Abstract
Enzyme-catalyzed therapeutic activation (ECTA) is a novel prodrug strategy to overcome drug resistance resulting from enzyme overexpression. β-Lactamase overexpression is a common mechanism of bacterial resistance to β-lactam antibiotics. We present here the results for one of the β-lactamase ECTA compounds, NB2001, which consists of the antibacterial agent triclosan in a prodrug form with a cephalosporin scaffold. Unlike conventional β-lactam antibiotics, where hydrolysis of the β-lactam ring inactivates the antibiotic, hydrolysis of NB2001 by β-lactamase releases triclosan. Evidence supporting the proposed mechanism is as follows. (i) NB2001 is a substrate for TEM-1 β-lactamase, forming triclosan with a second-order rate constant (kcat/Km) of greater than 77,000 M−1 s−1. (ii) Triclosan is detected in NB2001-treated, β-lactamase-producing Escherichia coli but not in E. coli that does not express β-lactamase. (iii) NB2001 activity against β-lactamase-producing E. coli is decreased in the presence of the β-lactamase inhibitor clavulanic acid. NB2001 was similar to or more potent than reference antibiotics against clinical isolates of Staphylococcus aureus (including MRSA), Staphylococcus epidermidis, Streptococcus pneumoniae, vancomycin-resistant Enterococcus faecalis, Moraxella catarrhalis and Haemophilus influenzae. NB2001 is also active against Klebsiella pneumoniae, Enterobacter aerogenes, and Enterobacter cloacae. The results indicate that NB2001 is a potent, broad-spectrum antibacterial agent and demonstrate the potential of ECTA in overcoming β-lactamase-mediated resistance.
Increased resistance of bacterial infections to antibiotic treatment has been extensively documented (30) and has now become a generally recognized medical problem, particularly with nosocomial infections (9, 21, 23, 29).
β-Lactam antibiotics are among the most widely used antimicrobial agents. Mechanistically, they act by covalently binding to penicillin-binding proteins in the cytoplasmic membrane of bacteria, thereby inhibiting peptidoglycan transpeptidase and eventually leading to cell death (32).
A common mechanism for bacterial resistance to β-lactam antibiotics is via production of β-lactamases, which catalyze the hydrolysis of the amide bond of the β-lactam ring, resulting in ring opening and antibiotic inactivation. Numerous β-lactamases exist, encoded either by chromosomal genes or by transferable genes located on plasmids or transposons (3, 17). Four molecularly distinct β-lactamase classes (A, B, C, and D) have been defined based on amino acid and nucleotide sequence analysis (18). Of these, class A, to which the plasmid-mediated TEM-1 enzyme belongs, is the most prevalent in clinical isolates (26).
The classical approach to circumvent resistance has been to develop new β-lactam antibiotics resistant to β-lactamase hydrolysis. However, new β-lactamases have risen to meet the challenge of new synthetic β-lactam derivatives (11, 14, 18, 25). A second approach has been to develop novel antibiotics. However, these agents are often more toxic than their predecessors, and many are not orally active. A third strategy is to develop β-lactamase inhibitors that have been successful primarily with class A enzymes. However, resistance to β-lactamase inhibitors via point mutations in the β-lactamase gene has been reported (25). Further, some β-lactamase inhibitors (e.g., clavulanic acid and sulbactam) induce β-lactamase expression, which diminishes their effectiveness (10, 15). Thus, a new approach is needed to intercept the established cycle of drug resistance.
Enzyme-catalyzed therapeutic activation (ECTA) is a novel prodrug strategy to overcome drug resistance resulting from enzyme overexpression. In this approach, specific enzyme substrates are designed for conversion by the overexpressed enzyme into cytotoxic agent(s). Based on the ECTA concept, β-lactamase can be used as an engine to generate antibacterial agents in drug-resistant microorganisms. We describe here the design, synthesis, and in vitro evaluation of the β-lactamase ECTA compound NB2001. The formation of triclosan from NB2001 via β-lactamase hydrolysis is demonstrated. The dependence of antibacterial activity on β-lactamase is demonstrated in an Escherichia coli model system, where NB2001 was more active against a TEM-1 β-lactamase-overproducing strain than a nonproducing strain; its antibacterial activity against the former was antagonized by β-lactamase inhibitors. Further in vitro studies showed NB2001 to be active against major gram-positive and gram-negative pathogens.
MATERIALS AND METHODS
Chemicals.
Chemical reagents and anhydrous solvents were purchased from Aldrich Chemicals (Milwaukee, Wis.). 7-Aminocephalosporanic acid was purchased from Sigma (St. Louis, Mo.) or Antibiotico (Milan, Italy), and cephalothin, oxacillin sodium salt, gentamicin sulfate, ampicillin sodium salt, and vancomycin hydrochloride were from Sigma. Nitrocefin was from Calbiochem (San Diego, Calif.). Sulbactam sodium was from Morepen Laboratories, Ltd. (New Delhi, India). Lithium clavulanate was a generous gift from Smithkline Beecham (West Sussex, United Kingdom), and triclosan was kindly provided by KIC Chemicals (Armonk, N.Y.).
Bacterial strains.
E. coli BL21(DE3) and E. coli N (Novablue) were obtained from Novagen (Madison, Wis.). The E. coli/TEM-1 clone was generated by transforming plasmid pcDNA3.1(−) (Invitrogen, San Diego, Calif.), which constitutively expresses TEM-1 β-lactamase, in E. coli N. The bacterial strains used for determining antimicrobial activity included Staphylococcus aureus strains 700698, 700699, 43300, 700787, 700788, 700789, 33591, 33592, 33593, 33594, 700260, 13301, 11632, and 14154 (American Type Culture Collection [ATCC], Manassas, Va.). A total of 300 recent clinical isolates tested in the Microbiology Reference Lab (MRL) in Herndon, Va., were procured from the MRL Bacterial Culture Collection and included S. aureus oxacillin-susceptible (n = 20), S. aureus oxacillin-resistant (n = 20), Staphylococcus epidermidis oxacillin-susceptible (n = 15), S. epidermidis oxacillin-resistant (n = 15), Streptococcus pneumoniae (n = 10), Enterococcus faecalis (n = 15), Enterobacter cloacae (n = 30), Enterobacter aerogenes (n = 30), E. coli (n = 30), Klebsiella pneumoniae (n = 30), Pseudomonas aeruginosa (n = 10), Haemophilus influenzae (n = 30), and Moraxella catarrhalis (n = 30) strains.
