Abstract
Mutations in the low-density lipoprotein receptor–related protein 5 gene (LRP5) cause autosomal recessive osteoporosis-pseudoglioma syndrome (OPPG). We sequenced the coding exons of LRP5 in 37 probands suspected of having OPPG on the basis of the co-occurrence of severe congenital or childhood-onset visual impairment with bone fragility or osteoporosis recognized by young adulthood. We found two putative mutant alleles in 26 probands, only one mutant allele in 4 probands, and no mutant alleles in 7 probands. Looking for digenic inheritance, we sequenced the genes encoding the functionally related receptor LRP6, an LRP5 coreceptor FZD4, and an LRP5 ligand, NDP, in the four probands with one mutant allele, and, looking for locus heterogeneity, we sequenced FZD4 and NDP in the seven probands with no mutations, but we found no additional mutations. When we compared clinical features between probands with and without LRP5 mutations, we found no difference in the severity of skeletal disease, prevalence of cognitive impairment, or family history of consanguinity. However, four of the seven probands without detectable mutations had eye pathology that differed from pathology previously described for OPPG. Since many LRP5 mutations are missense changes, to differentiate between a disease-causing mutation and a benign variant, we measured the ability of wild-type and mutant LRP5 to transduce Wnt and Norrin signal ex vivo. Each of the seven OPPG mutations tested, had reduced signal transduction compared with wild-type mutations. These results indicate that early bilateral vitreoretinal eye pathology coupled with skeletal fragility is a strong predictor of LRP5 mutation and that mutations in LRP5 cause OPPG by impairing Wnt and Norrin signal transduction.
Introduction
Osteoporosis-pseudoglioma syndrome (OPPG [MIM 259770]) is an autosomal recessive disorder generally characterized by congenital or infancy-onset visual loss and skeletal fragility recognized during childhood. Mutations in the low-density lipoprotein receptor–related protein 5 (encoded by LRP5) cause OPPG (Gong et al. 2001). OPPG is a rare disorder. With the assumption of an estimated population incidence of 1 per 2,000,000 and a carrier frequency of 1 per 700, ∼380,000 U.S. citizens are predicted to be carriers of deleterious LRP5 mutations. Heterozygous carriers of OPPG-causing mutations have reduced bone-mineral density (BMD) compared with age- and sex-matched controls (Gong et al. 2001; Lev et al. 2003), and LRP5 mutations have been found among individuals with “idiopathic” osteoporosis and/or skeletal fragility (Hartikka et al. 2005). Population-based studies suggest that common variants of LRP5 contribute to the normal population variation in BMD (Ferrari et al. 2004; Mizuguchi et al. 2004; Bollerslev et al. 2005). Two other phenotypes have been attributed to a mutation in LRP5. Heterozygous missense mutations in the receptor’s first six-bladed propeller domain can cause autosomal dominant disorders of high bone mass (HBM), in which BMD is several SDs above the mean (Boyden et al. 2002; Little et al. 2002; Van Wesenbeeck et al. 2003). These mutations may cause a gain of function in the receptor by altering a binding site for the receptor’s endogenous inhibitors (Boyden et al. 2002; Ai et al. 2005). Heterozygous and homozygous mutations have also been described in some patients with the eye disease familial exudative vitreoretinopathy (FEVR) (Jiao et al. 2004; Toomes et al. 2004; Qin et al. 2005), a locus heterogeneous disorder that can also be caused by mutations in a secreted ligand, Norrin (NDP) (Chen et al. 1993), and an LRP5 coreceptor, Frizzled 4 (FZD4) (Robitaille et al. 2002).
LRP5 is a member of the low-density lipoprotein receptor superfamily of cell-surface receptors. Similar to the prototype family member LDLR, LRP5 has been implicated in plasma lipid homeostasis in mice (Fujino et al. 2003; Magoori et al. 2003). However, the relevance of this function to human plasma lipid homeostasis has not yet been evaluated. The most important role of LRP5 in humans is as a cell-surface signaling receptor. LRP5 serves as a coreceptor with members of the frizzled family of seven-pass membrane receptors in transducing signal by two extracellular ligand classes, Norrin and Wnts. Evidence to support LRP5 function as a Norrin coreceptor derives from ex vivo cell signaling assays (Xu et al. 2004) and the aforementioned observation that mutations in NDP, FZD4, or LRP5 can each cause FEVR. Evidence to support LRP5 function as a coreceptor in canonical Wnt signaling also derives from ex vivo signaling assays (Tamai et al. 2000; Gong et al. 2001) and from observations of phenotypic overlap between knockout and transgenic mouse models involving Lrp5 (Kato et al. 2002; Babij et al. 2003; Holmen et al. 2004), its closely related family member Lrp6 (Holmen et al. 2004), and members of the Wnt ligand superfamily (Bennett et al. 2005). Current data suggest that the skeletal effects of LRP5 mutations result from altered Wnt signaling and that the altered visual effects result from altered Norrin signaling. However, this hypothesis has not been definitively proven.
Most clinical descriptions of patients with OPPG were published prior to the discovery of LRP5 (summarized by Gong et al. [1996]). With the identification of LRP5 as the responsible gene, it is now possible to define phenotypic features that are common to patients with mutation-confirmed OPPG, to describe the disease course in these individuals, to correlate their phenotype with their genotype, and to address whether OPPG exhibits locus heterogeneity. Since there is the prediction of a large number of OPPG carriers who are at increased risk of osteoporosis (Gong et al. 2001), it is also important to determine the functional consequences of specific missense variations in LRP5—in particular, whether they interfere with Wnt and/or Norrin signaling and whether their mechanism of mutational effect is to cause a simple loss of function or an interfering function within the signaling complex. We report the clinical features and the results of mutation detection by direct sequencing of PCR amplimers from genomic DNA in a cohort of 37 probands/families in whom OPPG was clinically suspected. We also describe the functional consequences of several missense mutations found in patients with OPPG on LRP5 receptor trafficking and on Wnt and Norrin signal transduction, and we compare them with wild-type (WT) protein and missense mutants that have been associated with FEVR.
