Abstract
After a single oral dose of praziquantel with 250 ml of grapefruit juice, the area under the concentration-time curve and the maximum concentration in plasma of praziquantel (Cmax) were significantly increased (Cmax for water treatment, 637.71 ± 128.5 ng/ml; and Cmax for grapefruit juice treatment, 1,037.65 ± 305.7 ng/ml, P < 0.05). No statistically significant differences were found in the time to maximum concentration of drug in plasma or elimination half-life.
Grapefruit juice increases the bioavailability of a variety of drugs (3, 4, 10, 11, 13, 14, 18), but it scarcely affects the elimination half-life (t1/2). These findings suggest that grapefruit juice alters the first-pass metabolism mainly by suppression of the cytochrome P450 enzyme CYP3A4 in the small intestine (1, 2, 15).
Praziquantel is an effective antihelmintic drug, widely used in the treatment of various parasitic diseases, including brain cysticercosis (7, 20). It has been shown that the drug undergoes extensive metabolism by cytochrome P450; therefore, the systemic bioavailability of praziquantel is low and variable despite its almost complete gastrointestinal absorption (17). The specific cytochrome P450 enzymes involved in the metabolism of praziquantel in humans have not been characterized (5), but a significant inhibition of praziquantel hydroxylation by compounds that are inhibitors for 3A isoforms of cytochrome P450 has been observed in microsomes isolated from rat liver (16). In previous studies in humans, it was shown that cimetidine (an inhibitor of the microsomal cytochrome P450 mixed-function oxidase system) or simultaneous food administration increased the levels in plasma of praziquantel (6, 12); therefore, the aim of this study was to investigate the effect of the ingestion of grapefruit juice on the pharmacokinetics of praziquantel.
Eighteen healthy male volunteers (age range, 23 to 37 years; mean, 28.8 ± 5.4 years) participated in the study. Medical examination before the study showed that they were healthy and had normal results in laboratory tests that included complete blood count, serum chemistry measurement, hepatic test, and urinalysis. The volunteers signed an informed consent after detailed explanation about the purpose and design of the study, as well as the possible risks. The study was approved by the local ethics committee.
The study was performed using a balanced randomized crossover design. Volunteers were separated in two groups of nine subjects each and received three tablets of 600 mg of praziquantel (Cisticid; Merck) with 250 ml of water or 250 ml of commercially squeezed grapefruit juice (Florida 7; lot L-2 05:51; Zano Alimentos S.A. de C.V., Mexico City, Mexico). The same lot number was used throughout the study. The interval between tests was 1 week. Blood samples were obtained through an indwelling catheter placed in the antecubital vein at 0.0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, and 8.0 h after the drug administration. Samples were centrifuged; the plasma was separated and stored at −4°C until analysis. Subjects did not take any other medication or alcohol for at least 7 days prior to the study. Each subject fasted overnight prior to the study; fasting was continued for the first 4 h after the administration of praziquantel. Smoking and consumption of beverages that contained caffeine were not allowed, but water was allowed ad libitum during the study.
The concentration of praziquantel in plasma was determined using a high-performance liquid chromatography assay as follows. To 1 ml of plasma, 50 μl of a solution containing the diazepam (10 μg/ml) plus 1 ml of 0.2 M sodium hydroxide was added, shaken on a vortex mixer for 15 s, and extracted by passage through a Sep Pack C18 cartridge. The sample was washed with 20 ml of phosphate buffer. The compounds were then eluted with 6 ml of ethyl acetate-diisopropyl ether (70:30 [vol/vol]). The sample was evaporated to dryness under a nitrogen stream at 25°C. The residue was dissolved in 100 μl of the mobile phase of acetonitrile-water (45:55). Aliquots of 50 μl were injected into a Hewlett-Packard high-performance liquid chromatography system equipped with a variable wavelength detector and a Spherisorb ODS2 column (inside diameter, 250 by 4.6 mm; particle size, 5 μm). The method was linear from 15.6 to 8,000 ng/ml. Sensitivity was 15.6 ng/ml, and the maximum coefficient of variation was 7%. The recovery ranged between 95 and 100%. Samples were stable for 2 months when stored at −4°C.
The maximum concentration of drug in plasma (Cmax) and the time to maximum concentration of drug in plasma (Tmax) were determined from the highest observed value in the individual plasma concentration-time profiles. The area under the concentration-time curve (AUC) was calculated from 0 to 8 h (AUC0-8) by means of the linear trapezoidal method, and the terminal first-order rate constant was estimated by the least-squares fit of the terminal concentration phase using the program Pkanalyst MicroMath Scientific Software for Windows (Salt Lake City, Utah). The AUC0-∞ was calculated from the relationships: AUC0-8 + C8h/elimination rate constant (kel). The t1/2 was calculated as ln 2/kel. Data are presented as mean values ± standard errors of the means. Results are shown in Table 1.
TABLE 1.