All bacteria were grown in cation-adjusted Mueller-Hinton (MH) broth, except for H. influenzae, which was grown in Haemophilus Test Medium broth, and Streptococcus pneumoniae, which was grown in cation-adjusted MH broth supplemented with 2 to 5% lysed horse blood.
Bacterial cultures were tested for β-lactamase production by use of nitrocefin according to the manufacturer's instructions.
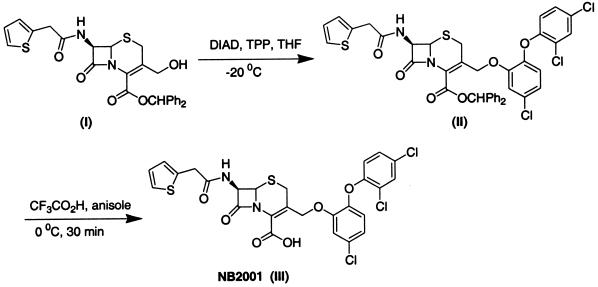
Synthesis of NB2001. (i) Diphenylmethyl 7-(2-thienylacetylamino)-3-{[5-chloro-2-(2,4-dichlorophenoxy)phenoxymethyl}-3-cephem-4-carboxylate (com-pound II [Fig. 1])
FIG. 1.
Outline of synthesis and chemical structure of NB2001 and its precursors. For details of this synthesis, see Materials and Methods.
A solution of diphenylmethyl 7-(2-thienylacetylamino)-3-hydroxymethyl-3-cephem-4-carboxylate (compound I in Fig. 1) (28) (0.52 g, 1.0 mmol) and triclosan (0.36 g, 1.25 mmol) in anhydrous THF (10 ml) was cooled to −20°C in a dry-ice bath under an argon atmosphere. A solution of triphenylphosphine (0.32 g, 1.25 mmol) in anhydrous THF (5.0 ml) was then added by using a syringe. After 10 min, a solution of diisopropyl diazodicarboxylate (0.25 g, 1.25 mmol) in THF (5.0 ml) was added slowly by using a syringe pump over 40 min. The reaction mixture was then poured into water and extracted twice with ethyl acetate (25 ml each time). The combined ethyl acetate extracts were washed sequentially with water and brine and dried over Na2SO4. Removal of volatiles, followed by purification on a silica gel column with 5% ethyl acetate in dichloromethane, provided compound II as light yellow solid (yield = 0.32 g, 32%).
(ii) 7-(2-Thienylacetylamino)-3-[5-chloro-2-(2,4-dichlorophenoxy)phenoxymethyl]-3-cephem-4-carboxylic acid (compound III in Fig. 1).
Compound II (0.75 g, 0.94 mmol) was dissolved in anhydrous anisole (4.0 ml) and cooled in an ice bath under an argon atmosphere. Trifluoroacetic acid (TFA; 9.0 ml) was then introduced slowly over 10 min. After 45 min, the volatiles were removed under reduced pressure with continuous stirring to give a light yellow residue. Ether (10 ml) was added, and the mixture was stirred at 0°C for 1 h. The resultant light yellow precipitate was filtered, washed with cold ether (10 ml), and dried under vacuum to obtain NB2001 as a light yellow solid (melting point, 105°C). The yield was 0.37 g (64%). 1H NMR (dimethyl sulfoxide [DMSO]-d6, 500 MHz): δ 3.10 (1H, d, J = 16.5 Hz), 3.75 (2H, qAB, J = 15.17, 4.75 Hz), 4.80 (1H, d, J = 12.0 Hz), 4.97 (1H, d, J = 12.0 Hz), 5.03 (1H, d, J = 4.0 Hz), 6.80 (1H, d, J = 8.7 Hz), 6.92 to 6.95 (2H, m), 7.09 to 7.07 (1H, m), 7.16 (1H, d, J = 8.7 Hz), 7.29 to 7.31 (1H, m), 7.31 to 7.37 (1H, m), 7.72 (1H, d, J = 2.8 Hz), and 9.11(1H, d, J = 8.3 Hz). Infrared analysis (neat) 1,767, 1,715, 1,662, and 1,495 cm−1. The purity of NB2001 was 98.2% as determined by high-pressure liquid chromatography (HPLC).
Chemical analyses.
Flash chromatography was performed on Merck grade 60 silica gel (230 to 400 mesh). Infrared spectra were measured neat on a Nicolet Avatar 320 spectrophotometer and are reported as per-centimeter values. Proton nuclear magnetic resonance spectra were recorded in a Varian Associates Gemini (Bruker Instruments) spectrometer operating at 500 MHz, and chemical shifts were reported relative to an internal tetramethylsilane reference at δ = 0.0 ppm. Melting points are uncorrected.
Susceptibility testing.
MICs of antimicrobial compounds were determined by the broth microdilution method (range, 0.016 to 128 μg/ml) in 96-well microtiter plates, according to National Committee for Clinical Laboratory Standards guidelines (5). Stock solutions of test compounds were prepared in water or DMSO, depending on their solubility. In the latter case, DMSO concentration in the incubation mixture did not exceed 0.5%. The bacterial inoculum was 5 × 105 CFU/ml, and growth was monitored by measuring the increase in the optical density at 600 nm (OD600) with a microplate reader (Tecan SpectraFluor Plus). MIC was defined as the lowest antibiotic concentration at which bacteria growth (OD600 > 0.05, i.e., a value equal to visible growth) was inhibited after 18 to 24 h of incubation at 35°C.
Time-kill kinetics.