Material and Methods
Recruitment of Study Participants
Individuals clinically suspected of having OPPG were invited to participate in the present study. Informed consent was obtained from all study participants. The institutional review board at University Hospitals of Cleveland approved the study. All participants, or their physicians, were asked to complete a detailed questionnaire about clinical features and disease course (the clinical data form used in the study is accessible from the Warman lab Web site).
Mutation Detection in LRP5, LRP6, FZD4, and NDP by Use of Genomic DNA
Participant DNA was extracted and quantified using standard techniques. Genomic DNA (50 ng) was amplified by PCR in 25-μl reactions by use of intronic primers that flank each exon. Primer sequences and locations relative to the exon splice sites for the 23 LRP5 coding exons are available on request. Standard PCR conditions (94°C for 5 min, 35 cycles of 94°C for 1 min, 57°C for 50 s, and 72°C for 50 s, followed by a single 72°C 10-min extension) were used, with the following exceptions: reactions for exons 1, 4, and 5 contained 10% dimethyl sulfoxide, and the reaction for exon 21 contained 10% enhancer solution (Invitrogen). Primer annealing temperatures were 70°C for exons 1 and 23 and were 60°C for exons 4, 5, and 21. Amplification primer pairs for exons 1 and 3–9 were designed to avoid amplifying the LRP5 pseudogene on chromosome 22.
Amplicons were sequenced with BigDye 1.1 or 3.1 chemistry (Applied Biosystems) on an ABI 3100 or 3730 sequencer. Sequencing primers were usually the same primers used in PCR. Internal primers were used in some sequencing reactions. The ABI Sequence Analysis Software (v5.1) was used, and sequence electropherograms were also visually inspected for quality and for evidence of heterozygous changes and were electronically aligned with genomic sequences from human clones AC024124 and AC024123, to look for other types of mutations. Mutations identified in each proband were confirmed by sequencing amplimers from the proband’s parents or a sibling. For two mutations for which a relative was unavailable, the probands’ mutations were confirmed by sequencing independent amplimers, to exclude the possibility that the mutations were created during PCR. We did not look for mutations in ethnically/geographically matched controls, to differentiate a disease-causing mutation from a low-frequency polymorphism.
In four probands for whom only one putative disease-causing allele in LRP5 was identified, the 23 coding exons of LRP6, the two coding exons of FZD4, and the two coding exons of NDP were also PCR amplified and sequenced (primers and conditions are available on request). FZD4 and NDP were also sequenced for the seven probands for whom no LRP5 mutations were found.
Creation of Expression Constructs for LRP5
Construction of full-length human LRP5 (WT-LRP5) and a truncated form of human LRP5 that lacks the transmembrane and cytoplasmic domains but has a myc and His6 epitope at the C-terminus (LRP5N-myc) have been described elsewhere (Ai et al. 2005). Putative disease-causing missense mutations were introduced into WT-LRP5 by site-directed mutagenesis (Quickchange [Stratagene]). Smaller restriction fragments were subcloned from the expression construct, were used as the template for mutagenesis, were sequence verified, and were then shuttled into the original expression vectors. For example, the T244M mutation was introduced into a 1.3-kb EcoRI/SalI restriction fragment containing the first epidermal growth factor–like (EGF-like) domain, and the S356L, T390K, G404R, D434N, G520V, and G610R mutations were individually introduced into a 700-bp SalI/XhoI restriction fragment containing coding sequence for part of the second EGF-like domain. FEVR-associated mutations T173M, R570Q, Y1168H, C1361G, and E1367K were made in a similar manner. Other expression vectors used in this study were mouse Wnt1-v5 (Ai et al. 2005), Wnt 10b (Bennett et al. 2005), Norrin and Frizzled 4 (Xu et al. 2004), MESD-C2 and RAP (Hsieh et al. 2003), Topflash (Korinek et al. 1997), and pRL-TK (Upstate Biotechnology).
Ex Vivo Reporter Assays for Wnt and Norrin Signal Transduction
HEK293T cells (American Type Culture Collection), cultured in Dulbecco’s modified essential medium containing 10% fetal bovine serum, were plated at 2.5×105 cells per well in 24-well plates 24 h prior to transfection. Transfection was done in serum-free media with Lipofectamine Plus (Invitrogen), in accordance with the manufacturer’s protocol. For Wnt-signaling assays, DNA transfections included the following expression constructs: Topflash (100 ng), pRL-TK (5 ng), MESD-C2 (20 ng), WT-LRP5 or LRP5 constructs containing missense mutations (30 ng), and Wnt1-v5 or Wnt10b (100 ng). For Norrin-signaling assays, DNA transfections included the following expression constructs: Topflash (100 ng), pRL-TK (5 ng), MESD-C2 (20 ng), WT-LRP5 or LRP5 constructs containing missense mutations (30 ng), Fzd4 (50 ng), and Norrin (50 ng). To assure that equal amounts of DNA were transfected in each experiment, pcDNA3.1-LacZ (Invitrogen) was added to make 255 ng the total amount of DNA per transfection. Cells were lysed 30 h after transfection. Firefly luciferase activity from the Topflash reporter was measured using a dual Luciferase assay kit (Promega) in a luminometer (Molecular Devices). Renilla luciferase activity from pRL-TK was measured, as an internal control for transfection efficiency. Each assay was performed in triplicate. Data from single experiments are reported in the “Results” section, but each experiment was performed three times with consistently reproducible results.
