Abstract
The pharmacodynamic profile of clarithromycin (CLR) was evaluated with a murine model of pneumonia. Eight Streptococcus pneumoniae isolates, including three macrolide-sensitive and five macrolide-resistant strains, were inoculated intratracheally into immunocompromised ICR mice as 108-CFU bacterial suspensions. Orally administered CLR daily doses ranging from 5 to 600 mg/kg of body weight were given over 5 days, during which animal survival was monitored. The bacterial density in lung tissues was examined after 24 h of CLR treatment and in control groups. Pharmacokinetic analysis of CLR in mice demonstrated that the regimen of 150 mg/kg twice a day was representative of human pharmacokinetics and was used to compare the efficacy of CLR against sensitive and resistant S. pneumoniae strains. Immunocompetent CBA/J mice were also infected and treated as described above and evaluated for bacterial density and survival to assess the effect of the presence of leukocytes. All three pharmacodynamic parameters, the duration (percent) of the time that serum CLR concentrations remain above the MIC (%T>MIC), the ratio of the area under the concentration-time curve from 0 to 24 h (AUC0-24) to the MIC, and the ratio of the maximum concentration of drug in serum to the MIC, were found to be closely correlated to CLR bacterial efficacy (P < 0.001). Furthermore, all parameters had close correlation to bacterial density (r2 = 0.72 to 0.82), median survival (r2 = 0.93 to 0.94), and total percent survival (r2 = 0.91 to 0.92). These in vivo data suggest that the bacterial activity of CLR is closely correlated with all three parameters over a wide range of exposures and, as a consequence of parameter interdependency, AUC0-24/MIC is the most reasonable predictor of antibiotic efficacy. In this neutropenic pneumonia model, CLR was less efficacious against S. pneumoniae strains for which MICs were ≥4 μg/ml. However, the presence of leukocytes in the immunocompetent mice resulted in improved bactericidal activity, relative to that in the neutropenic animals, despite an MIC of 4 μg/ml.
Streptococcus pneumoniae is a pathogen that continues to be commonly associated with community-acquired pneumonia. Since their introduction, macrolide antibiotics have been used to treat respiratory infections, including those caused by S. pneumoniae (25). Currently, the estimated incidence of S. pneumoniae in vitro resistance to the macrolide antibiotics is approximately 15 to 20% (6, 22). The mechanism of resistance to the macrolides is due primarily to the presence of either or both of two genes in pneumococci, mef(A) and erm(B) (19). For organisms harboring mef(A), low-level macrolide resistance (MICs, 1 to 16 μg/ml) are generally observed, whereas high-level resistance (MICs, ≥64 μg/ml) is typically seen in the presence of erm(B).
The pharmacodynamics (PD) of macrolides, clarithromycin (CLR) in particular, have not yet been fully studied, nor has the key surrogate marker been successfully elucidated. The topic of macrolide PD has received considerable attention recently, including in our facility (3, 5, 16; T. Mazzei, A. Novelli, S. Fallani, M. I. Cassetta, and S. Conti, 5th Int. Conf. Macrolides, Azalides, Streptogramins, Ketolides Oxazolidones, abstr. 09.01, 2000). With the advent of increasing in vitro resistance to macrolides, the impetus to determine which surrogate PD marker(s) provides a reasonable estimation of efficacy is intensifying.
Describing the PD of the new macrolides such as azithromycin and CLR seems difficult at best. The newer macrolides show extensive tissue distribution, and the pharmacokinetic profile and subsequent concentrations at the site of infection, in this case the epithelial lining fluid, are quite different for the available macrolides (17, 20). As a result, a more complete understanding of macrolide PD is required to differentiate appropriate therapies and is especially relevant due to the apparently high incidence of in vitro resistance to these commonly utilized agents.
In this study, a pneumonia model was used in an effort to mimic environmental conditions to which S. pneumoniae isolates are subjected during pneumonia and to better equate bacterial efficacy and clinical condition. While it is impossible and unrealistic to link in vivo model outcomes to clinical situations, it is important to design an experiment that allows investigators to obtain a reasonable estimation of drug activity during a specific form of infection, e.g., in pneumonia. It is well documented that mice are not susceptible to any but the most virulent strains of S. pneumoniae (2); therefore, immunosuppression is required in order to induce lethal infection (13, 24). Recently, one strain of mouse, CBA/J, has been found to be susceptible to strains of S. pneumoniae without the use of immunosuppressive agents and has been used successfully in a pneumonia model (21).
In this in vivo investigation, the rate and extent of resolution of infection due to macrolide-sensitive and macrolide-resistant pneumococci in immune-suppressed mice were assessed, and the pharmacokinetic profile of CLR was determined. The use of immunocompetent CBA/J mice allowed a comparison to these results. Using the observed pharmacokinetic parameters along with bacterial and survival assessments, we determined the PD profile of CLR over a wide range of exposures to identify which of the PD parameters would be a reliable predictor of efficacy.
MATERIALS AND METHODS
Antibiotics, bacteria and media.
CLR laboratory standard powder was supplied by Abbott Laboratories (Chicago, Ill.) and was used for all in vitro and in vivo testing. Clindamycin powder was obtained from Sigma Chemical Co. (St. Louis, Mo.).
Eight strains of S. pneumoniae were used throughout the study and included three macrolide-susceptible isolates, four mef(A) [formerly mef(E)] isolates, and one mef(A)/erm(B) [formerly erm(AM)]isolate (19). Isolates were stored in skim milk medium (BD Biosciences, Sparks, Md.) at −80°C and subcultured twice onto Trypticase soy agar with 5% sheep blood (BD Biosciences) before use in in vitro and in vivo experiments. CLR and clindamycin MICs were determined twice in duplicate according to NCCLS methods by microdilution (15).