Overnight liquid cultures of E. coli/ TEM-1 were diluted 100 times into MH broth and allowed to grow to exponential phase (OD600 = 0.6). Bacteria were next diluted into fresh medium to give a working concentration of 106 CFU/ml. NB2001 or triclosan was added to a final concentration corresponding to 2×, 4×, and 8× MIC, and the suspension was incubated at 37°C. Aliquots (0.1 ml) were removed at 1-h intervals for up to 6 h of incubation. Serial 10-fold dilutions down to 10−6 were prepared in saline and plated onto agar plates. The plates were incubated for 24 h at 37°C to obtain CFU counts.
Preparation of TEM-1 β-lactamase.
An N-terminal His-tagged TEM-1 construct, TEM-1/pET28b(+), was generated by subcloning TEM-1 into the NcoI and HindIII sites of pET28b(+) vector. TEM-1 was prepared by transforming TEM-1/pET28b(+) into E. coli BL21(DE3) (Novagen) strain. After induction with IPTG (isopropyl-β-d-thiogalactopyranoside), TEM-1 was purified by affinity chromatography on a Ni2+ His-binding metal chelation resin (Novagen). The Ni2+ His-binding metal chelation column was washed with 20 mM Tris (pH 7.9)-5 mM imidazole-0.5 M NaCl. TEM-1 was eluted with 20 mM Tris (pH 7.9), 100 mM imidazole, and 0.5 M NaCl at room temperature and dialyzed against 100 mM Tris (pH 8.0). Aliquots were stored at −80°C.
β-Lactamase assays.
Initial assessment of partially purified β-lactamase TEM-1 was by use of the chromogenic substrate nitrocefin on a Tecan Spectrafluor Plus with a 495 cutoff filter on the excitation path. Nitrocefin in concentrations ranging from 2 to 100 μM, diluted from a 10 mM stock in DMSO, was added to 100 mM potassium phosphate (pH 7.2) and 1 mM EDTA and incubated at 37°C for 15 min. After thermal equilibrium, 0.25 μg of TEM-1 β-lactamase/ml was added and the hydrolysis product, nitrophenol, was monitored at 486 nm. The extinction coefficient, ɛ, of nitrophenol (15,900 M−1 cm−1) was determined from a standard curve of the total hydrolysis of nitrocefin and found to be consistent with published values (1).
Enzyme hydrolysis of NB2001 was determined by fixed-time assays of varied concentrations of NB2001, followed by the addition of a 1% TFA quench. The hydrolysis product, triclosan, was separated from NB2001 by use of an HP1100 series HPLC equipped with an Alltech Adsorbosphere HS(C18) 5-ml column (50 by 4.6 mm). The mobile phase was isocratic, containing 55% acetonitrile and 0.1% TFA, producing retention times of 20.4 and 24.1 min for triclosan and NB2001, respectively. The flow rate was 1 ml min−1. Quantitation of triclosan was based on the A260 integrated peak area compared to triclosan standards.
Detection of triclosan formed from NB2001 in E. coli/TEM-1.
Overnight cultures of both wild-type E. coli N and the β-lactamase-producing strain E. coli/TEM-1 were diluted 100 times with fresh Luria broth (LB) medium, and cells were grown at 37°C with shaking at 230 rpm for 4 h. Cells were then diluted with fresh LB medium containing 100 μM NB2001 until the OD600 reached 0.2. Culture aliquots were removed at different time intervals, and the cells were pelleted by centrifugation at 10,000 × g for 2 min at 4°C. The supernatant was removed and combined with 500 μl of acetonitrile. The pelleted bacterial cells were resuspended in 100 μl of phosphate-buffered saline (pH 8.0; Gibco-BRL) and subjected to three cycles of freeze-thawing, followed by the addition of 500 μl of acetonitrile. Both pellet and supernatant extracts were centrifuged at 10,000 × g for 2 min at 4°C. Then, 550 μl of the resultant supernatant was transferred into a new Eppendorf tube and vacuum dried. Dried pellets were resuspended in 50 μl of H2O, and 100 μl of acetonitrile was added. Finally, 75 μl of the resulting solution was analyzed by HPLC as described above.
RESULTS
Design and synthesis of NB2001.
The generic design of β-lactamase ECTA compounds includes a cephalosporin backbone with a prodrug form of an antibacterial agent incorporated at the 3′ position of the cephem nucleus. Hydrolysis by β-lactamase triggers release of the antibacterial agent. One of our lead compounds, NB2001, was prepared by the condensation of cephalothin with triclosan under Mitsunobu reaction conditions (28), followed by deprotection (Fig. 1). The purity of NB2001 was 98.2% as determined by HPLC.
NB2001 hydrolysis by TEM-1 β-lactamase.
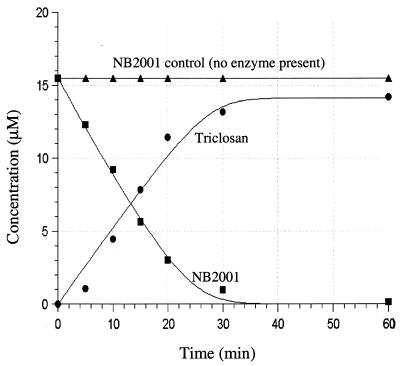
NB2001 was examined as a substrate of TEM-1 β-lactamase and compared to nitrocefin, a widely used chromogenic β-lactamase substrate with the same C-7 side chain as NB2001. As shown in Table 1, the kinetic parameters of NB2001 were comparable to nitrocefin. Interestingly, nitrocephin hydrolysis was slower than that reported in the literature, probably due to the fact that the TEM-1 enzyme was His tagged. In a further study, TEM-1 β-lactamase-mediated hydrolysis of NB2001 was found to be associated with the concomitant production of triclosan (Fig. 2).
TABLE 1.
Comparison of the β-lactamase TEM-1 kinetic constants for NB2001 and nitrocefina
| Substrate | kcata (s−1) | Kma (μM) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| NB2001 | 0.50 | 6.5 | 7.7 × 104 |
| Nitrocefin | 3.3 | 15 | 22 × 104 |
The assay conditions for NB2001 (fixed-time assay) and nitrocefin (continuous assay) hydrolyses are described in Materials and Methods. The kinetic constants kcat and Km for NB2001 were determined from a progress curve analysis fit to the integrated Michaelis-Menten equation at 16 μM NB2001 and 12.5 nM TEM-1 β-lactamase.