Assay to Assess Trafficking of WT and OPPG LRP5 Constructs That Lack the Transmembrane and Cytoplasmic Domains
WT-LRP5N-myc or LRP5N-myc constructs that contained OPPG missense mutations were used. These constructs lack the transmembrane and cytoplasmic domain and should be secreted into the conditioned medium of expressing cells if properly trafficked. HEK293T cells were cultured as described above. Cells were plated at 5×105 cells per well in six-well culture plates 24 h prior to transfection. Each well was transfected with LRP5N-myc (200 ng), MESD-C2 (200 ng), and RAP (200 ng), with use of Lipofectamine Plus, and was maintained in 1 ml of serum-free medium. LRP5N-myc protein was recovered from the medium after 48 h and from the cell lysate, by scraping the cell layer after the addition of 1 ml RIPA buffer (50 mM Tris; pH 8; 150 mM NaCl; 1% NP-40; 0.5% deoxycholate; 0.1% sodium dodecyl sulfate). Twenty microliters of conditioned medium or cell lysate were mixed with 5 μl of 5× SDS-PAGE–loading buffer, were subjected to reducing SDS-PAGE, and were detected by the monoclonal anti-myc antibody 9E10 (Santa Cruz Biotech). To demonstrate that the secreted forms of LRP5 are posttranslationally modified, we mixed 17.5 μl of LRP5N-myc that contained conditioned medium with 1 μl Tris (1 M; pH 8.0), 1 μl β-mercaptoethanol, and 0.5 μl SDS (10%) and heated at 99°C for 5 min. We then added 25 μl water, 5 μl NP-40 (6%), and 5 μl N-glycosidase F (1 unit/μl) (Roche) and incubated overnight at room temperature. A control reaction was performed by adding 5 μl water instead of 5 μl N-glycosidase. The control and N-glycosidase digested samples were mixed with 25 μl 4× SDS-PAGE loading buffer, and 25 μl were subjected to reducing SDS-PAGE and were detected using the anti-myc antibody.
Results
LRP5 Mutations in Patients Suspected to Have OPPG
Probands from 37 families were suspected of having OPPG (table 1). PCR amplification and direct sequencing of amplimers for all LRP5 coding exons led to the identification of 11 nonsense, 11 frameshift, 2 splice-site, and 20 missense mutations, all of which we assumed were disease causing (table 1 and fig. 1A). Several other missense mutations were identified that have an appreciable frequency in unaffected controls and were not considered to be disease causing (data not shown). Of the 37 probands, 12 were homozygous for the disease-causing mutations, and 14 of the 37 were compound heterozygous for two disease-causing mutations. A single heterozygous mutation was detected in 4 of the 37 probands; these mutations were unlikely to cause OPPG via a dominant mechanism, since they were also found in an asymptomatic carrier parent or sibling. Of the 37 probands, 7 had no identified mutant LRP5 alleles.
Table 1.
Characteristics of Study Families
|
Phenotype (Age First Recognized) |
||||||||
| Mutation Type and Familya | Consanguinityb | DNA Changec | Protein Change | Sex of Proband | Eye | Skeletal | Mental Retardationd | Atypical Findingsof Affectedsiblings or parentse |
| Homozygous mutations: | ||||||||
| OP236* | Y | 29G→A | W10X | F | Pseudoglioma (1 mo) | Craniotabes (birth) | No | |
| OP342*,f | Y | 3804delA | T1268fsX1438 | M | Hyperplastic vitreous (3 mo) | Osteoporosis and fractures (10–11 years) | No | |
| OP642* | N | 3804delA | T1268fsX1438 | M | Vitreoretinal dysplasia (birth) | Fractures (3 years) | Moderate | Mother has a mild degree of degenerative change in her retina |
| OP345*,g | N | 1467delG | L489fsX529 | M | Microphthalmia (4 mo) | Fractures (1 year) | No | |
| OP346*,h | Y | 1708C→T | R570W | F | Rentrolental fibroplasia (5 years) | Osteoporosis (8 years) | No | Brother was blind at birth and had vertebral compression fractures noted in adulthood |
| OP352* | Y | 2151_2152insT | D718fsX718 | M | Pseudoglioma (3 mo) | Craniotabes (9 mo) | Moderate | |
| OP417*,i | Y | 2557C→T | Q853X | M | Pseudoglioma (1 mo) | Fractures (4 years) | No | |
| OP445* | N | 1481G→A | R494Q | F | Posterior synechiae (birth) | Fractures (7 years) | No | |
| OP457* | Y | 1282C→T | R428X | M | Pseudoglioma (birth) | Fractures (<10 years) | Mild | |
| OP502j | Y | 1058G→A | R353Q | M | Blind (6 years) | Osteoporosis (20 years) | No | Brother was blind at age 8 mo and had osteoporosis noted in adulthood |
| OP616 | N | 920C→T | S307F | M | Blind (birth) | Fractures (5 years) | Severe | Sister had retrolental fibroplasia at 1 year, fractures, and severe retardation |
| OP637 | Y | 1000_1004dupAGGAC | T335fsX385 | M | PHPV (birth) | Fractures (1 year) | No | |
| Compound heterozygous mutations: | ||||||||
| OP238 | N | 1210 G→A 2718_2721delTATG | G404R C906fsX958 |
M | Exudative retinopathy (2 years) | Fractures (3 years) | No | |
| OP344*,k | N | 765G→A 1067C→T | W255X S356L |
F | Retrolental mass (1 mo) | Fractures (2 years) | No | |
| OP347*,l | N | 789C→A 2202G→A | C263X W734X | M | Turbidity (2 mo) | Fractures (4 years) | No | |
| OP348* | N | 1453G→T 4600C→T | E485X R1534X | F | Vitreoretinal disease (6 wk) | Fractures (2 years) | No | |
| OP349m | Y | 1300G→A 1559G→T | D434N G520V |
F | PHPV (birth) | Fractures (9 years) | No | |
