Abstract
The resistance of bacterial biofilms to physical and chemical agents is attributed in the literature to various interconnected processes. The limitation of mass transfer alters the growth rate, and physiological changes in the bacteria in the film also appear. The present work describes an approach to determination of the mechanisms involved in the resistance of bacteria to quaternary ammonium compounds (benzalkonium chloride) according to the C-chain lengths of those compounds. For Pseudomonas aeruginosa CIP A 22, the level of resistance of the bacteria in the biofilm relative to that of planktonic bacteria increased with the C-chain length. For cells within the biofilm, the exopolysaccharide induced a characteristic increase in surface hydrophilicity. However, this hydrophilicity was eliminated by simple resuspension and washing. The sensitivity to quaternary ammonium compounds was restored to over 90%. Staphylococcus aureus CIP 53 154 had a very high level of resistance when it was in the biofilm form. A characteristic of bacteria from the biofilm was a reduction in the percent hydrophobicity, but the essential point is that this hydrophobicity was retained after the biofilm bacteria were resuspended and washed. The recovery of sensitivity was thus only partial. These results indicate that the factors involved in biofilm resistance to quaternary ammonium compounds vary according to the bacterial modifications induced by the formation of a biofilm. In the case of P. aeruginosa, we have underlined the involvement of the exopolysaccharide and particularly the three-dimensional structure (water channels). In the case of S. aureus, the role of the three-dimensional structure is limited and drastic physiological changes in the biofilm cells are more highly implicated in resistance.
Bacterial biofilms associated with surfaces are complex three-dimensional structures in which bacteria are embedded in a matrix principally made of extracellular polymers. Biofilm formation may be separated into the primary attachment of bacteria to surfaces, followed by proliferation of the attached bacterial cells, which leads to the accumulation of multilayered clusters of cells and glycocalyx formation. This mode of growth has often been implicated in surface contamination that leads to resistance to particular physical and/or chemical treatments (7, 23). This mode of growth is also considered essential for infections that occur and persist at various sites in the human body, especially in association with medical devices (6). Such infections are characteristically resistant to antibiotics as well as to host defenses (7, 16). Many in vitro (2, 36, 39) and in vivo (35) studies have attributed the resistance to the biofilm's mode of growth (slow growth rate, adhesion steps), but it is now thought that there are a number of contributory factors. The exopolysaccharide (EPS) matrix may exclude and/or influence the penetration of antimicrobial agents (11, 30). It has also been demonstrated that the abiotic part of the biofilm plays a part in resistance because of its potent neutralization effect on many compounds. Chlorine is known for its susceptibility to organic and inorganic matter (41). Therefore, the production of EPS can dramatically affect the activity of chlorine (34). On the other hand, recent work has shown that a large number of genes and regulatory systems are activated during biofilm formation. Some of these are associated with the adhesion step (17, 31, 38), whereas others are associated with colonization and maturation of the biofilm (10, 13). This leads to different phenotypes between planktonic and biofilm cells. The mechanism(s) involved in biofilm maturation and particularly in biofilm resistance to biocides may involve a wide variety of profound changes. The present study was performed to characterize the variations in the susceptibilities of two different mature biofilms (biofilms of Pseudomonas aeruginosa and Staphylococcus aureus) versus that of planktonic cells of P. aeruginosa and S. aureus to cationic surfactants (quaternary ammonium compounds [QACs]) according to the C-chain lengths of the surfactants. The two species selected, P. aeruginosa and S. aureus, are opportunistic human pathogens that are increasingly the cause of hospital-acquired infections, which is strongly correlated with the increasing rate of use of implantable medical devices like catheters (21, 27). Modification of the surface hydrophobicities of planktonic and biofilm cells and the persistence of resistance after disruption of the three-dimensional structure were also determined to focus on the possibility that phenotypic changes limit the efficacies of the chemicals tested against the two different biofilms.
MATERIALS AND METHODS
QACs.
N-Alkylbenzyldimethyl ammonium chlorides were prepared by the reaction between the N,N-alkyldimethylamine and benzyl chloride in refluxing butanone (unpublished data). They were recrystallized from the butanone and were obtained in a highly purified form (purity, >98%). The formula for the QACs tested is (C9H13N-CnH2n+1)Cl, where n is equal to 8, 10, 12, 14, 16, or 18. The compounds tested, named C8, C10, C12, C14, C16, and C18, were dissolved at the appropriate concentrations in sterile distilled water.