FIG. 2.
Time course of TEM-1-catalyzed formation of triclosan from NB2001. Assays were conducted at 37°C in 100 mM potassium phosphate buffer (pH 7.2) with 1 mM EDTA and 0.5 μg of TEM-1 β-lactamase/ml. A hydrolysis product (triclosan) was separated from NB2001 by HPLC as described in Materials and Methods. In the absence of TEM-1 β-lactamase, NB2001 was stable for at least 5 h at 37°C.
Triclosan formation from NB2001 by TEM-1-producing E. coli.
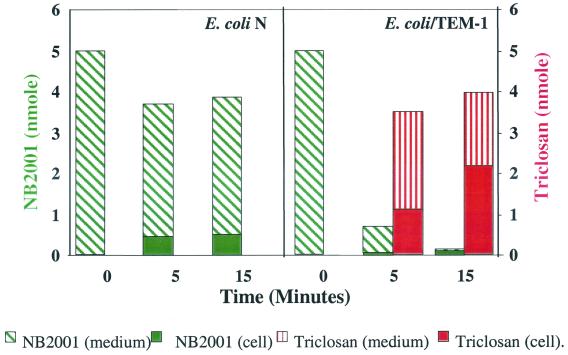
Triclosan formation by β-lactamase was examined in an E. coli model system established in our laboratory. We used the cloned β-lactamase-producing strain, E. coli/TEM-1, and its parental strain E. coli N, differing only in the expression of TEM-1 β-lactamase. As shown in Fig. 3, 80% of NB2001 was degraded, and an equimolar amount of triclosan was produced in E. coli/TEM-1 after 5 min, whereas no triclosan was detected in E. coli N after up to 15 min of incubation. Thus, triclosan production from NB2001 is dependent on β-lactamase.
FIG. 3.
Formation of triclosan from NB2001 in E. coli. E. coli N and β-lactamase-expressing E. coli/TEM-1 were treated with NB2001 under the conditions described in Materials and Methods. Culture aliquots were removed at 0, 5, and 15 min. The amount of NB2001 and triclosan was assessed by HPLC.
Dependence of antibacterial activity of NB2001 on β-lactamase.
This was initially demonstrated in the β-lactamase-producing strain E. coli/TEM-1. As shown in Table 2, NB2001 was 32-fold more potent against the E. coli/TEM-1 than against the β-lactamase-negative strain E. coli N. The increased susceptibility of E. coli/TEM-1 to NB2001 was abolished in the presence of the β-lactamase inhibitor clavulanate (Table 2), suggesting that it was dependent on β-lactamase.
TABLE 2.
Activity of NB2001 with or without β-lactamase inhibitors against wild-type and β-lactamase-producing E. coli
| Strain | Relevant phenotype | MIC (μg/ml)a of:
|
|||
|---|---|---|---|---|---|
| NB2001
|
Cephalothin | Triclosan | |||
| Alone | With 4 μg of CLA/ml | ||||
| E. coli N | BLA− | 0.5 | 0.5 | 8 | 0.03 |
| E. coli/TEM-1 | BLA+ | 0.016 | 0.25 | >64 | 0.03 |
| E. coli 25922 | BLA− | 1 | 0.5 | 16 | 0.13 |
BLA, β-lactamase; CLA, lithium clavulanate. Lithium clavulanate (4 μg/ml) had no effect on cells when tested alone.
Activity of NB2001 against S. aureus.
Most β-lactam-resistant isolates of S. aureus produce β-lactamase (16, 17). We compared the in vitro antibacterial activity of NB2001 against 12 ATCC S. aureus strains to a panel of reference antimicrobial agents, including vancomycin, the antibiotic of choice for clinical applications. The MIC50 of NB2001 was ≤0.016 μg/ml, which is at least 32-fold lower than vancomycin (MIC50 = 0.5 μg/ml) (Table 3). NB2001 exhibited higher activity against most β-lactamase-producing S. aureus strains than the nonproducing ATCC 700698, although the β-lactamase nonproducer was also sensitive to triclosan (MIC = 0.063 μg/ml). Predictably, NB2001 was not very active against two β-lactamase-producing, but relatively triclosan-resistant strains ATCC 33594 and ATCC 13301 (Table 3).
TABLE 3.
Antibacterial activities of NB2001 and reference antibiotics against S. aureus
| S. aureus type and ATCC no. | β-Lactamase statusa | MIC (μg/ml) of:
|
|||
|---|---|---|---|---|---|
| NB2001 | Vancomycin | Oxacillin | Triclosan | ||
| Methicillin resistant | |||||
| 700698 | Neg | 4 | 2 | >32 | 0.063 |
| 700789 | Pos | 0.031 | 2 | 32 | ≤0.016 |
| 700787 | Pos | ≤0.016 | 8 | >32 | ≤0.016 |
| 700788 | Pos | ≤0.016 | 2 | >32 | ≤0.016 |
| 33591 | Pos | ≤0.016 | 0.25 | 8 | ≤0.016 |
| 33593 | Pos | ≤0.016 | 0.5 | 8 | ≤0.016 |
| Methicillin sensitive | |||||
| 33594 | Pos | 8 | 0.5 | 0.25 | 4 |
| 13301 | Pos | 4 | 0.25 | 0.25 | 2 |
| 43300 | Neg/Pos | ≤0.016 | 0.5 | 2 | ≤0.016 |
| 33592 | Pos | ≤0.016 | 0.5 | 2 | ≤0.016 |
| 14154 | Pos | ≤0.016 | 0.5 | 0.5 | ≤0.016 |
| 11632 | Neg/Pos | ≤0.016 | 0.25 | 0.25 | ≤0.016 |
Neg, negative; Pos, positive; Neg/Pos, strains may have very low level expression of β-lactamase.
In vitro antibacterial activity of NB2001 against other pathogens.