| OP450m | N | 1042C→T in cis with 3337C→T 2047G→A | R348W in cis with R1113C D683N | F | Retinal detachments (3 mo) | Compressed vertebrae (11 years) | No | |
| OP482* | N | 2197T→C 2247delG | Y733H G749fsX797 | F | Vitreous hemorrhages (birth) | Craniotabes (birth) | No | Mother with Y733H has subtle evidence of FEVR |
| OP524 | N | 731C→T 3763+2T→C | T244M splice mutation |
M | Retrolental mass (birth) | Compressed vertebrae (4 mo) | No | |
| OP535 | N | 1750C→T 4512_4517delGGCCAC, insTGTACAACAT | Q584X P1504fsX1550 | M | Retrolental mass (5 wk) | Fractures (2 years) | Moderate | |
| OP579 | N | 4202G→A 4586+2T→C | G1401D splice mutation | F | Blind (birth) | Fractures (10 mo) | No | |
| OP608 | N | 2409_2503+79del 174 3232C→T | G804_G835delfsX49 R1078X | M | Blind (birth) | Fractures (2 years) | Moderate | |
| OP610 | N | 2247delG 2737_2738insT | G749fsX797 C913fsX985 | F | Pseudoglioma (birth) | Fractures (6 years) | No | |
| OP612 | N | 209_210TC→AA 1378G→A | F70X E460K | M | Blind (3 mo) | Compressed vertebrae (11 years) | No | |
| OP654 | N | 607G→A 1199C→A | D203N A400E | M | Pseudoglioma (birth) | Fracture (1 year) | No | |
| Single heterozygous mutations: | ||||||||
| OP240 | N | 1169C→A | T390K |
M | Pseudoglioma (5 wk) | Vertebral compression (2 years) | No | |
| OP343k | N | 4105_4106delAT | M1369fsX1370 | F | Posterior vascular anomaly (birth) | Fractures (11 years) | No | |
| OP350 | N | 3295G→T | D1099Y | F | Blind (3 years) | Fractures (<9 years) | Mild | |
| OP547 | N | 1828G→C | G610R |
M | PHPV | Fractures (4 years) | Mild | |
| No identified mutations: | ||||||||
| OP354 | Y | Heterozygous for LRP5 polymorphisms | F | Pseudoglioma (2 mo) | Fracture and pseudarthrosis (birth) | Mild | Brother had congenital heart defect, fractures, pseudarthroses, mild retardation, and retinopathy with temporal vascular changes detected at age 2 years | |
| OP474 | N | Homozygous for LRP5 polymorphisms | M | Congenital retinal folds (birth) | Fractures with osteoporosis (14 years) | No | ||
| OP549 | N | M | Bilateral glaucoma (birth), unilateral visual loss (34 years) | Fractures (7 years) | No | |||
| OP555 | N | M | Phthisis bulbi (birth) | Osteoporosis (2 years) | Mild | |||
| OP618 | N | M | Peters anomaly (birth) | Fractures (4 years) | No | |||
| OP624 | N | M | Retinal coloboma (birth) | Fractures (<15 years) | No | |||
| OP641 | N | M | Retinal detachment (5 years) | Fracture and osteoporosis (31 years) | Mild | |||
Families marked with an asterisk (*) had their mutations published by Gong et al. (2001).
Consanguinity is considered present when reported by the proband’s parents.
DNA mutations were identified by sequencing amplified genomic DNA fragments but are described relative to the Lrp5 cDNA sequence, with A as the first nucleotide of the ATG methionine translation initiation codon. Potential consequences at the protein level, if there was no nonsense-mediated mRNA decay, are described relative to the translated protein, with the methionine representing the first amino acid residue. Missense mutations in the underlined protein were tested for Wnt and Norrin signal transduction in 293T cells.
Mental retardation is considered to be independent of the proband’s visual loss.
Not all affected siblings are listed.
Clinical features of the participating probands were initially described by Beighton et al. (1985).
Clinical features of the participating probands were initially described by Frontali et al. (1985).
Clinical features of the participating probands were initially described by Somer et al. (1988).
Clinical features of the participating probands were initially described by Superti-Furga et al. (1986).
Clinical features of the participating probands were initially described by Lev et al. (2003).
Clinical features of the participating probands were initially described by De Paepe et al. (1993).
Clinical features of the participating probands were initially described by Swoboda and Grill (1988).
Clinical features of the participating probands were initially described by Zacharin and Cundy (2000).
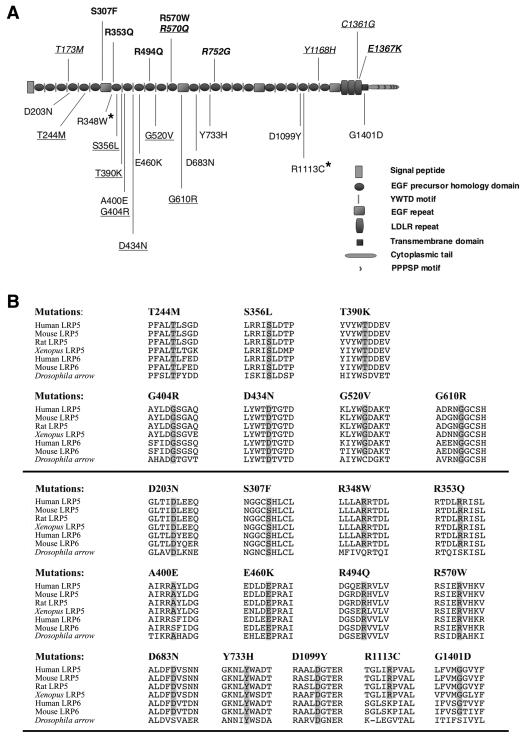
Figure 1.
Disease-associated missense mutations in LRP5. A, Schematic depiction of LRP5 protein and the sites of missense amino acid substitutions that have been associated with OPPG. Homozygous mutations in patients with OPPG are noted in bold above the protein. Heterozygous mutations in patients with OPPG are shown below the protein. Two missense mutations that occur in cis on a single allele are marked with an asterisk (*). Missense mutations that are associated with autosomal dominant FEVR are shown in italics, and mutations associated with autosomal recessive FEVR are in bold italics. All underlined mutations were tested ex vivo. B, Single-letter amino acid ClustalW alignments of residues surrounding the 20 OPPG-associated missense mutations for LRP5, LRP6, and arrow. The amino acid residues altered by the missense mutations are shaded in the species in which the residue is evolutionarily conserved. The seven putative OPPG-causing mutations that were tested ex vivo are shown in the upper half of the panel.