Bacterial strains.
The bacterial strains were provided by the Institut Pasteur Collection (Paris, France). They included two bacteria (P. aeruginosa CIP A 22 and S. aureus CIP 53 154) recommended by French standards for the evaluation of water-miscible antiseptics and the efficiencies of disinfectants. P. aeruginosa CIP A 22 and S. aureus CIP 53 154 were maintained on Trypticase soy agar (Biomérieux, Marcy l'Etoile, France) and were incubated under aerobic conditions at 37°C for 24 to 48 h. The bacterial suspensions were freshly prepared in sterile distilled water before each experiment.
Activities of the tested compounds against planktonic bacteria.
The assays for determination of the activities of the compounds were performed according to French and European standards, and the end of the contact between the microorganisms and the compounds was obtained by dilution-neutralization (3). The neutralizing medium was selected as indicated by the standards. Briefly, 1 ml of compound solution (or 1 ml of sterile distilled water, which was used to establish the harmlessness of the neutralizing medium) was added to 9 ml of neutralizing medium. After 10 min of contact, 1 ml of a bacterial suspension adjusted to 103 CFU/ml was added. After homogenization, 1 ml of each mixture was placed in duplicate on Trypticase soy agar medium, and the plates were incubated under the conditions described above. The neutralizing medium selected permitted at least 80% of the microorganisms to be recovered, indicating that it protected them from the antimicrobial effects of the products and was totally harmless. The method was validated for the neutralizing medium made of Trypticase soy broth containing 5% Tween 80 (Sigma, Saint Quentin Fallavier, France), 2% lecithin (Sigma), and 0.5% sodium thiosulfate (Sigma). For evaluation of the bactericidal efficiencies of the compounds against planktonic cells, 1 ml of a bacterial suspension adjusted to 108 CFU/ml was added to 9 ml of each dilution of the QACs (or 9 ml of distilled sterile water as a control). After 5 min of contact at 20°C, 1 ml of each mixture was transferred to 9 ml of neutralizing medium and allowed to remain in contact for 10 min after homogenization. Viable bacterial counts were determined by duplicate inclusion of serial dilutions of homogenized samples in Trypticase soy agar. The agar plates were incubated aerobically, as described above. The results were expressed as the log reduction (log reduction = log value for the control − log value for the assayed sample).
Biofilm formation.
Bacterial biofilms were obtained by a previously described procedure (32, 34). The nutrient broth used to promote bacterial adhesion and biofilm formation consisted of Vitamin Assay Casamino Acids (0.1 g/liter; Difco), yeast extract (0.1 g/liter), MgSO4 · 2H2O (0.2 g/liter), FeSO4 · 7H2O (0.0005 g/liter), anhydrous Na2HPO4 (1.25 g/liter), KH2PO4 (0.5 g/liter), and lactose (0.025 g/liter). This medium was circulated through a sterile loop of Tygon (Bioblock, Illikirch, France) tube (inner diameter, 6.4 mm) at 100 ml/min. The loop was maintained at 30°C and was connected to a discharge line and to a feeding tank (supply pump; feeding rate, 3 ml/min). After the bioreactor was filled with the adhesion broth, the loop was inoculated with 5 ml of a bacterial suspension containing about 108 CFU/ml. The circulation pump was run for 30 min to allow the cells to spread around the loop before the supply pump was turned on. After 65 h, stabilized and reproducible populations of adherent and evacuated bacteria were obtained for the two strains tested (32, 34).
Bactericidal activities of the tested compounds against the biofilm.
For evaluation of bactericidal activities of the tested compounds against the biofilm, samples of the Tygon tube (2-cm pieces cut in half lengthwise) were removed from the loop carrying the 65-h-old biofilm and were distributed among test tubes containing 10 ml of dilutions of the compounds to be tested. The control was dipped into sterile distilled water. After 5 min of contact at 20°C, the samples were removed and placed in 10 ml of neutralizing medium for 10 min. The adherent cells were immediately recovered by scraping them off with a sterile cutter. The portions of the Tygon tube and the corresponding neutralizing medium were then dispersed for 1 min with a vortex mixer (Bioblock) and by sonication (600 W; Vibra cell; Bioblock) at 45 W for 5 s. Viable bacterial counts were determined by placement of duplicate serial dilutions of homogenized samples on Trypticase soy agar. The agar plates were incubated aerobically as described above. The results were expressed as the log reduction (log reduction = log value for the control − log value for the assayed sample).