The antibacterial activity of NB2001 and comparator antimicrobial agents was evaluated with 300 recent clinical isolates, including S. aureus and other major human pathogens, at MRL. Antimicrobial comparator agents were chosen from appropriate drug classifications for the treatment of gram-positive or gram-negative infections. Generally, NB2001 demonstrated broad-spectrum activity against all species with the exception of P. aeruginosa (Tables 4 and 5).
TABLE 4.
In vitro activities of NB2001 and reference antimicrobial agents against recent clinical isolates of gram-positive bacteria (obtained from MRL)
| Organism (no. of strains)a and antimicrobial agent | MIC (μg/ml)b
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| MS S. aureus (20) | |||
| NB2001 | 0.06-0.25 | 0.12 | 0.25 |
| Vancomycin | 0.5-1 | 0.5 | 1 |
| Linezolid | 1-2 | 2 | 2 |
| Quinupristin-dalfopristin | ≤0.12-0.5 | 0.25 | 0.25 |
| Amoxicillin-clavulanate | 0.06-2 | 0.25 | 0.5 |
| Oxacillin | ≤0.06-2 | 0.12 | 0.25 |
| Ampicillin | ≤0.25-16 | 1 | 16 |
| MR S. aureus (20) | |||
| NB2001 | 0.06-1 | 0.12 | 0.5 |
| Vancomycin | 0.5-1 | 0.5 | 1 |
| Linezolid | 1-2 | 2 | 2 |
| Quinupristin-dalfopristin | 0.25-0.5 | 0.25 | 0.5 |
| Amoxicillin-clavulanate | 0.25-4 | 2 | 4 |
| Oxacillin | 4-16 | 8 | 8 |
| Ampicillin | ≤0.25->16 | 8 | >16 |
| MS S. epidermidis (15) | |||
| NB2001 | 0.015-0.12 | 0.06 | 0.06 |
| Vancomycin | 0.5-2 | 1 | 2 |
| Linezolid | 0.5-2 | 1 | 2 |
| Quinupristin-dalfopristin | ≤0.12-0.5 | ≤0.12 | 0.25 |
| Amoxicillin-clavulanate | 0.06-0.25 | 0.12 | 0.25 |
| Oxacillin | ≤0.06-0.12 | ≤0.06 | 0.12 |
| Ampicillin | ≤0.25-16 | ≤0.25 | 1 |
| MR S. epidermis (15) | |||
| NB2001 | 0.03-0.5 | 0.06 | 0.25 |
| Vancomycin | 1-2 | 1 | 2 |
| Linezolid | ≤0.25-1 | 1 | 1 |
| Quinupristin-dalfopristin | ≤0.12-0.25 | 0.12 | 0.25 |
| Amoxicillin-clavulanate | 0.25-4 | 1 | 2 |
| Oxacillin | 2->32 | 4 | 32 |
| Ampicillin | ≤0.25-8 | 2 | 8 |
| PS S. pneumoniae (4) | |||
| NB2001 | 0.12-0.25 | 0.12 | 0.25 |
| Vancomycin | 0.12-0.25 | 0.25 | 0.25 |
| Linezolid | 0.5-1 | 0.5 | 1 |
| Amoxicillin-clavulanate | ≤0.015-0.06 | ≤0.015 | 0.06 |
| Cefuroxime axetil | ≤0.12-0.5 | ≤0.12 | 0.5 |
| Ceftriaxone | ≤0.015-≤0.015 | ≤0.015 | ≤0.015 |
| Ampicillin | ≤0.25-≤0.25 | ≤0.25 | ≤0.25 |
| PR S. pneumoniae (6) | |||
| NB2001 | 1-8 | 2 | 8 |
| Vancomycin | 0.25-0.25 | 0.25 | 0.25 |
| Linezolid | 0.5-1 | 0.5 | 1 |
| Amoxicillin-clavulanate | 0.12-8 | 4 | 8 |
| Cefuroxime axetil | 1-32 | 8 | 32 |
| Ceftriaxone | 0.25-4 | 1 | 4 |
| Ampicillin | ≤0.25->16 | 4 | 16 |
| VS E. faecalis (10) | |||
| NB2001 | 4-8 | 4 | 8 |
| Vancomycin | 1-2 | 1 | 2 |
| Linezolid | 0.5-2 | 1 | 1 |
| Quinupristin-dalfopristin | 1-8 | 4 | 8 |
| Amoxicillin-clavulanate | 0.5-1 | 0.5 | 1 |
| Ampicillin | 1-2 | 1 | 2 |
| VR E. faecalis (5) | |||
| NB2001 | 2-8 | 4 | 8 |
| Vancomycin | 32->32 | 32 | >32 |
| Linezolid | 1-1 | 1 | 1 |
| Quinupristin-dalfopristin | 8->8 | >8 | >8 |
| Amoxicillin-clavulanate | 0.25-1 | 0.5 | 1 |
| Ampicillin | 0.5-2 | 1 | 2 |
MS, methicillin susceptible; MR, methicillin resistant; PS, penicillin susceptible; PR, penicillin resistant; VS, vancomycin susceptible; VR, vancomycin resistant; S. pneumoniae, Streptococcus pneumoniae; E. faecalis, Enterococcus faecalis.
MIC assays were performed at MRL.
TABLE 5.