Pharmacokinetic analysis.
Concentrations of CLR in serum were determined after single doses of 50, 100, or 150 mg/kg of body weight in 0.2 ml administered via oral gavage to infected, leukopenic ICR mice. The vehicle for dosing solutions was 95% ethanol and sterile 0.1 M phosphate buffer (pH 6.5) in a ratio of 1:10 in which the CLR powder was suspended and then sonicated for 30 min. Blood samples from two or three mice per time point were obtained via cardiac puncture after euthanasia through CO2 inhalation and cervical dislocation at 0.25, 0.5, 1, 2, 4, 6, 8, and 12 h after dosing. Blood samples were centrifuged for 10 min at 6,900 × g, and the serum was frozen at −80°C until assayed. A validated high-pressure liquid chromatography assay was used to determine CLR concentrations as previously reported (12). The standard curve ranged from 4.0 to 0.02 μg/ml, the limit of detection. Intra- and interrun variabilities of quality control samples were ≤6.7 and ≤5.3%, respectively.
CLR pharmacokinetic data analysis was performed using the nonlinear, mixed-effect model (double precision, version IV, level 1.0; NONMEM Project Group, San Francisco, Calif.). A two-compartment structural model with first-order absorption and elimination from the central compartment was used. The basic parameters were elimination clearance (CL), volume of distribution of the central compartment (Vc), volume of distribution of the peripheral compartment, intercompartment clearance, and the absorption constant (PREDPP subroutines ADAVA 4 and TRANS 4). The elimination constant was defined as CL/Vc. An exponential-error model was used to describe the interindividual variability in pharmacokinetic parameters. The residual variability in the model was described with an additive-error model. The first-order conditional estimation process was employed in NONMEM analyses.
After NONMEM analyses, the resulting population pharmacokinetic parameter estimates were used to simulate the pharmacokinetic profile of various dosing regimens using WinNonlin (version 3.0; Pharsight Corp., Mountain View, Calif.). The area under the concentration-time curve (AUC), maximum concentration of drug in serum (Cmax), time to Cmax, and half-life were then calculated from the respective simulated profiles.
While the extent of protein binding may substantially alter the magnitude of the PD parameter of interest, the degree of protein binding of CLR in mice is comparable to that in humans (William A. Craig, Madison, Wis., personal communication). Because the serum protein binding of CLR is comparable for the two species, the mouse data should be predictive of activity in humans without mathematical transformation. As a result, all pharmacokinetic and PD parameter estimates were calculated by using total CLR concentrations.
Pneumonia model.
Specific-pathogen-free outbred female ICR mice (Harlan Sprague Dawley, Inc., Indianapolis, Ind.) weighing approximately 25 g were used throughout the study. Animals were kept in accordance with National Research Council recommendations and were allowed food and water ad libitum (10). Mice were rendered leukopenic by intraperitoneal injection of cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, N.J.), 150 mg/kg in 0.2 ml on days −4 and −1 before inoculation (1, 11). The second subculture of S. pneumoniae strains was prepared less than 20 h before use in inoculum. Just prior to use, a suspension of 108 CFU of S. pneumoniae per ml was prepared in a 5% dextrose saline solution by adjusting to a 3.0 McFarland turbidity standard. Final inoculum densities (CFU per milliliter) were confirmed by serial dilution and culture of an aliquot of each inoculum prepared.
Mice were lightly anesthetized with isofluorane (2% vol/vol in 100% oxygen carrier) until the respiratory rate decreased upon visual inspection, at which time a 0.05-ml bacterial suspension was instilled by peroral administration. Tracheal inoculation was completed by blocking the nares of the animal, requiring inhalation through the mouth and thus drawing the inoculum into the lungs. Mice were allowed to fully recover from anesthesia in an oxygen-enriched chamber and were then randomized into survival, bacterial density, or control groups. CLR dosing began 12 to 14 h after inoculation (0 h dosing time) and continued for a total of 5 days. Control animals received blank vehicle.
Efficacy as assessed by bacterial density in lung tissue.
Cohorts of four to eight mice were randomized to the following groups: 0-h control, 24-h nontreated control, or 24-h CLR treatments. Three to five CLR treatment groups were studied per S. pneumoniae isolate. Doses of CLR ranged from 5 to 600 mg/kg every 24 h (q24h) administered as one to four doses daily. Just prior to the 0-h dosing, lung tissue from control animals was harvested and cultured. After 24 h of dosing, lung tissue was harvested and cultured from the control and treatment groups. After euthanasia, the pleural cavity was opened and all five lobes of the lung were removed aseptically, rinsed with sterile water, blotted, and placed in a sterile tube containing 0.5 ml of isotonic saline. Lung tissues were homogenized with a tissue homogenizer (PRO Scientific, Monroe, Conn.), and appropriate 10-fold dilutions made and plated onto agar media. All cultures were incubated at 35°C in 5% CO2 for 24 to 48 h, at which time the viable CFU were enumerated. Antibiotic carryover was minimized by the increased incubation time and CO2 and allowing the samples to absorb into the medium before streaking over the agar surface (14). The limit of detection of S. pneumoniae growth was 5 CFU/ml of lung homogenate except during testing of the sensitive S. pneumoniae strains, where the limit of detection was 500 CFU/ml due to antibiotic carryover with doses above 50 mg/kg.