Protein and polysaccharide levels.
Protein and polysaccharide levels were determined for planktonic cells and attached cells. For planktonic cells, a bacterial suspension at an absorbance of 70 to 75% (measured at 640 nm) was prepared. For biofilm cells, the samples of the Tygon tube (2-cm pieces cut in half lengthwise) were removed, the adherent cells were recovered by scraping the tubes with a sterile cutter, and the material was placed in 3 ml of distilled sterile water. The protein level was determined by the method of Lowry et al. (24) by using bovine serum albumin as a standard. The polysaccharide level was evaluated by the phenol-sulfuric assay procedure by using glucose as a standard (14).
Recovery of sensitivity to QACs after disruption of biofilms.
Samples of the Tygon tube (2-cm pieces cut in half lengthwise) were removed from the loop carrying the biofilm. The attached cells were immediately recovered in a phosphate-buffered (PBS) solution at 0.15 mol/liter by scraping the tube with a sterile cutter. The test was performed as described above for determination of the activities of the compounds against planktonic cells directly after disruption of the biofilm or after three washes in a PBS solution at 0.15 mol/liter. The percent recovery of sensitivity to QAC after disruption of the biofilm was expressed as (log reduction of cells after disruption of the biofilm/log reduction of planktonic cells) × 100.
Evaluation of hydrophobicity of planktonic cells.
Evaluation of hydrophobicity was done by the microorganism adherence to hydrocarbons (MATH) method first described by Rosenberg et al. (33) and modified by Mozes and Rouxhet (28). Cell suspensions were prepared at 108 CFU/ml in PBS at 0.15 mol/liter. They were directly used for the MATH assay or were first washed three times in a PBS solution at 0.15 mol/liter. A total of 1.2 ml of each cell suspension was placed into a glass test tube, and 0.6 ml of hexadecane was then added. After the tubes were allowed to rest for 10 min, each glass tube was vortexed for 2 min and was left for 15 min, during which time the two phases separated completely. The absorbance of the aqueous phase was then measured (λ = 640 nm). The blank consisted of PBS without cells. The results are expressed as the proportion of the cells which were excluded from the aqueous phase, determined by the equation [(A0 − A)/A0] × 100, where A0 and A are the initial and final optical densities of the aqueous phase, respectively.
Evaluation of hydrophobicity of biofilm cells.
Samples of the Tygon tube (15- to 20-cm pieces cut in half lengthwise) were removed from the loop carrying the biofilm. The biofilm cells were immediately recovered in a PBS solution at 0.15 mol/liter by scraping the tube with a sterile cutter. The test was then performed as described above directly after disruption of the biofilm or after three washes.
Scanning electron microscopy observations.
Biofilm samples were prepared for scanning electron microscopy by fixation in 2.5% glutaraldehyde, dehydration in ethanol, and air drying. The dried samples were mounted onto aluminum stubs, sputter coated with gold and palladium (60:40), and imaged with a Hitachi S-450 electron microscope (Centre de Microscopie Électronique Appliquée à la Biologie, Université de Médecine, Toulouse, France).