LRP5 has a closely related paralog, LRP6, which also serves as a Wnt receptor. Mice completely lacking Lrp6 are nonviable (Pinson et al. 2000); mice doubly heterozygous for mutations in Lrp5 and Lrp6 have low bone mass (Holmen et al. 2004). Therefore, we sequenced LRP6 in the four probands with OPPG for whom we could detect only a single mutant LRP5 allele, to look for digenic inheritance; we found no LRP6 mutations. We did not sequence LRP6 in the seven probands with no detected LRP5 mutation, given the embryonic lethality in the Lrp6 homozygous mutant mice and the lack of reported eye or severe skeletal phenotypes in the Lrp6 heterozygous mice. Frizzled 4 and LRP5 act as coreceptors for Norrin, and mutations in either LRP5 or FZD4 have been found to cause FEVR (Toomes et al. 2004; Xu et al. 2004; Qin et al. 2005). Therefore, we also sequenced FZD4 and NDP in the four probands with single LRP5 mutations and in the seven probands who had no identified LRP5 mutations. No FZD4 or NDP mutations were found.
Clinical Characteristics in Probands With and Without LRP5 Mutations
Congenital, childhood-onset, or childhood-recognized ocular disease was reported for all 30 probands with identified LRP5 mutations. The severity of ocular disease in patients for whom original ophthalmologic records were available ranged from phthisis bulbi to less-severe vitreoretinal findings, such as persistent hyperplasia of the primary vitreous (PHPV), congenital retinal folds, and exudative retinopathy. The majority of probands with mutations were congenitally blind in both eyes; some were congenitally blind in one eye and visually impaired in the other or were moderately visually impaired in both eyes. Most adult probands with LRP5 mutations—and their affected adult siblings—were blind by age 15 years, and all were blind by age 25 years. Four of the seven probands without LRP5 mutations had ocular defects that had not been previously described in OPPG, including isolated cataract, retinal coloboma, Peters anomaly, and unilateral eye involvement. The male:female ratio is 3:2 in probands with LRP5 mutations and 6:1 in probands without identified mutations. It remains possible that mutation in an X-linked gene, such as NDP, may be responsible for some instances of disease in these probands. However, we did not find an NDP coding-sequence mutation in any of the patients, and osteoporosis and skeletal fragility have not been described in males with Norrie disease.
Skeletal disease was apparent by adolescence in 29 of 30 probands with LRP5 mutations and in nearly all affected siblings. One proband (family OP346) has an affected sibling in whom skeletal disease was recognized in his 20s (table 1). Another proband and his sibling (family OP502) were both found to be osteoporotic in their 20s (table 1). Among all patients with LRP5 mutations, the skeletal disease was characterized by fractures and/or radiologically determined severe osteoporosis. Importantly, skeletal disease was recognized during the first 2 years of life in fewer than half of the probands with identified mutations. In several asymptomatic infants and toddlers, osteoporosis or vertebral compressions were found incidentally when radiographs were obtained for other reasons (e.g., suspected pneumonia or constipation). We observed no consistent difference in clinical and radiographic skeletal features between probands for whom LRP5 mutations were and were not found.
Cognitive impairment, independent of visual impairment, was reported for 8 of 30 probands with identified mutations. There were no significant differences in the rates of cognitive impairment between probands with homozygous (4 of 12 probands), compound heterozygous (2 of 14 probands), or single identified (2 of 4 probands) LRP5 mutations. Three of seven probands without identified mutations were also reported to be cognitively impaired.
Functional Analysis of LRP5 Receptors with Missense Mutations
We hypothesized that most nonsense and frameshift mutations would result in nonsense-mediated mRNA decay or would produce truncated proteins that would be improperly trafficked within the cell. The consequences of missense mutations, which accounted for 20 of the 44 putative disease-causing mutations, were less clear. Alignment of the amino acid residues affected by the missense mutations against LRP5 sequence from mouse, rat, and Xenopus laevis, LRP6 sequence from human and mouse, and arrow sequence from Drosophila melanogaster demonstrated complete conservation of these residues within LRP5 orthologs and near-complete conservation within LRP6 across vertebrates and arrow in fruit fly (fig. 1B).
Since conservation can suggest but neither prove causality of a missense change nor predict the precise mechanism by which a missense change will exert an effect, we studied several of the mutant proteins in transiently transfected 293T cells, to determine their ability to traffic through the cell and to transduce Wnt and Norrin signal. We expressed a myc-epitope–tagged version of LRP5 that lacks the transmembrane and cytoplasmic domains and should be secreted into the conditioned medium when normally trafficked. The version of this construct that contained the WT sequence was able to traffic through the cell, be posttranslationally modified by N-linked glycosylation, and be secreted (fig. 2). We then studied seven different mutant proteins whose missense mutations affect the first or second six-bladed propeller domains within the protein. One mutant, T390K, was expressed within the cell but was unable to traffic normally. Several other mutants—T244M, G404R, D434N, and G610R—appeared to traffic less well than did the WT protein. Two mutants, S356L and G520V, appeared to traffic comparably to the WT protein (fig. 2A). All secreted OPPG mutant proteins appeared to be posttranslationally modified, similar to WT LRP5 (fig. 2B and data not shown).
Figure 2.
Trafficking and posttranslational modification of OPPG-LRP5 mutants in 293T cells. A, WT and OPPG-causing LRP5 constructs that express a myc-tagged, truncated polypeptide lacking the transmembrane and cytoplasmic domains (LRP5N-myc) were transiently transfected into 293T cells. Western-blot analyses were performed to detect the recombinant protein in the conditioned medium (“CM”) and cell lysate (“Lysate”) with use of an anti-myc antibody. Note the relative efficiencies of different mutants to transit the cell and be secreted into the conditioned medium compared with the WT protein. T390K is present in the cell lysate but is not secreted into the conditioned medium, whereas G520V is secreted into the conditioned medium at rates comparable to WT. Equal loading of cell lysate and conditioned medium in each lane is demonstrated by immunodetection of cell lysate with an anti-tubulin antibody, D-10-HRP (Santa Cruz Biotechnology), and Coomassie staining of conditioned medium, respectively. B, Western-blot analysis of conditioned medium (“CM”) and cell lysate (“LY”) from 293T cells expressing WT or G520V LRP5N-myc protein. Note that, when the conditioned medium was digested with N-glycosidase (“DG”), the molecular weight of the secreted protein was decreased. Other OPPG-associated mutant proteins were tested in the same assay and gave similar results (data not shown).