In vitro activities of NB2001 and reference antimicrobial agents against gram-negative bacteria
| Organism (no. of strains)a and antimicrobial agent | MIC (μg/ml)b
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| BP M. catarrhalis (20) | |||
| NB2001 | ≤0.004-0.015 | ≤0.004 | ≤0.004 |
| Ceftriaxone | ≤0.015-0.5 | ≤0.015 | 0.25 |
| Imipenem | ≤0.03-0.12 | ≤0.03 | 0.06 |
| Ciprofloxacin | 0.008-0.03 | 0.015 | 0.015 |
| Amoxicillin-clavulanate | ≤1-≤1 | ≤1 | ≤1 |
| Sulfamethoxazole | 0.06-0.5 | 0.12 | 0.25 |
| Ampicillin | ≤0.12-2 | 0.5 | 2 |
| BN M. catarrhalis (20) | |||
| NB2001 | 0.015-0.06 | 0.03 | 0.03 |
| Ceftriaxone | ≤0.015-≤0.015 | ≤0.015 | ≤0.015 |
| Imipenem | ≤0.03-≤0.03 | ≤0.03 | ≤0.03 |
| Ciprofloxacin | 0.008-0.03 | 0.015 | 0.015 |
| Amoxicillin-clavulanate | ≤1-≤1 | ≤1 | ≤1 |
| Sulfamethoxazole | 0.03-0.12 | 0.06 | 0.12 |
| Ampicillin | ≤0.12 | ≤0.12 | ≤0.12 |
| BP H. influenzae (20) | |||
| NB2001 | 0.12-2 | 0.5 | 1 |
| Ceftazidime | ≤0.03-0.12 | 0.06 | 0.06 |
| Ceftriaxone | ≤0.015-0.03 | ≤0.015 | ≤0.015 |
| Imipenem | 0.25-0.5 | 0.5 | 0.5 |
| Ciprofloxacin | 0.004-0.015 | 0.008 | 0.015 |
| Amoxicillin-clavulanate | ≤1-4 | ≤1 | 4 |
| Sulfamethoxazole | 0.06-16 | 0.25 | 16 |
| Ampicillin | 8->32 | >32 | >32 |
| BN H. influenzae (10) | |||
| NB2001 | 2-4 | 2 | 4 |
| Ceftazidime | ≤0.03-0.12 | 0.06 | 0.06 |
| Ceftriaxone | ≤0.015-≤0.015 | ≤0.015 | ≤0.015 |
| Imipenem | 0.06-0.5 | 0.25 | 0.5 |
| Ciprofloxacin | 0.008-0.015 | 0.008 | 0.008 |
| Amoxicillin-clavulanate | ≤1-≤1 | ≤1 | ≤1 |
| Sulfamethoxazole | 0.12-1 | 0.5 | 1 |
| Ampicillin | ≤0.12-0.25 | ≤0.12 | 0.25 |
| E. aerogenes (30) | |||
| NB2001 | 0.5-16 | 4 | 8 |
| Ceftazidime | 0.12->32 | 32 | >32 |
| Ceftriaxone | 0.03-64 | 16 | 32 |
| Imipenem | 0.25-2 | 1 | 2 |
| Ciprofloxacin | 0.008->4 | 0.015 | 1 |
| Amoxicillin-clavulanate | 32->32 | 32 | >32 |
| Sulfamethoxazole | 0.03-32 | 0.12 | 0.5 |
| Ampicillin | 32->32 | >32 | >32 |
| Gentamicin | 0.25-2 | 0.5 | 1 |
| E. cloacae (30) | |||
| NB2001 | 0.5->32 | 2 | 8 |
| Ceftazidime | 0.12->32 | >32 | >32 |
| Ceftriaxone | 0.03->64 | 32 | >64 |
| Imipenem | 0.25-2 | 0.5 | 2 |
| Ciprofloxacin | ≤0.002->4 | 0.03 | 2 |
| Amoxicillin-clavulanate | 4->32 | >32 | >32 |
| Sulfamethoxazole | 0.03-32 | 0.06 | 0.5 |
| Ampicillin | 2->32 | >32 | >32 |
| Gentamicin | 0.25->16 | 0.5 | 4 |
| E. coli (30) | |||
| NB2001 | 1-16 | 4 | 8 |
| Ceftazidime | 0.06->32 | 0.25 | 32 |
| Ceftriaxone | ≤0.015->64 | 0.06 | 8 |
| Imipenem | 0.06-0.5 | 0.12 | 0.25 |
| Ciprofloxacin | 0.004->4 | 0.008 | >4 |
| Amoxicillin-clavulanate | 2-32 | 4 | 32 |
| Sulfamethoxazole | ≤0.015->32 | 0.06 | >32 |
| Ampicillin | 1->32 | 32 | >32 |
| Gentamicin | 0.25->16 | 0.5 | 2 |
| K. pneumoniae (30) | |||
| NB2001 | 1->32 | 8 | 16 |
| Ceftazidime | ≤0.03->32 | 32 | >32 |
| Ceftriaxone | ≤0.015->64 | 4 | >64 |
| Imipenem | 0.06-1 | 0.12 | 1 |
| Ciprofloxacin | 0.008->4 | 0.12 | >4 |
| Amoxicillin-clavulanate | ≤1->32 | 8 | 32 |
| Sulfamethoxazole | 0.06->32 | 0.5 | >32 |
| Ampicillin | 16->32 | >32 | >32 |
| Gentamicin | 0.25->16 | 4 | >16 |
| P. aeruginosa (10) | |||
| NB2001 | >32->32 | >32 | >32 |
| Ceftazidime | 1->32 | 4 | >32 |
| Ceftriaxone | 2->64 | 64 | >64 |
| Imipenem | 0.25->16 | 2 | 16 |
| Ciprofloxacin | 0.12-4 | 1 | 2 |
| Amoxicillin-clavulanate | 4->32 | >32 | >32 |
| Sulfamethoxazole | >32 | 8 | 16 |
| Ampicillin | 4->32 | >32 | >32 |
| Gentamicin | 1->16 | 2 | 16 |
BP, β-lactamase positive; BN, β-lactamase negative; E. cloacae, Enterobacter cloacae, E. aerogenes, Enterobacter aerogenes.
MIC assays were performed at MRL.
NB2001 inhibited all 70 strains of staphylococci tested (including methicillin-resistant S. aureus and S. epidermidis) at a concentration of 1 μg/ml or less, being equipotent to quinupristin-dalfopristin and more active than vancomycin, linezolid, amoxicillin-clavulanate, cefuroxime axetil, and ceftriaxone (Table 4). It also demonstrated activity against Enterococcus faecalis, including vancomycin-resistant strains (MIC50 = 4 μg/ml), although this was substantially less than other against gram-positive bacteria, probably reflecting the low levels of β-lactamase in that organism. Nevertheless, the data highlight the potential of NB2001 against this clinically significant problem pathogen. Streptococcus pneumoniae, the major pathogen for community-acquired pneumonia with increasing resistance to commonly used antibiotics (22), was also susceptible to NB2001 (MIC50 = 1 μg/ml).