RESULTS
P. aeruginosa.
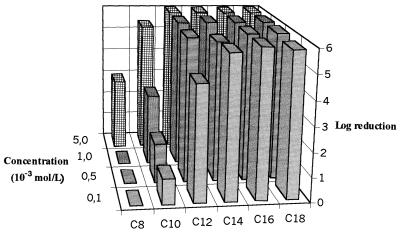
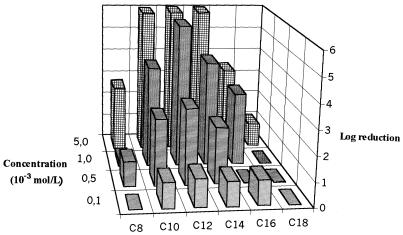
The variations in logarithmic reductions according to QAC concentration are presented in Fig. 1 and 2 for planktonic and biofilm P. aeruginosa cells, respectively. High levels of bactericidal activity (>5 logs) were observed against planktonic cells even at low concentrations (10−4 mol/liter) for C12 to C18 compounds. A gradual loss of activity was noted with a reduction in the C-chain length, and for the short-chain compounds (C8 and C10), a dilution effect appeared. Biofilm assays were characterized by resistance to the six QACs tested, with the logarithmic reductions being lower than 2 even at 10−4 mol/liter. The optimal efficiency was observed for C12. The loss of activity was thus dramatic for C14 to C18 compounds, and the loss of activity increased according to the C-chain length. In contrast, the bactericidal activities of short-chain QACs (C8 and C10 compounds) were maintained, even if the level of activity was low. The 65-h-old P. aeruginosa biofilm was characterized by very much higher protein (50 times) and polysaccharide (400 times) contents than planktonic cells. The protein levels were 61.0 ± 0.5 and 3,080 ± 760 ng/106 CFU for planktonic cells and biofilms, respectively; and polysaccharide levels were 8.9 ± 7.4 and 3,800 ± 1,000 ng/106 CFU for planktonic cells and biofilms, respectively (the values are means ± standard deviations; n = 3 for planktonic cells and 25 for the biofilm). The results are expressed according to the number of cultivable cells and so indicate the levels of accumulation of proteins and polysaccharides in the three-dimensional matrix. The results for P. aeruginosa adherence to hexadecane are presented in Table 1 according to whether the cells were planktonic or were from the biofilm and whether they were washed. After three washes, the hydrophobicities of planktonic cells and those obtained from the biofilm were similar. However, assays performed with unwashed bacteria indicated a higher degree of hydrophilicity of biofilm bacteria (20.4%) than of planktonic cells (41.4%) (P = 0.002) and also a higher degree of hydrophilicity of unwashed bacteria than of washed biofilm cells (42.3%; P = 0.05). The hydrophobicity of planktonic cells was not modified by washing. Therefore, the variations in surface properties observed for P. aeruginosa biofilm cells seem to be linked to the extracellular matrix.
FIG. 1.
Variations in bactericidal efficiencies (log reductions) of QACs against planktonic cells of P. aeruginosa (n = 2).
FIG. 2.
Variations in bactericidal efficiencies (log reductions) of QACs against a P. aeruginosa biofilm (n = 2).
TABLE 1.
Adherence of P. aeruginosa planktonic cells or biofilm to hexadecane
| Cell | % Adherencea
|
P | |
|---|---|---|---|
| Planktonic cells | Biofilm | ||
| Unwashed | 41.4 ± 4.6 | 20.4 ± 2.8 | 0.002b |
| Washed | 38.2 ± 4.8 | 42.3 ± 4.7 | >0.05b |
| P | >0.05c | 0.05c | |
Values are means ± standard deviations (n = 14 for planktonic cells and 10 for the biofilm).
Unpaired t test.
Paired t test.
The rates of recovery of sensitivity to the C12 and C14 compounds after disruption of the biofilm were as follows: 93 and 92%, respectively, for washed bacteria and 100 and 100%, respectively, for unwashed bacteria (the values are means; n = 2). Therefore, total recovery of sensitivity to C12 and C14 was obtained for washed bacteria, but important but incomplete (92%) recovery was obtained for unwashed bacteria.
S. aureus.