The change in bacterial density was calculated by subtracting the mean log10 CFU/ml of lung homogenate of the control mice obtained just prior to antibiotic administration (0 h) from the log10 CFU/ml of lung homogenate of each nontreated or CLR-treated mouse at the end of 24 h of therapy. The mean change in bacterial density of each group value was used in PD analyses.
Efficacy assessment using survival analysis.
Groups of 19 to 25 ICR mice were similarly infected with each test strain for each CLR treatment group for evaluation of survival over 120 h of therapy. Each CLR treatment regimen group consisted of 14 or 15 mice, with three to five CLR treatment groups tested per isolate. Dosages of CLR were similar to those used in the bacterial density studies. The remaining animals received an equal volume of dosing vehicle and served as the control population for each isolate. The percent survival (%S) was calculated at the end of 120 h of therapy and the median survival time (MST) was calculated over 120 h. In addition, preliminary experiments monitored survival over 120 h for control animals not inoculated with S. pneumoniae to confirm that death was not related to immunosuppression or processes other than S. pneumoniae infection.
Although death has historically been used as an end point for studies of this type, this end point is not suitable in the present era of animal research. Therefore, our study method has been modified to meet contemporary standards, which have been recently employed in studies conducted at our institution (9). Although our experiment may lead to death, we make every attempt to lessen the duration of pain and suffering by utilizing the following approaches: (i) the animals were monitored not less than thrice daily by members of the study team with training and experience in recognizing the signs of illness and abnormal behavior, and (ii) animals which appeared to have substantial alterations in posture (e.g., abnormal posture or head tucked into abdomen), coat, exudate around eyes and/or nose, and breathing or movement were removed from the group housing and were euthanized. Therefore, although the term “mortality” is used as an end point for this study, it should be understood that death due to the natural infection process and euthanasia were considered the same end point for experimental and statistical purposes.
Additionally, an inbred strain of mice, CBA/J (female; average, 22 g; National Cancer Institute/Charles River Laboratories, Frederick, Md., and The Jackson Laboratory, Bar Harbor, Maine), was used as comparative immunocompetent animals. Unlike the ICR mice, these mice are susceptible to certain strains of both penicillin-susceptible and -resistant S. pneumoniae without the use of immunosuppressive agents (21). These animals were used in the same procedures as the ICR mice but with selected S. pneumoniae isolates (S. pneumoniae 21, 100, and 102) without the use of cyclophosphamide, except in one run, when S. pneumoniae 21 was utilized to directly compare with immunocompromised ICR mice. Groups of five or six mice per run were used for lung cultures and survival controls and groups of ten were used for survival during CLR treatment.
PD and statistical analysis.
Spearman's correlation coefficient test was used to evaluate the following relationships in the data obtained from the ICR mice: (i) mean change in log10 CFU versus total %S or MST; (ii) mean change in log10 CFU with duration (percent) of the time that serum CLR concentrations remain above the MIC (%T>MIC), AUC from 0 to 24 h (AUC0-24)/MIC, and Cmax/MIC; (iii) total %S with %T>MIC, AUC0-24/MIC, and Cmax/MIC; and (iv) MST with %T>MIC, AUC0-24/MIC, and Cmax/MIC. A P value of <0.01 was considered significant.
Furthermore, these relationships were characterized by nonlinear regression using a sigmoidal Emax model. The MST was determined by Kaplan-Meier analysis limited to 120 h. The corresponding PD parameters of the CLR treatments that inhibit the log-phase growth of the S. pneumoniae isolate at 24 h are termed bacteriostatic; treatments which decrease the log10 CFU by 1 log (or more) are designated bactericidal. These PD parameter values were determined from the line of the Emax curve. For survival studies, the point at which the Emax sigmoidal curve reached a horizontal plateau is the PD parameter value defining efficacy.
RESULTS
Median MICs of CLR and clindamycin and resistance phenotypes for all S. pneumoniae isolates are recorded in Table 1. Penicillin interpretations for the organisms ranged from sensitive to resistant. Median CLR MICs were used in calculating PD values; for S. pneumoniae 106, an MIC of 128 μg/ml was used. The selected test organisms represent a wide range of sensitivities to CLR and thus allow the opportunity for PD modeling over a representative range of anticipated values in clinical practice.
TABLE 1.
MICs and genotypes for S. pneumoniae isolates
| Straina | Median MIC (μg/ml) of:
|
||
|---|---|---|---|
| Clindamycin | CLR | Genotype | |
| 21 | 0.03 | 0.03 | Non-mef(A) or -erm(B) |
| 75 | 0.09 | 0.03 | Non-mef(A) or -erm(B) |
| 84 | 0.09 | 0.03 | Non-mef(A) or -erm(B) |
| 100 | 0.06 | 1.0 | mef(A)b |
| 102 | 0.06 | 4.0 | mef(A)b |
| 87 | 0.09 | 4.0 | mef(A) |
| 109 | 0.06 | 8.0 | mef(A) |
| 106 | >16.0 | >128.0 | mef(A) and erm(B)b |
Internal strain designation.
Genotype provided by Abbott Laboratories.