Praziquantel pharmacokinetic parameters in healthy volunteers after 1,800-mg dose given with 250 ml of water or grapefruit juiceb
| Product tested or parameter | Cmax (ng/ml) | AUC0-8 (ng · h/ml) | Tmax (h) | t1/2 (h) | kel |
|---|---|---|---|---|---|
| Water | 637.71 (128.35) | 1,387.80 (240.46) | 1.77 (0.24) | 1.78 (0.17) | 0.45 (0.04) |
| Grapefruit juice | 1,037.65 (305.37) | 2,639.43 (786.98) | 2.10 (0.16) | 1.67 (0.11) | 0.44 (0.02) |
| Statistical comparisona | P = 0.0208 | P = 0.0204 | NS | NS | NS |
The Wilcoxon signed-rank test (n = 18) was used: water versus grapefruit juice, P < 0.05. NS, not statistically significant.
Values in table are given as means (standard errors of the means).
The pharmacokinetic parameters were analyzed by the analysis of variance of two one-sided tests to calculate bioequivalence (P < 0.05), including effects due to sequences, subjects, periods, and treatments. The 90% confidence intervals of the log-transformed data were calculated using the BIOPAK program (version 4.0) using the water treatment as the reference.
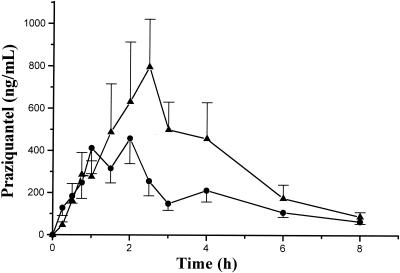
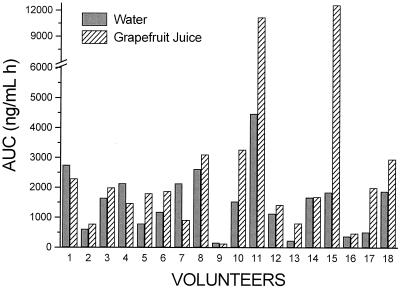
The mean drug plasma concentration-time profiles are shown in Fig. 1; compared with the Cmax of the water treatment group, the Cmax of praziquantel was significantly increased after administration of grapefruit juice (1.62-fold; 90% confidence interval, 1.05 to 2.0). The mean AUC of praziquantel increased 1.90-fold (1,387.8 ± 240.46 with water and 2,639.4 ± 786.9 with grapefruit juice). Individual AUC0-∞ data for treatments are shown in Fig. 2. As found in previous studies, a great interindividual variation on praziquantel disposition was observed; values found were 1,536.6 ± 254.9 and 2,821.7 ± 808.9 ng·h/ml when it was administered with water and grapefruit juice, respectively. When compared with results for water treatment, the 90% confidence interval was 1.03 to 2.47. The mean Tmax values of praziquantel were 1.77 and 2.10 h, and the t1/2 values were 1.78 and 1.67 h with water and with grapefruit juice, respectively. No significant differences were observed in kel, Tmax, and t1/2 between treatments at P < 0.05.
FIG. 1.
Concentration in plasma (mean values + standard errors of the means) of praziquantel in 18 healthy volunteers after a single oral dose of 1,800 mg of praziquantel administered either with 250 ml of grapefruit juice (▴) or water (•).
FIG. 2.
Individual AUC0-∞ of praziquantel when it was administered with grapefruit juice or water.
Grapefruit juice is known to interact with a broad range of drugs. It has been shown that grapefruit juice did not decrease the interindividual variation observed between subjects in AUC or Cmax. Therefore, it appears that drug concentrations obtained by a given dose are more difficult to predict if the drug is taken with grapefruit juice (9). The results of the present study showed that grapefruit juice increased the praziquantel concentration in plasma in most subjects. The mean Cmax increased 160%, and the AUC increased 190%. The effect of grapefruit juice on the bioavailability of praziquantel showed an interindividual variability similar to that found with other drugs (11, 13).
Ours findings may be clinically important because the ingestion of a normal-sized glass of grapefruit juice (nutrition facts: 115 kcal for serving, 1.5% of total fat daily allowance, and 7% of total carbohydrate daily allowance) may increase the bioavailability of praziquantel. When comparing our data with those obtained in previous studies, we found that the influence of grapefruit juice on the AUC of praziquantel is comparable to that observed with cimetidine and lower than that observed with food (1.65- and 2.7-fold for AUC, respectively) (6, 12).
Grapefruit juice did not prolong the praziquantel half-life; it seems that grapefruit juice modified the absorption phase. The biotransformation of praziquantel seems to be mediated mainly by CYP3A4. As grapefruit juice selectively inhibits the CYP3A4-mediated drug metabolism in the small intestine (8, 15, 19), the increase in the bioavailability of praziquantel in this study suggests that it could also be metabolized in the small intestine. This is the mechanism proposed for the increased oral bioavailability observed for other drugs metabolized by CYP3A4 when taken with grapefruit juice; however, more studies are needed to determine the mechanisms involved in its biotransformation.
Results suggest that joint administration of praziquantel and grapefruit juice could lead to a further improvement in the effectiveness of praziquantel therapy.