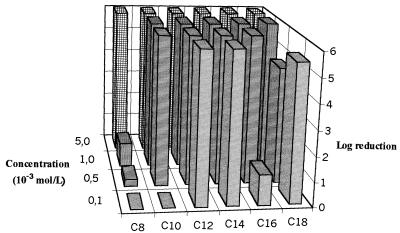
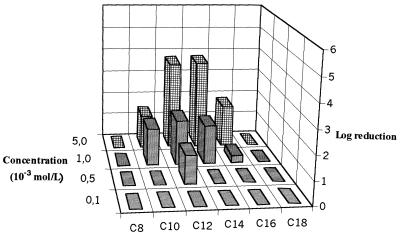
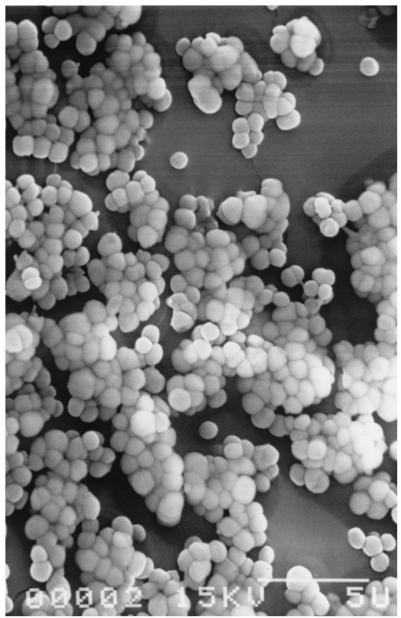
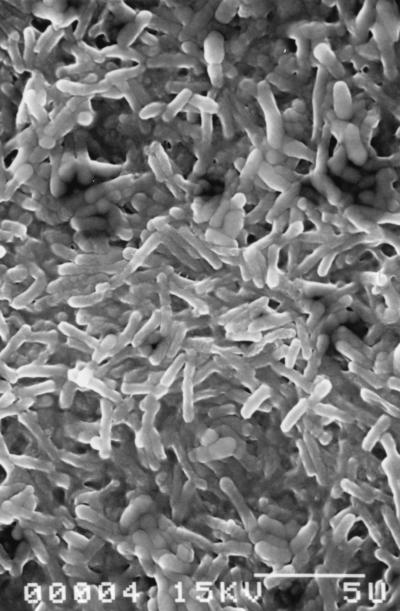
Planktonic S. aureus CIP 53 154 was characterized by susceptibility to the C12 and C14 QACs, with the logarithmic reductions being greater than 6 at 10−4 mol/liter (Fig. 3). A paradoxical effect was observed for the C18 compound, and further experiments are required to explain the effect, despite the possible problem with the dissolution or distribution of this hydrophobic compound in an aqueous solution. Figure 4 presents the variations in logarithmic reductions for the S. aureus biofilm according to the QAC concentration. These results show a more dramatic loss of sensitivity to QAC in comparison with that of the P. aeruginosa biofilm, with logarithmic reductions being lower than 2 even at 10−3 mol/liter. The best efficiency was always observed for the C12 and C14 compounds. The mature biofilm had significantly higher levels of protein (9 times) and polysaccharide (70 times) than the planktonic cells. The protein levels were 98.2 ± 11.2 and 896 ± 280 ng/106 CFU for planktonic cells and the biofilm, respectively; and polysaccharide levels were 12.0 ± 9.2 and 901 ± 270 ng/106 CFU for planktonic cells and the biofilm, respectively (the values are means ± standard deviations; n = 3 for planktonic cells and 7 for the biofilm). Note the similarity in protein and polysaccharide levels for planktonic S. aureus and P. aeruginosa cells (see above). In contrast, the values of both parameters were very much higher for the P. aeruginosa biofilm than for the S. aureus biofilm. These results corroborate the scanning electron microscopy observations for the two 65-h biofilms, which show a lower level of colonization of the Tygon tube surface for S. aureus (Fig. 5), which was characterized by cluster formation, than for P. aeruginosa, which was characterized by a total overlap of the cells (Fig. 6). These observations correspond to the cultivable counts determined for the two mature biofilms, i.e., 7.5 to 8 log CFU/cm2 for the P. aeruginosa biofilm and 6 to 6.5 log CFU/cm2 for the S. aureus biofilm. The levels of adhesion to hexadecane (Table 2) indicate that planktonic S. aureus cells are more hydrophobic than planktonic P. aeruginosa cells, and there was no difference between washed (88.3%) and unwashed cells (79.5%) (P > 0.05). Washed biofilm cells are significantly (P = 0.002) more hydrophilic (69.8%) than planktonic ones (88.3%). For unwashed biofilm bacteria (hydrophobicity, 69.2%), the decrease in hydrophobicity (biofilm versus planktonic) is not significant (P > 0.05) but is sufficient to suppress the difference between washed and unwashed bacteria.
FIG. 3.
Variations in bactericidal efficiencies (log reductions) of QACs against planktonic cells of S. aureus (n = 2).
FIG. 4.
Variations in bactericidal efficiencies (log reductions) of QACs against an S. aureus biofilm (n = 2).
FIG. 5.
A 65-h-old biofilm of. S. aureus observed by scanning electron microscopy. Bar, 5 μm.
FIG. 6.
A 65-h-old biofilm of P. aeruginosa observed by scanning electron microscopy. Bar, 5 μm.
TABLE 2.
Adherence of S. aureus planktonic cells or biofilm to hexadecane
| Cell | % Adherencea
|
P | |
|---|---|---|---|
| Planktonic cells | Biofilm | ||
| Unwashed | 79.5 ± 6.6 | 69.2 ± 2.6 | >0.05b |
| Washed | 88.3 ± 2.5 | 69.8 ± 5.3 | 0.002b |
| P | >0.05c | >0.05c | |
Values are means ± standard deviations (n = 18 for planktonic cells and 13 for the biofilm).