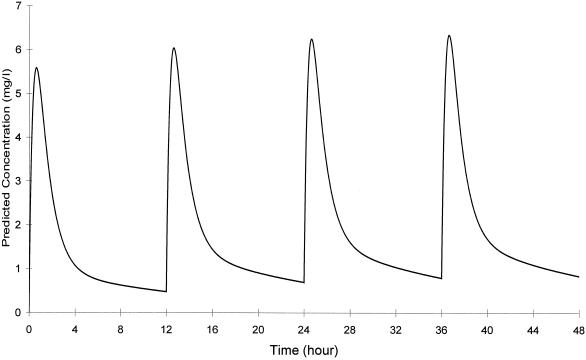
The steady-state pharmacokinetic parameters of CLR in ICR mice were as follows: AUC0-12 = 24 μg·h/ml and Cmax = 6.3 μg/ml for the 150-mg/kg dose; time to Cmax = 0.6 h; and half-life = 10.5 h. Dosages ranging from 5 to 600 mg/kg q24h administered in one to four doses were used to effect a variety of CLR exposures and a broad range of calculated PD values. The dosing regimen of 150 mg/kg twice a day (BID) produced an AUC which most closely reflected those seen after oral administration of 500 mg BID in humans (12, 25). A simulated pharmacokinetic profile is shown in Fig. 1.
FIG. 1.
Simulated pharmacokinetic profile of CLR in infected, immunocompromised ICR mice after a 150-mg/kg dose.
The mean bacterial density from the 0-h control ICR animals was 6.33 ± 0.81 log10 CFU (range, 4.9 to 7.4). The average change in log10 CFU in the untreated control groups at 24 h was an increase of 1.01 ± 1.10 (−0.2 to +2.9), with counts at 7.34 ± 0.44, ranging from 6.8 to 7.9. For all CLR treatment groups, the overall mean change in log10 CFU ranged from −3.6 to +1.5. The total number of treatment and control groups was 42.
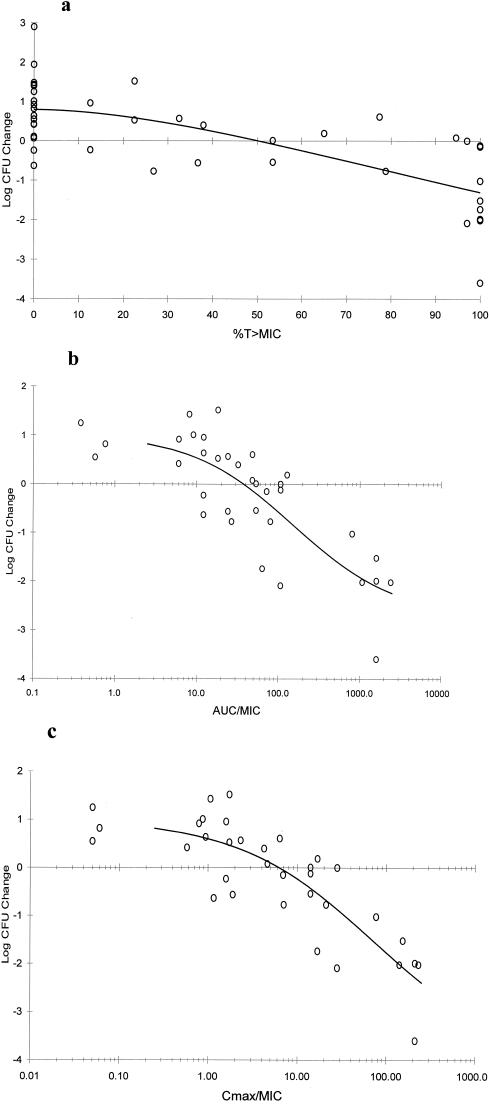
All three PD parameters exhibited a significant correlation with the change in log10 CFU (P < 0.001). Figure 2 shows sigmoidal Emax model characterization curves of the relationships between change in log10 CFU in lung tissue versus %T>MIC, AUC0-24/MIC, and Cmax/MIC, with correlation values (r2) of 0.72, 0.79, and 0.82, respectively. Efficacy as identified by a bacteriostatic effect and determined by Emax fit was detected at approximately 50% T>MIC, while an AUC0-24/MIC of approximately 40 and a Cmax/MIC approaching 7 appeared to be bacteriostatic. A T>MIC of 90%, an AUC0-24/MIC of 200, and a Cmax/MIC of 12, however, are more consistent with bactericidal effects.
FIG. 2.
Change in CFU recovered from lung tissue (n = 42) for all S. pneumoniae strains (n = 8) after 24 h versus %T>MIC (r2 = 0.72) (a), AUC0-24/MIC (r2 = 0.79) (b), and Cmax/MIC (r2 = 0.82) (c). Each point represents four to eight mice; data for controls and treatment groups with mean AUC0-24/MIC or Cmax/MIC values of <0.1 are not shown (range, −0.24 to +2.90).