REFERENCES
- 1.Bailey, D. G., J. D. Spence, C. Munoz, and J. M. O. Arnold. 1991. Interaction of citrus juices with felodipine and nifedipine. Lancet 337:268-269. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, D. G., J. M. O. Arnold, A. Strong, C. Munoz, and J. D. Spence. 1993. Effect of grepefruit juice and naringin on nisoldipine pharmacokinetics. Cin. Pharmacol. Ther. 54:589-594. [DOI] [PubMed] [Google Scholar]
- 3.Bailey, D. G., J. M. O. Arnold, and J. D. Spence. 1994. Grapefruit juice and drugs. How significant is the interaction? Clin. Pharmacokinet. 26:91-98. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, D. G., J. M. O. Arnold, and J. D. Spence. 1998. Grapefruit juice interactions. Br. J. Clin. Pharmacol. 46:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertz, R., and R. Granneman. 1997. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin. Pharmacokinet. 32:210-258. [DOI] [PubMed] [Google Scholar]
- 6.Castro, N., R. Medina, J. Sotelo, and H. Jung. 2000. Bioavailability of praziquantel increases with concomitant administration of food. Antimicrob. Agents Chemother. 44:2903-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Brutto, O., J. Sotelo, and G. C. Roman. 1993. Therapy for neurocysticercosis: a reappraisal. Clin. Infect. Dis. 17:730-735. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, D. J., M. E. Fitzsimmons, E. G. Schuetz, K. Yasuda, M. P. Ducharme, L. H. Warbasse, P. M. Woster, J. D. Shuetz, and P. Watkins. 1999. 6′7′-Dihydroxybergamottin in grapefruit juice and Seville orange juice: effects on cyclosporine disposition, enterocyte CYP3A4, and P-glycoprotein. Clin. Pharmacol. Ther. 65:237-244. [DOI] [PubMed] [Google Scholar]
- 9.Fuhr, U. 1998. Drug interactions with grapefruit juice. Extent, probable mechanism and clinical relevance. Drug Saf. 18:251-272. [DOI] [PubMed] [Google Scholar]
- 10.Garg, S. K., N. Kumar, V. K. Bhargava, and S. K. Prabhakar. 1998. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin. Pharmacol. Ther. 64:286-288. [DOI] [PubMed] [Google Scholar]
- 11.Gross, A. S., Y. D. Goh, R. S. Addison, and G. M. Shenfield. 1999. Influence of grapefruit juice on cisapride pharmacokinetics. Clin. Pharmacol. Ther. 65:395-401. [DOI] [PubMed] [Google Scholar]
- 12.Jung, H., R. Medina, N. Castro, T. Corona, and J. Sotelo. 1997. Pharmacokinetic study of praziquantel administered alone and in combination with cimetidine in a single-day therapeutic regimen. Antimicrob. Agents Chemother. 41:1256-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupferschmidt, H. H. T., H. R. Ha, W. H. Ziegler, P. J. Meier, and S Krähenbühl. 1995. Interaction between grapefruit juice and midazolam in humans. Clin. Pharmacol. Ther. 58:20-28. [DOI] [PubMed] [Google Scholar]
- 14.Lilja, J. J., K. T. Kivisto, J. T. Backman, T. S. Lamberg, and P. J. Neuvonen. 1998. Grapefruit juice substantially increases plasma concentrations of busipirone. Cin. Pharmacol. Ther. 64:655-660. [DOI] [PubMed] [Google Scholar]
- 15.Lown, K. S., D. G. Bailey, R. J. Fontana, S. K. Janardan, C. H. Adair, L. A. Fortlage, M. B. Brown, W. Guo, and P. B. Watkins. 1997. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J. Clin. Investig. 99:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masimirembwa, C. M., and J. A. Hasler. 1994. Characterization of praziquantel metabolism by rat liver microsomes using cytochrome P450 inhibitors. Biochem. Pharmacol. 48:1779-1783. [DOI] [PubMed] [Google Scholar]
- 17.Patzschke, K., J. Putter, F. A. Wegner, and H. W. Diekmann. 1979. Serum concentrations and renal excretion in humans after oral administration of praziquantel--results of three determination methods. Eur. J. Drug Metab. Pharmacokinet. 3:149-156. [DOI] [PubMed] [Google Scholar]
- 18.Rau, S. E., J. R. Bend, M. O. Arnold, L. T. Trand, J. D. Spence, and D. G. Bailey. 1997. Grapefruit juice-terfenadine single-dose interaction: magnitude, mechanism, and relevance. Clin. Pharmacol. Ther. 61:401-409. [DOI] [PubMed] [Google Scholar]
- 19.Soons, P. A., B. A. Vogels, M. C. Roosemalen, H. C. Schoemaker, E. Uchida, B. Edgar, J. Lundahl, A. F. Cohen, and D. D. Breimer. 1991. Grapefruit juice and cimetidine inhibit stereoselective metabolism of nitredipine in humans. Clin. Pharmacol. Ther. 50:394-403. [DOI] [PubMed] [Google Scholar]
- 20.Sotelo, J., F. Escobedo, J. Rodríguez, B. Torres, and F. Rubio. 1984. Therapy of parenchymal brain cysticercosis with praziquantel. N. Engl. J. Med. 310:1001-1007. [DOI] [PubMed] [Google Scholar]