Unpaired t test.
Paired t test.
After biofilm cells had been resuspended, determination of the bactericidal efficiencies of C12 and C14 indicated that the recovery of sensitivity was poor for unwashed bacteria (39 and 28%, respectively [the values are means; n = 2]). The addition of three washes led to better recoveries of efficiency (84 and 61%, respectively), although it was still partial. These results indicate a partial effect of the extracellular part of the biofilm, but it is not the only phenomenon involved. These results indicate that the extracellular matrix is only partially implicated in S. aureus biofilm resistance to QACs and that modifications of the parietal structure are involved.
DISCUSSION
P. aeruginosa is able to rapidly produce structured biofilms under the conditions described above. In the course of continuous urine flow, Goto et al. (20) also noted that P. aeruginosa cells adherent to a Teflon catheter formed microcolonies at 6 h and a biofilm at 24 h. This maturation is characterized by EPS accumulation and is characterized overall by a three-dimensional structure that has been well defined by Costerton et al. (9). The negatively charged EPS produced by P. aeruginosa has been shown to bind to positively charged antibiotics such as aminoglycosides, but the resulting reduction in the diffusion coefficient within the biofilm has been shown to be insufficient to explain the observed changes in the susceptibility of the biofilm (29). The mucoid glycocalyx associated with the P. aeruginosa biofilm also protects the stuck cells against neutral antibiotics, indicating that factors other than charge are responsible for the reduced efficacies of antibiotics (22). Costerton (8) observed that β-lactam antibiotics can penetrate the entire thickness of the biofilm and so concluded that protection of biofilm cells is not really linked to slime. This observation is certainly true for hydrophilic antibiotics able to pass through the water channels of P. aeruginosa biofilms. Because of this specific architecture of the P. aeruginosa biofilm, molecules like the C8 and C10 QACs have ready access to the water channel systems, and this is aided by convective flow, which leads to preservation of their bactericidal activities. In contrast, an increase in the hydrophobicity of a molecule corresponds to a progressive loss of bactericidal efficiency against a P. aeruginosa biofilm according to the C-chain length of the QAC. Concerning N-alkylbenzyldimethyl ammonium chlorhydrate (40% C12, 50% C14, 10% C16), Gelinas and Goulet (19) also indicated that it was more readily neutralized by organic matter than other disinfectants, a phenomenon which could be attributed to the patterns of adsorption of the QAC. A second hypothesis in biofilm resistance concerns the microenvironment of the cells. When bacteria undergo nutrient deprivation they respond in a number of ways, leading to a population of cells with physiological functions and cell envelopes peculiar to each nutrient limitation (15) and, therefore, the adoption of atypical phenotypes. In the same way, reports of cell density-regulated phenotypes in biofilms (4) and the presence of N-acylhomoserine lactone-dependent regulation in biofilms (26) have led to demonstration of the role of quorum sensing in the final maturation of biofilms (13); this is especially the case for P. aeruginosa cells (12, 18, 27). The bacteria in a biofilm adopt a phenotype profoundly different from that of their planktonic counterparts, and 30 to 40% of the proteins in the cell envelope are different. Therefore, current work focuses on the specific role of this genetic switch in biofilm resistance, especially resistance to antibacterial compounds with specific targets. Concerning nonspecific bactericidal products like QACs, modification of the phenotype of attached P. aeruginosa cells does not seem to play a key role in the reduction of the efficiencies of the compounds. The disruption of the three-dimensional structure alone and the elimination of EPS by washing induce a loss of the cell surface-specific hydrophilicity and restoration of the major part of the efficiency of QACs. These results are in agreement with the loss of resistance to sodium dodecyl sulfate of a lasI mutant P. aeruginosa biofilm that expressed no structure (13) and with the increase in the level of resistance to chlorine observed with mucoid P. aeruginosa strains (34).
