed] [Google Scholar]
- KLEIN P. D., JOHNSON R. M. Phosphorus metabolism in unsaturated fatty acid-deficient rats. J Biol Chem. 1954 Nov;211(1):103–110. [PubMed] [Google Scholar]
- Lusena C. V., Depocas F. Heterogeneity and differential fragility of rat liver mitochondria. Can J Biochem. 1966 May;44(5):497–508. doi: 10.1139/o66-060. [DOI] [PubMed] [Google Scholar]
- MORIN R. J., ALFIN-SLATER R. B. FACTORS AFFECTING HEPATIC PHOSPHOLIPID SYNTHESIS. Metabolism. 1964 Jan;13:69–74. doi: 10.1016/s0026-0495(64)80011-1. [DOI] [PubMed] [Google Scholar]
- PASCAUD M. LES PHOSPHOLIPIDES DE LA CELLULE H'EPATIQUE. INTERPR'ERATION FONCTIONNELLE DE LEUR RENOUVELLEMENT. II. RENOUVELLEMENT DES ACIDES GRAS DES PHOSPHOGLYCERIDES. Biochim Biophys Acta. 1964 Oct 2;84:528–537. [PubMed] [Google Scholar]
- ROODYN D. B., REIS P. J., WORK T. S. Protein synthesis in mitochondria. Requirements for the incorporation of radioactive amino acids into mitochondrial protein. Biochem J. 1961 Jul;80:9–21. doi: 10.1042/bj0800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN O., STEIN Y. METABOLISM IN VITRO OF PALMITIC AND LINOLEIC ACID IN THE HEART AND DIAPHRAGM OF ESSENTIAL FATTY ACID-DEFICIENT RATS. Biochim Biophys Acta. 1964 Dec 2;84:621–635. doi: 10.1016/0926-6542(64)90022-8. [DOI] [PubMed] [Google Scholar]
- STEIN Y., STEIN O. The incorporation and disappearance of fatty acids in the rat epididymal fat pad studied by the in vivo incubation technique. Biochim Biophys Acta. 1962 Jun 18;60:58–71. doi: 10.1016/0006-3002(62)90372-4. [DOI] [PubMed] [Google Scholar]
- THOMPSON R. C., BALLOU J. E. Studies of metabolic turnover with tritium as a tracer. IV. Metabolically inert lipide and protein fractions from the rat. J Biol Chem. 1954 Jun;208(2):883–888. [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Mitochondrial changes in the liver of essential fatty acid-deficient mice. J Cell Biol. 1963 Feb;16:281–296. doi: 10.1083/jcb.16.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