To assess the effect of these mutations on signal transduction, we coexpressed full-length, untagged WT LRP5 (or full-length untagged mutant LRP5) with Wnt ligand (or Norrin ligand) and measured β-catenin–mediated signaling, using the Topflash reporter assay (white bars in fig. 3A and 3B). WT-LRP5 was able to transduce Wnt signal, as indicated by the large fold increase in Topflash reporter activity. However, the mutant LRP5 receptors T244M, S356L, T390K, and G520V were unable to transduce Wnt1 or Wnt10b signal. The mutants G404R and D434N had <50% the activity, and the mutant G610R had 60% the activity of WT-LRP5. The phenotype of the probands with these latter three mutations was not clinically milder than that of probands with other LRP5 mutations; each was blind and had severe skeletal disease.
Figure 3.
OPPG-causing missense mutations impair Wnt and Norrin signaling. Fold-induction of luciferase activity in 293T cells expressing WT or OPPG-causing missense mutants are expressed in combination with Wnt1-v5 (A), Wnt10b (B), and Norrin and Fzd4 (C). Thirty hours after transfection, firefly luciferase activity was measured and normalized to Renilla luciferase activity. Note that four mutants have no signal transduction and that three (G404R, D434N, and G610R) have markedly reduced signal transduction (white bars); WT-LRP5 signal transduction was not inhibited when coexpressed with an OPPG mutant (gray bars), which indicates that the OPPG mutants do not exert a dominant negative effect (A and B). C, OPPG mutants have reduced ability to transduce Norrin signal. D, Western blot of cell lysates from transfected 293T cells used in the Wnt1-v5 signal transduction assay shown in panel A, demonstrating comparable expression of WT and OPPG-causing LRP5 receptors. Lysates were separated by SDS-PAGE and were immunodetected with an anti-LRP5/LRP6 antibody, 3801–100 (Biovision). An anti-tubulin antibody was used to demonstrate that comparable amounts of cell lysates were loaded.
Carriers of OPPG mutations often have significantly reduced BMD. Furthermore, some LRP5 mutations that cause FEVR appear to act in a dominant manner. Therefore, we coexpressed WT and mutant LRP5, to determine whether the heterozygote phenotype of LRP5 mutations was likely to be due to functional haploinsufficiency or a dominant negative effect. When coexpressed with WT LRP5, none of the mutant proteins interfered with WT Wnt signal transduction (gray bars in fig. 3A and 3B). This would argue against a dominant negative effect for these mutations on Wnt signaling.
We also assessed the effects of the LRP5 mutations on Norrin signaling. Norrin also activates the β-catenin–mediated signaling pathway, for which the Topflash reporter assay is a useful readout (Xu et al. 2004). Each of the LRP5 mutants, including those that appear to traffic normally through the cell, had a significantly reduced ability to transduce Norrin signal (fig. 3C). This result is consistent with the hypothesis that visual loss in patients with OPPG results from defective Norrin signaling, although this result does not preclude defective Wnt signaling from also affecting eye development.
Three LRP5 missense mutations have been identified in patients with autosomal recessive FEVR who were not reported to have skeletal involvement. We tested two of these mutants, along with three missense mutants that have been associated with dominantly inherited FEVR, for their ability to transduce Wnt and Norrin signal (fig. 4). One dominant mutant, Y1168H, was unable to transduce Wnt or Norrin signal. One recessive mutant, R570Q, had significantly reduced Wnt and Norrin signal transduction, and one dominant mutant, C1361G, had mildly reduced Wnt and Norrin signal transduction. However, the remaining dominant and recessive mutants behaved like WT LRP5 in these assays.
Figure 4.
FEVR-causing missense mutations variably affect Wnt and Norrin signaling. Fold-induction of luciferase activity in 293T cells expressing WT or FEVR-causing missense mutants in combination with Norrin and Fzd4 (A) or Wnt1-v5 (B). Mutants associated with autosomal recessive FEVR are underlined. Note that the Y1168H mutant could not transduce Norrin or Wnt signal, and mutants T173M and E1367K could transduce signal from both ligands (white bars). Coexpression of WT and FEVR-causing mutant receptors did not interfere with the WT receptor’s ability to transduce Norrin or Wnt-v5 signal (gray bars), which indicates that these mutations do not have dominant negative effects.
Discussion
We sequenced LRP5 in 37 probands who had been referred with a suspected diagnosis of OPPG. We found 44 likely disease-causing mutations of 64 anticipated mutant alleles (we anticipated fewer mutant alleles because several families were consanguineous), for a mutation-detection rate of ∼70%. For those four probands in whom only one heterozygous change was identified, we assume that an undetected second mutation exists in the other allele. Mutations affecting LRP5 that would have been missed in our mutation-detection strategy include exon deletions, intron mutations that affect splicing, coding mutations that could not be PCR amplified because of primer annealing-site polymorphisms, and mutations that affect regulatory regions. Another possible explanation for the presence of only a single mutant allele is digenic inheritance, which has been observed in other human diseases (Kajiwara et al. 1994; Katsanis et al. 2001; Gabriel et al. 2002), including FEVR (Qin et al. 2005). We sequenced three additional genes, LRP6, FZD4, and NDP, that have skeletal or ocular findings when disrupted in mice (Richter et al. 1998; Holmen et al. 2004; Xu et al. 2004), but we found no additional mutations. However, it remains possible that mutant alleles of genes encoding other Wnt signaling components, such as Wnt ligands and other Frizzled receptors, could cause OPPG in combination with a heterozygous mutation in LRP5.