Attached S. aureus cells growing in nutrient-rich batch culture were found to go through the same growth phases as equivalent planktonic cultures and to possess high levels of viability (39). Biofilm formation in S. aureus is not as well defined as that in gram-negative bacteria and coagulase-negative staphylococci (Staphylococcus epidermidis) (25). Nevertheless, some attachment factors have been identified (37), like polysaccharide intercellular adhesin (10). In gram-positive bacteria, the signaling molecules are not homoserine lactones but hydrophobic peptides (8), which also lead to a wide range of modifications in gene expression. However, one of the most important observations that we made is that the two mature biofilms have very different structures, with the persistence of individual microcolonies for the S. aureus biofilm and the lowest protein and polysaccharide levels for the S. aureus biofilm. Despite this “poor” S. aureus biofilm structure, its formation led to more drastic resistance to QACs than the resistance of the P. aeruginosa biofilm. The adhesion of S. aureus to a solid substrate followed by cell-cell adhesion corresponds to a significant reduction of cell surface hydrophobicity which is not modified either by disruption of the biofilm or by washing. The percentages of recovery of sensitivity indicate a partial role of EPS (after washing) but a very important role of phenotypic modifications, the numbers of which increase with the molecule's hydrophobicity. Akiyama et al. (1) reported on the resistance to antimicrobial agents of an immature biofilm of S. aureus formed on coverslips after incubation for 24 h at 37°C. This observation for such a young biofilm structure could be linked to an earlier physiological modification of the attached cells and could corroborate the roles of parameters other than the final structure in the resistance of S. aureus biofilms.
Thus, the presence of a glycocalyx will sometimes alter susceptibility or will sometimes be negligible, depending on the circumstances (5), including the bacterial species concerned. The structure and organization of microbial biofilms are determined largely by surface properties, the nature and availability of nutrients, and fluid dynamic forces (40). With this in mind, the mode of biofilm formation could modify responses to antimicrobial agents. The results presented here describe possible mechanisms of resistance of biofilms and demonstrate the variabilities in the ways that microorganism adapt and decrease their susceptibilities according to environmental factors.
In a recent review, Costerton (8) underlines the fact that planktonic cells appear to “explore” the surface that they encounter in a species-specific series of behaviors, leading to the formation of isolated microcolonies or cell layers. This kind of species-specific behavior may certainly be extended to the mechanisms implicated in biofilm resistance if we consider that the EPS polymers produced by different species of bacteria are different, thus leading to different immediate surroundings and to the upregulation of different genes. Therefore, we must consider species-specific factors as well as molecule-specific factors in biofilm resistance patterns, especially for the development of new therapeutic concepts.
Acknowledgments
This work was supported by the Medlore Company.
REFERENCES
- 1.Akiyama, H., O. Yamasaki, H. Kanzaki, J. Tada, and J. Arata. 1998. Effects of sucrose and silver on Staphylococcus aureus biofilms. J. Antimicrob. Chemother. 42:629-634. [DOI] [PubMed] [Google Scholar]
- 2.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Eradication of biofilm cells of Staphylococcus aureus with tobramycin and cephalexin. Can. J. Microbiol. 38:618-625. [DOI] [PubMed] [Google Scholar]
- 3.Association Française de Normalisation. 1998. Antiseptiques et désinfectants. AFNOR, Paris, France.
- 4.Batchelor, S. E., M. Copper, S. R. Chabra, L. A. Glover, G. S. A. B. Stewart, P. Williams, and J. I. Prosser. 1997. Cell-density-regulated recovery of starved biofilm population of ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 63:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bown, M. R. W., and P. Gilbert. 1993. Sensitivity of biofilms to antimicrobial agents. J. Appl. Bacteriol. S74:87-97. [DOI] [PubMed] [Google Scholar]
- 6.Cook, G., J. W. Costerton, and R. O. Darrouiche. 2000. Direct confocal microscopy studies on the bacterial colonization in vitro of a silver-coated heart valve sewing cuff. Int. J. Antimicrob. Agents 13:169-173. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, J. W. 1999. Introduction to biofilm. Int. J. Antimicrob. Agents 11:217-221. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korder, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 10.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intracellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darouiche, R. O., A. Dhir, A. J. Miller, G. C. Landon, I. I. Raad, and D. M. Muscher. 1994. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J. Infect. Dis. 170:720-723. [DOI] [PubMed] [Google Scholar]