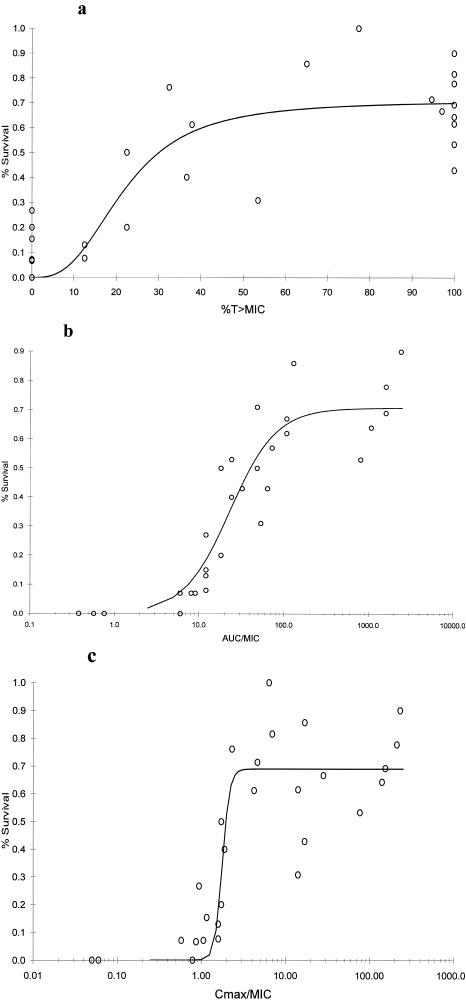
The relationships between %S of the ICR mice at 120 h and each PD parameter defined by the sigmoidal Emax model are depicted in Fig. 3; a significant correlation was noted (P < 0.001), with r2 for all Emax curves being 0.89 to 0.92. Mortality of control animals by 120 h was >93%, with the exclusion of S. pneumoniae 84, where 50% mice survived over the observation period. The %S data from S. pneumoniae 84 were not included in analyses, leaving a total of 36 data collection groups. Maximum %S as defined by the Emax model for all three PD parameters approached only 70% and further exemplifies the rigorous antibiotic challenge represented by this neutropenic murine model.
FIG. 3.
Cumulative %S for seven S. pneumoniae strains over 120 h versus %T>MIC (r2 = 0.90) (a), AUC0-24/MIC (r2 = 0.92) (b), and Cmax/MIC (r2 = 0.89) (c). Each point (n = 36) represents 5 to 10 mice (controls) or 14 or 15 mice (treatment groups); data for controls and treatment groups with mean AUC0-24/MIC or Cmax/MIC values of 0.0 are not shown (range, 0 to 30%).
Upon visual inspection of survival curves, a %T>MIC of ≥80%, an AUC0-24/MIC of approximately 200, and a Cmax/MIC of 2 represent PD parameter efficacy values as delineated by Emax model curves. Regardless of significant r2 values, a wide range of survival (40 to 90%) was noted at the maximally observed PD values.
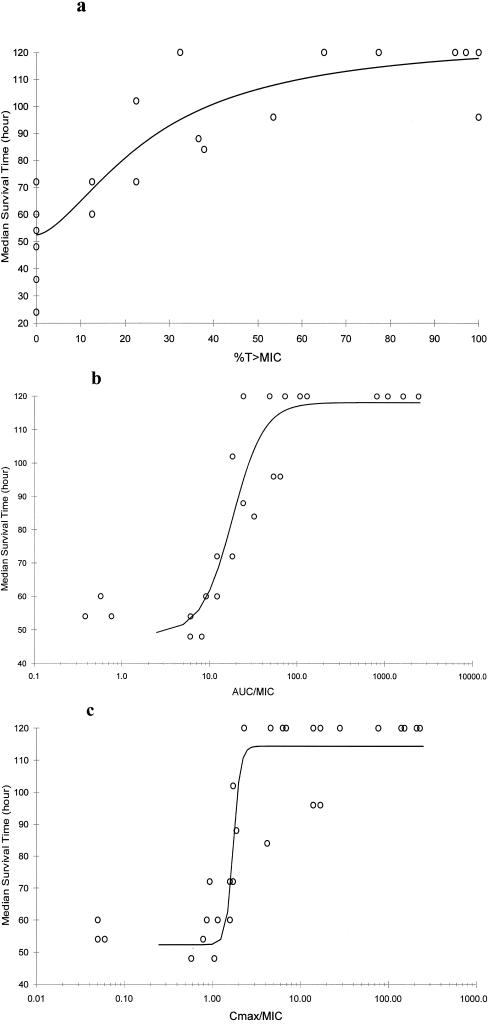
The average MST for all ICR mice in the control groups was 47.1 ± 14.9 h (range, 24 to 72). The MST for CLR-treated groups ranged between 24 and 120 h, with 120 h used as the limit of observation. As with the change in log10 CFU and %S, all PD parameters demonstrated a statistically significant correlation with MST (P < 0.001). Emax correlation values revealed virtually identical r2 values of 0.93 to 0.94 (Fig. 4). Efficacy values are quantified by a T>MIC of 70%, an AUC0-24/MIC of 100, and a Cmax/MIC of 3 for MST.
FIG. 4.
Median survival time over 120 h versus %T>MIC (r2 = 0.94) (a), AUC0-24/MIC (r2 = 0.94) (b), Cmax/MIC (r2 = 0.93) (c). Each point (n = 36) represents 5 to 10 mice (controls) or 14 or 15 mice (treatment groups) for S. pneumoniae isolates (n = 7); data for controls and treatment groups with mean AUC0-24/MIC or Cmax/MIC values of 0.0 are not shown (range, 24 to 72 h).
The correlation between the change in log10 CFU and %S (linear regression [r] = 0.64) in the ICR mice was significant (P = 0.002). A bacteriostatic effect was predictive of roughly 60% survival. The MST was also closely associated with the change in log10 CFU (r = 0.61; P = 0.001).
Based on these data, not one of the three PD parameters is an outstanding candidate as predictor of efficacy when measured by antibacterial activity or survival in this model of S. pneumoniae pneumonia in ICR mice. In fact, all appear to be equally predictive, with the exception of %T>MIC compared to the change in log10 CFU. While the r2 value for AUC0-24/MIC is certainly higher that for Cmax/MIC for %S and median survival time, the correlation numbers are close. By observation of r2 and by visual inspection of the graphs, these two PD parameters are comparable. Considering the correlation values, the PD parameter which most consistently appears to have the best fit with outcome measurements is AUC0-24/MIC, but Cmax/MIC is a close second. Given the less definitive curves with %T>MIC versus both the change in log10 CFU and survival, the default parameter of the AUC0-24/MIC should be chosen as the parameter foretelling the extent and rate of infection resolution due to CLR therapy, with positive outcomes predicted at values of >100.