In addition to finding likely loss-of-function alleles, such as those with nonsense and frameshift mutations, we identified 20 missense mutations in LRP5. We assume that these are disease-causing because they alter amino acid residues that are highly conserved across species. However, this does not prove causality, since mutations affecting highly conserved amino acid residues can occur without any functional consequence. For example, residues in the highly conserved catalytic domain of Thermus aquaticus DNA polymerase I could be mutated without disrupting enzymatic activity (Patel and Loeb 2000). Additionally, two missense mutations, each affecting a conserved residue, are present on the same allele in siblings with OPPG (family OP450) (table 1). Therefore, to determine whether missense mutations in LRP5 cause a loss of function, we utilized a cell-based reporter assay to measure the ability of mutant receptors to transduce Wnt signal. Although we have not tested all 20 missense mutants, the first 7 mutants we did test all had impaired Wnt signal transduction. This result supports the hypothesis that the skeletal phenotype in OPPG is due to reduction in Wnt signaling.
The hypothesis that impairment of Wnt signal transduction is specific to OPPG-causing missense mutations is supported by studies of LRP5 mutants that cause the opposite skeletal phenotype, autosomal dominant HBM. When seven HBM-causing LRP5 mutants were tested in this assay, none exhibited reduced Wnt signal transduction (Ai et al. 2005). Therefore, this assay should be useful for determining whether a missense mutation identified in a person with OPPG or idiopathic osteoporosis is disease causing.
The availability of an allelic series of mutations in LRP5 that affect Wnt signal transduction enabled us to ask whether all OPPG mutations affect receptor function in the same way. By expressing mutant receptors in transiently transfected cells, we observed that some mutations impaired receptor trafficking, whereas others did not. These latter mutations may impair signal transduction at the level of ligand binding, coreceptor interaction, or recruitment of cytoplasmic factors. Importantly, we found no evidence of a dominant negative effect in any of the mutants tested thus far. Since low bone mass has been observed in obligate carriers of nonsense, frameshift, and missense mutations (authors’ unpublished data), functional haploinsufficiency appears to be the common mechanism of mutational effect associated with isolated osteoporosis.
Within the developing eye, LRP5 may transduce Norrin signal rather than Wnt signal. Mutations in NDP cause blindness associated with PHPV (Sims 2004), which has also been observed in patients with OPPG (Steichen-Gersdorf et al. 1997). A second eye disease, FEVR, is locus heterogeneous, with mutations identified in NDP, FZD4, and LRP5 (Toomes et al. 2004). In mice and in ex vivo studies these three proteins interact in a signal transduction pathway (Xu et al. 2004). This led us to determine whether OPPG-causing mutations affect Norrin-signal transduction and whether FEVR-associated mutations affect Wnt-signal transduction. All seven OPPG-causing missense mutants impaired Norrin signaling (fig. 3C). Surprisingly, there was great variability in the ability of the FEVR-causing mutant receptors to transduce Wnt and Norrin signal (fig. 4). For example, one mutant, Y1168H, which is associated with autosomal dominant FEVR, was unable to transduce Wnt and Norrin signal, whereas the mutant R570Q, which is associated with autosomal recessive FEVR, had residual Wnt- and Norrin-signal transduction. Another homozygous mutation affecting this residue (R570W) is present in siblings with OPPG (family OP346) (table 1). We suspect that, when evaluated, the patients with homozygous R570Q FEVR will have significantly reduced BMD. Several other FEVR mutations had no effect on Wnt or Norrin signaling. We do not know whether these results imply that these latter mutations are non–disease causing or that the cell-based assay is insensitive. The T173M mutation may be an example of the former possibility, since it was found in an elderly patient with retinal folds who had no family history of FEVR (Toomes et al. 2004); additionally, the mutated residue is not evolutionarily conserved. In support of the latter possibility are several FEVR mutations that alter highly conserved residues and the observation that disease-causing mutations in Norrin have had variable effects on signal transduction in a similar assay (Xu et al. 2004).
Our results do not help elucidate the mechanism by which heterozygous mutations in LRP5 cause autosomal dominant FEVR in some families but little eye disease among the carrier parents and siblings of patients with OPPG. Because obligate OPPG carriers have not complained of visual impairment, few eye exams have been performed (10 parents underwent formal ophthalmologic assessment in our study, and only 2 have subtle evidence of retinal disease [families OP642 and OP482] [table 1]). This contrasts with FEVR, which had been considered a highly penetrant disorder (Toomes et al. 2004). Penetrance could be higher in FEVR-affected families with FZD4 mutations than in families with LRP5 mutations or could seem to be higher because family members have undergone studies, such as fluorescein angiography, to detect subtle signs of disease. Similarly, we do not know which patients with FEVR may be at increased risk for osteoporosis as are OPPG carriers, although a recent study reported that five of seven patients with FEVR due to LRP5 mutation had BMD >1 SD below the mean, whereas zero of five patients with FEVR due to FZD4 mutation had comparably reduced BMD (Qin et al. 2005).
The results of this study have several practical implications for patients and families suspected of being affected by OPPG or of having deleterious LRP5 mutations. First, infancy-onset visual loss that is not associated with vitreoretinal disease seems unlikely to be due to LRP5, since mutations were not found in the four probands who lacked vitreoretinal pathology. Second, radiographs and quantitative BMD measurements should be performed in infants and children who have eye features of OPPG, since skeletal features such as vertebral compressions may be present but may not be clinically apparent in young children; early diagnosis of skeletal disease is important so that affected individuals can take advantage of emerging therapies for improving bone strength (Zacharin and Cundy 2000). Third, cognitive problems that are independent of visual impairment occur in only a minority of individuals with OPPG and do not correlate with the type of LRP5 mutation. Fourth, most patients with typical features of OPPG have detectable LRP5 mutations; however, we cannot exclude locus heterogeneity or digenic inheritance as accounting for a minority of cases of OPPG. Last, the ability to functionally test a mutation’s effect on Wnt- and possibly Norrin-signal transduction may help determine whether a variant identified in patient with OPPG, idiopathic osteoporosis, or FEVR is disease causing.
Acknowledgments
We thank our patients and their families, for participating in this study, and Drs. Jeremy Nathans, Bernadette Holdener, Hans Clevers, and Ormond MacDougald, for sharing reagents. Dr. Warman is an investigator with the Howard Hughes Medical Institute and the recipient of a Clinical Scientist in Translational Research Award from the Burroughs Wellcome Fund. Both organizations supported this work.