- 12.Davies, D. G., and G. G. Geesey. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 14.Dubois, M., K. A. Gilles., J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugar and related substance. Anal. Chem. 28:350-355. [Google Scholar]
- 15.Evans, D. J., D. G. Allison, M. R. Brown, and P. Gilbert. 1991. Susceptibility of Pseudomonas aeruginosa and Escherichia coli towards ciprofloxacin: effect of specific growth rate. Antimicrob. Agents Chemother. 27:177-184. [DOI] [PubMed] [Google Scholar]
- 16.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fournier, B., and C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 128:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 19.Gelinas, F., and J. Goulet. 1983. Neutralization of the activity of eight disinfectants by organic matter. J. Appl. Bacteriol. 54:243-247. [DOI] [PubMed] [Google Scholar]
- 20.Goto, T., Y. Nakame, M. Nishida, and Y. Ohi. 1999. Bacterial biofilms and catheters in experimental urinary tract infection. Int. J. Antimicrob. Agents 11:227-231. [DOI] [PubMed] [Google Scholar]
- 21.Grosserode, M. H., and R. P. Wenzel. 1991. The continuing importance of staphylococci as major hospital pathogens. J. Hosp. Infect. 19(Suppl. B):3-17. [DOI] [PubMed] [Google Scholar]
- 22.Hodges, N. A., and C. A. Gordon. 1991. Protection of Pseudomonas aeruginosa against ciprofloxacin and β-lactams by homologous alginate. Antimicrob. Agents Chemother. 35:2450-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis, K. 2001. The riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry, O. R., N. H. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:256-275. [PubMed] [Google Scholar]
- 25.Mack, D. 1999. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect. 43:S113-S125. [DOI] [PubMed] [Google Scholar]
- 26.McLean, R. J. C., M. Whiteley, D. J. Stickler, and W. C. Fuqua. 1997. Evidence of autoinducer activity in naturally occurring biofilm. FEMS Microbiol. Lett. 154:259-263. [DOI] [PubMed] [Google Scholar]
- 27.Morris, N. S., D. J. Stickler, and R. J. C. McLean. 1999. The development of bacterial biofilms on indwelling urethral catheters. World J. Urol. 7:345-350. [DOI] [PubMed] [Google Scholar]
- 28.Mozes, N., and P. G. Rouxhet. 1987. Methods for measuring hydrophobicity of microorganisms. J. Microbiol. Methods. 6:99-112. [Google Scholar]
- 29.Nichols, W. W., S. M. Darrington, M. P. E. Slack, and H. L. Walmsley. 1988. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 32:1518-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols, W. W. 1991. Biofilms, antibiotics and penetration. Rev. Med. Microbiol. 2:177-181. [Google Scholar]
- 31.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 32.Pineau, L., C. Roques, J. Luc, and G. Michel. 1997. Automatic washer disinfector for flexible endoscopes: a new evaluation process. Endoscopy 29:372-379. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg, M., D. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 34.Samrakandi, M. M., C. Roques, and G. Michel. 1997. Influence of trophic conditions on exopolysaccharide production: bacterial biofilm susceptibility to chlorine and monochloramine. Can. J. Microbiol. 43:751-758. [DOI] [PubMed] [Google Scholar]
- 35.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 36.Suci, P. A., J. D. Vrany, and M. W. Mittelman. 1998. Investigation of interactions between antimicrobial agents and bacterial biofilms using attenuated total reflection Fourier transform infrared spectroscopy. Biomaterials 19:327-339. [DOI] [PubMed] [Google Scholar]
- 37.Switalski, L. M., J. M. Patti, W. Butcher, A. G. Gristina, P. Speziale, and M. Höök. 1993. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol. Microbiol. 7:99-107. [DOI] [PubMed] [Google Scholar]
- 38.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, I., F. Paul, D. Lloyd, R. Jepras, I. Critchley, M. Neuman, J. Warrack, T. Giokarini, A. J. Hayes, P. F. Randerson, and W. A. Venables. 1999. Flow cytometry and other techniques show that Staphylococcus aureus undergoes significant physiological changes in the early stages of surface-attached culture. Microbiology 145:1325-1333. [DOI] [PubMed] [Google Scholar]
- 40.Wood, P., D. E. Caldwell, E. Evans, M. Jones, D. R. Korber, G. M. Wolfhaardt, M. Wilson, and P. Gilbert. 1998. Surface-catalysed disinfection of thick Pseudomonas aeruginosa biofilms. J. Appl. Microbiol. 84:1092-1098. [DOI] [PubMed] [Google Scholar]
- 41.Xu, X., P. S. Stewart, and X. Chen. 1996. Transport limitation of chlorine disinfection of Pseudomonas aeruginosa entrapped in alginate beads. Biotechnol. Bioeng. 49:93-100. [DOI] [PubMed] [Google Scholar]