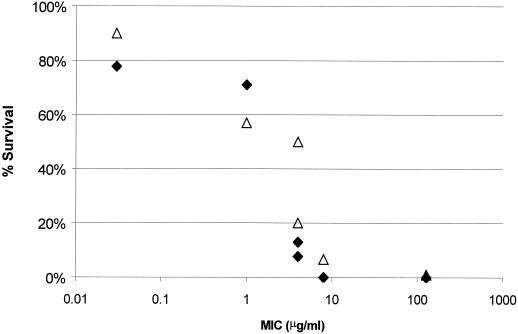
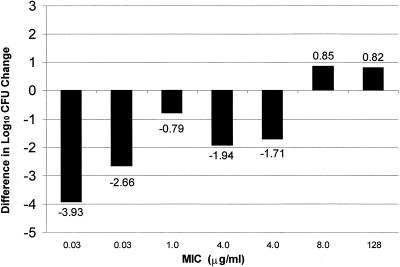
Infection with macrolide-nonsusceptible S. pneumoniae isolates for which CLR MICs were ≥4 μg/ml led to decreased survival (Fig. 5) after the regimen of 150 mg/kg q12h, which resembles 500 mg BID in humans in this model. An increased dosage of 150 mg/kg thrice daily did not improve survival for the isolates for which MICs were 8 and 128 μg/ml. When the bactericidal activity of CLR after the dose of 150 mg/kg q12h was evaluated, killing of pneumococci for which MICs were <4 μg/ml was observed (Fig. 6). After 24 h, this treatment resulted in a 0.8- to 3.9-log decrease in CFU compared to CFU levels in the nontreated animals. The difference in change in log10 CFU between treatment and control groups at 24 h revealed that CLR is less effective against macrolide-resistant S. pneumoniae isolates for which MICs were >4 μg/ml. At MICs above 4 μg/ml, the 150-mg/kg BID CLR treatment resulted in a decreased bacterial clearance. This treatment in animals infected with S. pneumoniae for which MICs were 8 and 128 μg/ml resulted in growth of approximately 0.8 log CFU, comparable to the nontreated animals.
FIG. 5.
Cumulative %S over 120 h after CLR treatment of 150 mg/kg BID (⧫) or 150 mg/kg TID (▵) after infection with S. pneumoniae (six strains) versus MIC (each point represents 14 or 15 mice).
FIG. 6.
Difference in log10 CFU change in lung tissue at 24 h between CLR-treated (150 mg/kg BID) and nontreated (control) neutropenic ICR mice.
In contrast to the ICR mice, immunosuppression in the CBA/J mice was not required for them to succumb to lethal infection with these penicillin- and macrolide-resistant S. pneumoniae isolates. The mean bacterial density at 0 h in the control CBA/J mice was 6.0 ± 1.5 log10 CFU (range, 4.0 to 7.7), similar to that in the ICR mice. The average change in log10 CFU in the nontreated groups at 24 h was an decrease of 0.51 ± 1.0, ranging from +1.0 to −1.8, with counts of 5.45 ± 2.0 (range, 2.9 to 8.4) for all three S. pneumoniae strains. Only the most virulent of the three S. pneumoniae isolates, S. pneumoniae 21, had increased bacterial density at 24 h of approximately 1 log; CFU of both S. pneumoniae 100 and 102 decreased roughly 1 log. For S. pneumoniae 100 and 102 at 24 h, bacterial counts were 4.11 ± 1.55 and 4.98 ± 1.24 log10 CFU, a decline of −1.20 ± 0.57 and −0.72 ± 0.59, respectively. S. pneumoniae 21 counts increased 0.85 ± 0.26 log in the CBA/J mice, similar to the 1-log increase seen in ICR mice at 24 h.
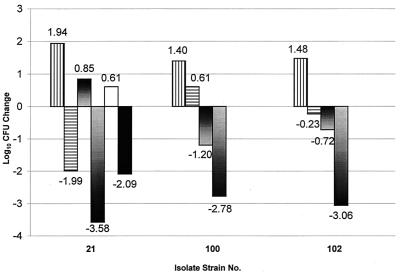
The CLR treatment regimen of 150 mg/kg q12h was administered to nonneutropenic CBA/J mice for comparison to immunocompromised ICR mice. After this treatment in the CBA/J mice, the mean overall change in log10 CFU in lung tissue extended from −2.5 to −4.0. In comparison to the leukopenic ICR mice, a further decrease in bacterial density was observed in the CBA/J mice (Fig. 7) after infection with all three S. pneumoniae isolates. Overall differences between the ICR and CBA/J mice after this specific dose ranged from approximately 2 to 3.5 logs compared to the 0-h bacterial counts, indicating the major role of leukocytes in the clearance of bacteria from the lungs. The dissimilarity in bacterial recoveries at 24 h between the two murine species may have been anticipated owing to the presence of leukocytes. Nevertheless, a frank reduction of log CFU between the two species of mice resulting from the CLR treatment calculated as the difference between the bacterial density in the 24 h control group and the CLR treatment group for each species showed the following decrease of bacterial density in CBA/J mice: for S. pneumoniae 21, −0.5 log; for S. pneumoniae 100, −0.8 log; and for S. pneumoniae 102, −0.6 log.