NB2001 was also active against gram-negative species (Table 5). Its MIC50s against β-lactamase-producing M. catarrhalis and H. influenzae were at least fourfold superior to ciprofloxacin. Against Enterobacter aerogenes, Enterobacter cloacae, E. coli, and K. pneumoniae, NB2001 was more active than amoxicillin-clavulanate, ceftazidime, or ceftriaxone and less active than imipenem and ciprofloxacin. However, NB2001 showed no activity against P. aeruginosa (Table 5).
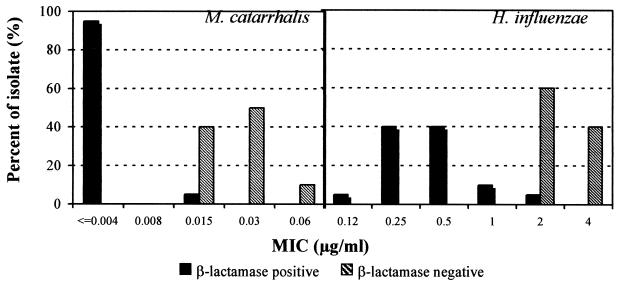
Of the 60 M. catarrhalis and H. influenzae isolates tested, 40 were β-lactamase-producing strains. The distribution of NB2001 MICs clearly showed greater activity against β-lactamase-producing strains than β-lactamase-negative ones (Fig. 4).
FIG. 4.
Susceptibility to NB2001 of β-lactamase-positive and -negative strains of M. catarrhalis and H. influenzae.
Bactericidal activity of NB2001.
We examined whether NB2001 was bactericidal or bacteristatic in E. coli/TEM-1. At concentrations up to eightfold its MIC, NB2001 showed no bactericidal activity after 6 h of incubation. Similarly, its toxophore triclosan was also clearly bacteristatic (Fig. 5).
FIG. 5.
Time-kill curve of NB2001 and triclosan against β-lactamase-producing E. coli. The compounds were added to log-phase cultures of E. coli/TEM-1 and incubated at 37°C. Culture aliquots were removed every hour up to 6 h, and the number of cells (CFU) was determined as described in Materials and Methods.
In a separate experiment, we performed a synergy study between cephalothin and triclosan in E. coli ATCC 25922 and found that the two agents have additive effects (data not shown).
DISCUSSION
ECTA is a novel prodrug strategy, whose effectiveness depends upon the activation of nontoxic compounds by bacterium-specific enzymes. We chose as a target ECTA enzyme β-lactamase, a well-characterized and widely prevalent bacterial enzyme expressed extracellularly or in the periplasmic space. A key disease resistance mechanism, overexpression of β-lactamase, was thereby exploited for the formation of antibacterial agents, and it became a marker of enhanced susceptibility to NB2001. This compound was synthesized as a proof-of-principle molecule for the ECTA technology.
The structure of NB2001 is similar to that of cephalothin and nitrocefin. It is composed of a cephem core with a 2-thienylacetylamino side chain at the 7-position, which is an important determinant of β-lactamase binding. In addition, NB2001 contains a prodrug form of the antimicrobial agent triclosan, which is converted to its fully active form by β-lactamase. Triclosan is a broad-spectrum agent that acts via inhibition of the enoyl-acyl carrier protein reductase (FabI) (7, 8, 12). Because of its high potency in bacteria and its low toxicity in humans, triclosan itself is widely used in formulations as diverse as antiseptic soap, toothpaste, cosmetics, and household products (2).
NB2001 was designed to have a dual mode of action: cephalosporin activity against β-lactamase-negative organisms and triclosan-associated activity against β-lactamase-producing strains. As we have shown, NB2001 is a good substrate for β-lactamase TEM-1. The idea of multiple mechanisms of action for NB2001 is supported by the low frequency (<10−8) of bacteria resistant to NB2001 (unpublished data).
β-Lactamase ECTA compounds have enhanced activity against β-lactamase-producing strains but also good activity against β-lactamase-negative strains. For example, in E. coli N NB2001 has higher activity relative to cephalothin (Table 2). The higher activity might be due to triclosan production via hydrolysis by β-lactamases present in amounts undetectable by the nitrocefin test and/or acylation of target penicillin-binding proteins.
NB2001 had potent activity against S. aureus, a major human pathogen (19, 30) of increasing significance in nosocomial drug-resistant infections (6, 20, 27, 31). The compound was also active against a number of clinically important gram-positive and -negative pathogens. Its high potency against problem pathogens such as methicillin-resistant staphylococci (S. aureus and S. epidermidis) and vancomycin-resistant Enterococcus faecalis underscores its potential for clinical use.
P. aeruginosa is known to be resistant to a variety of antibiotics. The primary reason for the multidrug resistance of P. aeruginosa is its outer membrane (4) in combination with multidrug efflux pumps (13, 23). We have confirmed both of these reasons to account for the low activity of NB2001 against P. aeruginosa (unpublished data).
Antibiotics used in the treatment of most common bacterial infections attack only a few distinct targets in the pathogen (24), such as cell wall synthesis, protein synthesis, or DNA gyrase. ECTA represents a novel approach in that bacterium-specific, enzymatic antibiotic activation allows the use of antibiotics with toxic liabilities, thus expanding the number of bacterial targets. In addition, as the case of NB2001 clearly shows, the ECTA approach is an effective way of harnessing drug resistance caused by enzyme overexpression.
Acknowledgments
We gratefully acknowledge Nafsika Georgopapadakou for helpful discussions and valuable aid during the revision of the manuscript. Thanks are also extended to Analia Bueno for technical contributions in the evaluation of NB2001.