FIG. 7.
Mean change in log10 CFU in lung tissue at 24 h after CLR (150 mg/kg BID), shown by bars (left to right for each strain), in immunocompromised ICR mice (n = 4), ICR untreated controls (n = 4), immunocompetent CBA/J mice (n = 6 to 10), and CBA/J untreated controls (n = 4 to 6). The black bar and white bar represent the immunocompromised CBA/J mice at 24 h after CLR (150 mg/kg BID; n = 5) and untreated controls (n = 5), respectively.
A group of five CBA/J mice were also tested after induction of leukopenia. This group of leukopenic CBA/J mice demonstrated a 2-log decrease in CFU after infection with S. pneumoniae 21, not unlike the leukopenic ICR mice. This bacterial killing is indicative of the activity of the CLR without the augmented bacterial clearance of S. pneumoniae by leukocytes.
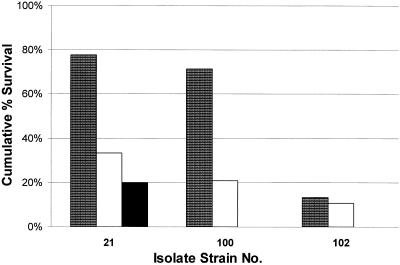
As with the ICR mice, survival of CBA/J mice in CLR treatment groups was monitored over 5 days. Mortality of control animals was 98% within the observation period. Survival of the CLR-treated groups varied from 10 to 40% at the end of 120 h. Survival was not enhanced by the presence of white cells compared to that of the leukopenic ICR mice after a CLR treatment of 150 mg/kg BID (Fig. 8). These results were confirmed by duplicating experiments and a repeat of the experiment with CBA/J mice from a second vendor. Excessive mucus was present in the pleural cavities of infected control and treatment animals; subsequently, lungs were examined for histological changes by an outside animal testing facility (An-Path Laboratory, Farmington, Conn.). Changes in lung tissue consistent with an overwhelming pneumococcal pneumonia were present and reported as severe fibrinous pleuritis. The presence of white cells may have brought about a decrease in mean bacterial densities but may have also augmented the immune response sufficiently to result in the buildup of mucus and purulent fluid in the lungs, with death ensuing.
FIG. 8.
Cumulative %S at 120 h after CLR (150 mg/kg BID) between immunocompromised ICR mice (patterned bars; n = 14 or 15), immune competent CBA/J mice (clear bars, n = 19 to 32), and immunocompromised CBA/J mice (solid bar, n = 11).
DISCUSSION
There is a general lack of consensus on the classification of macrolides as concentration-dependent or concentration-independent antimicrobials. The PD profile of erythromycin demonstrates that the duration of time above the MIC (%T>MIC) is the best predictor of efficacy, whereas AUC0-24/MIC was shown to be the better correlate for azithromycin (5, 7). CLR is considered concentration dependent by some investigators (Mazzei et al., 5th Int. Conf. Macrolides, Azalides, Streptogramins, Ketolides Oxazolidones) and concentration independent by others (4). Further, some investigators consider CLR to be positioned between erythromycin and azithromycin, having elements of both concentration-dependent and independent bacterial killing (7, 18).
Applying the most commonly used PD parameters over a wide range of CLR exposures, we have demonstrated that %T>MIC, AUC0-24/MIC, and Cmax/MIC are closely correlated to bacterial density and survival. In this model of murine pneumonia, the sigmoidal Emax correlation values were high from the observed %S data; however, variability remained after maximum efficacy. Perhaps the better measure is the MST, where efficacy was more clearly delineated by the Emax curve. As a consequence of parameter interdependency, AUC0-24/MIC is the most reasonable predictor of antibiotic efficacy as measured by the change in log10 CFU and MST. An AUC0-24/MIC of 100 predicts both a bactericidal response and a maximum MST.
Survival in the CBA/J mice was unexpectedly low, as these mice have been used successfully in a pneumonia model by Tateda et al. (21). The inoculum used in the present study, however, was 3 log10 CFU/ml higher and may have produced an overwhelming, acute infection in contrast to the model used by Tateda et al. Furthermore, those investigators did not begin antibiotic administration until 48 to 96 h after inoculation, which further indicates a less severe infection.
CLR is known to have extensive tissue distribution, and the pharmacokinetic profile and subsequent concentrations at the site of infection, in this case the epithelial lining fluid, may be the crucial factor for extracellular pathogens such as S. pneumoniae. PD parameters based on levels in serum may ultimately be shown not to be the optimum models for these drugs (16). Concentrations in epithelial lining fluid that are 10- to 20-fold higher than levels in serum in humans have been reported (17, 20). In one study, Veber et al. also demonstrated elevated levels of CLR in lungs of S. pneumoniae-infected mice on the order of 12-fold (23). Usual serum AUC values in humans after a 500-mg dose are 19 to 22 μg·h/ml (12, 25). A typical regimen of 500 mg BID results in an AUC24 below 50 μg·h/ml; thus, S. pneumoniae strains for which MICs are greater than 0.5 μg/ml would be predicted to fail with this regimen, based on the PD values above. Knowing that actual concentrations at the site of infection are manyfold higher than concentrations in serum, S. pneumoniae isolates for which MICs are increased may be adequately treated with typical regimens. This phenomenon was also evident in our neutropenic pneumonia model, as doses simulating those in humans were efficacious, as defined by improved survival and bactericidal activity with MICs up to 4 μg/ml, eightfold higher than the predicted 0.5 μg/ml.