REFERENCES
- 1.Banerjee, S., U. Pieper, G. Kapadia, L. K. Pannell, and O. Herzberg. 1998. Role of the omega-loop in the activity, substrate specificity, and structure of class A β-lactamase. Biochemistry 37:3286-3296. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava, H. N., and P. A. Leonard. 1996. Triclosan: applications and safety. Am. J. Infect. Control. 24:209-218. [DOI] [PubMed] [Google Scholar]
- 3.Bush, K. 2001. New β-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 4.Chen, H. Y., M. Yuan, and D. M. Livermore. 1995. Mechanisms of resistance to β-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J. Med. Microbiol. 43:300-309. [DOI] [PubMed] [Google Scholar]
- 5.Ferraro, M. J. 2000. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 5th ed. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 6.Haley, R. W., A. W. Hightower, R. F. Khabbaz, C. Thornsberry, W. J. Martone, J. R. Allen, and J. M. Hughes. 1982. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann. Intern. Med. 97:297-308. [DOI] [PubMed] [Google Scholar]
- 7.Heath, R. J., J. R. Rubin, D. R. Holland, E. Zhang, M. E. Snow, and C. O. Rock. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274:11110-11114. [DOI] [PubMed] [Google Scholar]
- 8.Heath, R. J., Y. T. Yu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 273:30316-30320. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N., and M. A. Pfaller. 1998. Bacterial resistance: a worldwide problem. Diagn. Microbiol. Infect. Dis. 31:379-388. [DOI] [PubMed] [Google Scholar]
- 10.Kazmierczak, A., X. Cordin, J. M. Duez, E. Siebor, A. Pechinot, and J. Sirot. 1990. Differences between clavulanic acid and sulbactam in induction and inhibition of cephalosporinases in enterobacteria. J. Int. Med. Res. 18:67D-77D. [PubMed] [Google Scholar]
- 11.Knox, J. R., P. C. Moews, and J. M. Frere. 1996. Molecular evolution of bacterial β-lactam resistance. Chem. Biol. 3:937-947. [DOI] [PubMed] [Google Scholar]
- 12.Levy, C. W., A. Roujeinikova, S. Sedelnikova, P. J. Baker, A. R. Stuitje, A. R. Slabas, D. W. Rice, and J. B. Rafferty. 1999. Molecular basis of triclosan activity. Nature 398:383-384. [DOI] [PubMed] [Google Scholar]
- 13.Li, X. Z., D. Ma, D. M. Livermore, and H. Nikaido. 1994. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to β-lactam resistance. Antimicrob. Agents Chemother. 38:1742-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livermore, D. M., M. Akova, P. J. Wu, and Y. J. Yang. 1989. Clavulanate and β-lactamase induction. J. Antimicrob. Chemother. 24(Suppl. B):23-33. [DOI] [PubMed] [Google Scholar]
- 16.Maranan, M. C., B. Moreira, S. Boyle-Vavra, and R. S. Daum. 1997. Antimicrobial resistance in staphylococci. Epidemiology, molecular mechanisms, and clinical relevance. Infect. Dis. Clin. North Am. 11:813-849. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros, A. A. 1984. β-Lactamases. Br. Med. Bull. 40:18-27. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros, A. A. 1997. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):S19-S45. [DOI] [PubMed] [Google Scholar]
- 19.Muder, R. R., C. Brennen, M. M. Wagener, R. M. Vickers, J. D. Rihs, G. A. Hancock, Y. C. Yee, J. M. Miller, and V. L. Yu. 1991. Methicillin-resistant staphylococcal colonization and infection in a long-term care facility. Ann. Intern. Med. 114:107-112. [DOI] [PubMed] [Google Scholar]
- 20.Mulligan, M. E., K. A. Murray-Leisure, B. S. Ribner, H. C. Standiford, J. F. John, J. A. Korvick, C. A. Kauffman, and V. L. Yu. 1993. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am. J. Med. 94:313-328. [DOI] [PubMed] [Google Scholar]
- 21.Murray, B. E. 1997. Antibiotic resistance. Adv. Intern. Med. 42:339-367. [PubMed] [Google Scholar]
- 22.Musher, D. M. 2000. Streptococcus pneumoniae, p. 2130-2139. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed., vol. 2. Churchill Livingstone, Philadelphia, Pa. [Google Scholar]
- 23.Nakae, T. 1997. Multiantibiotic resistance caused by active drug extrusion in Pseudomonas aeruginosa and other gram-negative bacteria. Microbiologia 13:273-284. [PubMed] [Google Scholar]
- 24.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas-Chanoine, M. H. 1997. Inhibitor-resistant β-lactamases. J. Antimicrob. Chemother. 40:1-3. [DOI] [PubMed] [Google Scholar]
- 26.Opal, M. O., K. H. Mayer, and A. A. Medeiros. 2000. Mechanisms of bacterial antibiotic resistance, p. 236-253. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed., vol. 1. Churchill Livingstone, Philadelphia, Pa. [Google Scholar]
- 27.Peacock, J. E., Jr., D. R. Moorman, R. P. Wenzel, and G. L. Mandell. 1981. Methicillin-resistant Staphylococcus aureus: microbiologic characteristics, antimicrobial susceptibilities, and assessment of virulence of an epidemic strain. J. Infect. Dis. 144:575-582. [DOI] [PubMed] [Google Scholar]
- 28.Randall, J. L., and J. E. Godlewski. September 1998. Process for making quinolonyl lactam antimicrobials and novel intermediate compounds. U.S. patent 5,801,242.
- 29.Schaechter, M. 1993. Teaching microbiology to medical students. Infect. Agents Dis. 2:394-397. [PubMed] [Google Scholar]
- 30.Steinberg, J. P., C. C. Clark, and B. O. Hackman. 1996. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin. Infect. Dis. 23:255-259. [DOI] [PubMed] [Google Scholar]
- 31.Waldvogel, F. A. 2000. Staphylococcus aureus, p. 2069-2091. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed., vol. 2. Churchill Livingstone, Philadelphia, Pa. [Google Scholar]
- 32.Waxman, D. J., and J. L. Strominger. 1983. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu. Rev. Biochem. 52:825-869. [DOI] [PubMed] [Google Scholar]