Additionally, in immunocompetent animals a pronounced bactericidal activity was observed relative to the neutropenic animals despite a CLR MIC of 4 μg/ml. As a result, these data call into question the appropriateness of the current designations utilized for in vitro susceptibility testing for CLR. Moreover, this apparent discrepancy between CLR in vitro resistance as defined by the NCCLS and the clinical outcome of patients receiving this agent was recently highlighted by Gotfried (8).
CLR does not appear to have the individual features of either concentration- or time-dependent antibiotics. The parameter AUC0-24/MIC seems best suited as a predictive index for CLR, as it takes both the time above the MIC and the peak concentration into account. Further PD studies with CLR which utilize concentrations at the site of infection are warranted if the association between in vitro susceptibility testing and clinical outcome is to be completely understood.
Acknowledgments
We thank the research group at the University of Iowa, in particular Angela Brueggemann, for assistance in the application of this animal model. We also acknowledge two sources of institutional assistance: Research Administration for statistical analysis and the Animal Research Facility for their procurement and care of the animals. C. H. Nightingale has a financial relationship with Abbott Laboratories.
This project was supported by a grant (128148) from Abbott Laboratories.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azoulay-Dupuis, E., V. Rieux, M. Muffat-Joly, J. P. Bedos, E. Vallee, C. Rivier, R. Isturiz, C. Carbon, and P. Moine. 2000. Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob. Agents Chemother. 44:1575-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauerfeind, A., R. Jungwirth, and E. Eberlein. 1995. Comparative pharmacodynamics of clarithromycin and azithromycin against respiratory pathogens. Infection 23:316-320. [DOI] [PubMed] [Google Scholar]
- 4.Carbon, C. 1998. Pharmacodynamics of macrolides, azalides, and streptogramins: effect on extracellular pathogens. Clin. Infect. Dis. 27:28-32. [DOI] [PubMed] [Google Scholar]
- 5.den Hollander, J. G., J. D. Knudsen, J. W. Mouton, F. Fuursted, N. Frimodt-Moller, H. A. Verbrugh, and F. Espersen. 1998. Comparison of pharmacodynamics of azithromycin and erythromycin in vitro and in vivo. Antimicrob. Agents Chemother. 42:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doern, G. V., M. A. Pfaller, K. Kugler, J. Freeman, and R. N. Jones. 1998. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the Sentry antimicrobial surveillance program. Clin. Infect. Dis. 27:764-770. [DOI] [PubMed] [Google Scholar]
- 7.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl. 3):38-44. [PubMed] [Google Scholar]
- 8.Gotfried, M. H. 2000. Comparison of bacteriologic eradication of Streptococcus pneumoniae by clarithromycin and reports of increased antimicrobial resistance. Clin. Ther. 22:2-14. [DOI] [PubMed] [Google Scholar]
- 9.Hamm, T. E. 1995. Proposed institutional animal care and use committee guidelines for death as an end point in rodent studies. Contemp. Top. Lab. Anim. Sci. 34:69-71. [Google Scholar]
- 10.Institute of Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 11.Joly-Guillou, M.-L., M. Wolff, J.-J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy, M. K., R. C. Owens, Jr., X. Xu, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1998. Comparison of bacterial activity after multidose administration of clarithromycin, azithromycin, and cefuroxime axetil against Streptococcus pneumoniae. Int. J. Antimicrob. Agents 10:279-283. [DOI] [PubMed] [Google Scholar]
- 13.Moine, P., E. Vallee, E. Azoulay-Dupuis, P. Bourget, J.-P. Bedos, J. Bauchet, and J.-J. Pocidalo. 1994. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob. Agents Chemother. 38:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved standard. NCCLS document M26-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 5th ed. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Nightingale, C. H. 1997. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr. Infect. Dis. J. 16:438-443. [DOI] [PubMed] [Google Scholar]
- 17.Patel, K. B., D. Xuan, P. R. Tessier, J. H. Russomanno, R. Quintiliani, and C. H. Nightingale. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Periti, P., and T. Mazzei. 1999. Clarithromycin: pharmacokinetic and pharmacodynamic interrelationships and dosage regimen. J. Chemother. 11:11-27. [DOI] [PubMed] [Google Scholar]
- 19.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodvold, K. A., M. H Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tateda, K., K. Takashima, H. Miyazaki, T. Matsumoto, T. Hatori, and K. Yamaguchi. 1996. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob. Agents Chemother. 40:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornsberry, C., P. Ogilive, J. Kahn, Y. Mauriz, and the Laboratory Investigator Group. 1997. Surveillance of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States in 1996-1997 respiratory season. Diagn. Microbiol. Infect. Dis. 29:249-257. [DOI] [PubMed] [Google Scholar]
- 23.Veber, B., E. Vallee, J. M. Desmonts, J. J. Pocidalo, and E. Azoulay-Dupuis. 1993. Correlation between macrolide lung pharmacokinetics and therapeutic efficacy in a mouse model of pneumococcal pneumonia. J. Antimicrob. Chemother. 32:473-482. [DOI] [PubMed] [Google Scholar]
- 24.Woodnutt, G., and V. Berry. 1999. Two pharmacodynamic models for assessing the efficacy of amoxicillin-clavulanate against experimental respiratory tract infection caused by strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuckerman, J. M., and K. M. Kaye. 1995. The newer macrolides: azithromycin and clarithromycin. Infect. Dis. Clin. N. Am. 9:731-745. [PubMed] [Google Scholar]