Members of the OPPG Collaborative Group are Konrad Oexle, Institute of Clinical Genetics, Medical Faculty “Carl Gustav Carus” University of Dresden, Dresden; Bryan D. Hall, Departments of Pediatrics and Genetics, College of Medicine, University of Kentucky, Lexington; Anne De Paepe, Ghent University Hospital, Department of Medical Genetics, Ghent; Bruno Dallapiccola, Istituto CSS-Mendel, Rome; Hannu Somer, Division of Neurology, University of Helsinki, Helsinki; Richard Boles, Division of Medical Genetics, Children's Hospital Los Angeles, Keck School of Medicine at the University of Southern California, Los Angeles; Tim Cundy, Department of Medicine, Faculty of Medical & Health Sciences, University of Auckland, Auckland; Ab Jans, Observation Center for Mentally Retarded Children, Hondsberg La Salle, Oisterwijk, The Netherlands; Andrea Superti-Furga, Centre for Pediatrics and Adolescent Medicine, Freiburg University Hospital, Freiburg, Germany; Elisabeth Steichen-Gersdorf, Department of Pediatrics, University of Innsbruck, Innsbruck; Tom Letteboer, Department of Medical Genetics, University Medical Centre, Utrecht; Chong Ae Kim, Genetics Unit, Instituto da Criança, University of São Paulo, São Paulo; Margaret Zacharin, Department of Endocrinology and Diabetes, Royal Children’s Hospital, Parkville, Australia; Marie Lambert, Department of Genetics, and Emmanuelle Lemyre, Medical Genetics Division, Pediatric Department, Hôpital Sainte-Justine, University of Montreal, Montreal; Raoul C. M. Hennekam, Melissa Lees Halfhide and Louise Wilson, Institute of Child Health, Great Ormand Street Hospital, Jeremy Allgrove, Department of Paediatric Endocrinology, St. Bartholomew’s Hospital, and Department of Paediatrics, Newham General Hospital, London; Kim Keppler-Noreuil, Department of Pediatrics, University of Iowa Hospitals and Clinics, Iowa City; Dorit Lev, Institute of Medical Genetics, Wolfson Medical Center, Holon, Israel; Marybeth Hummel, Department of Pediatrics, West Virginia University School of Medicine, Charleston; Jennifer A. Batch, Department of Endocrinology, Royal Children’s Hospital, Brisbane; Barbara Floege, Herzogenrath, Germany; Didier Lacombe, Department Genetique Medicale, Hôpital Pellegrin-Enfants, Bordeaux; Ahmad Teebi, Section of Clinical Genetics & Dysmorphology, The Hospital for Sick Children and University of Toronto, Toronto; Bruno Leheup, Service de Medecine Infantile III et Genetique Clinique, Hôpital Universitaire de Nancy, Nancy, France; Bassam Y. Abu-Libdeh, Pediatrics & Genetics, Makassed Hospital, Jerusalem; Luisa Bonafe, Division of Molecular Pediatrics, Centre Hospitalier Universitaire Vaudois, Lausanne; Emanuela Manfredi, Genetic Consulting Service, Ospedale Niguarda Ca’ Granda, Milan; Chris Sharp, Charles Salt Centre, Robert Jones & Agnes Hunt Orthopaedic Hospital National Health Service Trust, Gobowen, United Kingdom; Carol Gardiner, Clinical Genetics Service, Nottingham City Hospital, Nottingham, United Kingdom; Bruria Ben-Zeev, Department of Pediatrics, Tel Aviv University, Ramat Aviv, Israel; Valerie Cormier-Daire, Department of Medical Genetics and INSERM U393, Hôpital Necker, Paris; Susanne Kjaergaard, The John F. Kennedy Institute, Glostrup, Denmark; Emma Wakeling, North West Thames Regional Genetics Service, North West London Hospitals National Health Service Trust, Middlesex, United Kingdom; and Matthew L. Warman, Department of Genetics and Center for Human Genetics, Case School of Medicine and University Hospitals of Cleveland, Cleveland.
Web Resources
The URLs for data presented herein are as follows:
- Warman lab at Case Western Reserve University, http://genetics.case.edu/warmanlab/ (for the Department of Genetics Web site)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OPPG) [PubMed]
References
- Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML (2005) Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol 25:4946–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974 [DOI] [PubMed] [Google Scholar]
- Beighton P, Winship I, Behari D (1985) The ocular form of osteogenesis imperfecta: a new autosomal recessive syndrome. Clin Genet 28:69–75 [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollerslev J, Wilson SG, Dick IM, Islam FM, Ueland T, Palmer L, Devine A, Prince RL (2005) LRP5 gene polymorphisms predict bone mass and incident fractures in elderly Australian women. Bone 36:599–606 [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521 [DOI] [PubMed] [Google Scholar]
- Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW (1993) A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet 5:180–183 [DOI] [PubMed] [Google Scholar]
- De Paepe A, Leroy JG, Nuytinck L, Meire F, Capoen J (1993) Osteoporosis-pseudoglioma syndrome. Am J Med Genet 45:30–37 [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour J-P, Dermitzakis ET, Rizzoli R, Antonarakis SE (2004) Polymorphisms in the low-density lipoprotein receptor–related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet 74:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontali M, Stomeo C, Dallapiccola B (1985) Osteoporosis-pseudoglioma syndrome: report of three affected sibs and an overview. Am J Med Genet 22:35–47 [DOI] [PubMed] [Google Scholar]
- Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT (2003) Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci USA 100:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Salomon R, Pelet A, Angrist M, Amiel J, Fornage M, Attie-Bitach T, Olson JM, Hofstra R, Buys C, Steffann J, Munnich A, Lyonnet S, Chakravarti A (2002) Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat Genet 31:89–93 [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, et al (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed] [Google Scholar]
- Gong Y, Vikkula M, Boon L, Liu J, Beighton P, Ramesar R, Peltonen L, Somer H, Hirose T, Dallapiccola B, De Paepe A, Swoboda W, Zabel B, Superti-Furga A, Steinmann B, Brunner HG, Jans A, Boles RG, Adkins W, van den Boogaard MJ, Olsen BR, Warman ML (1996) Osteoporosis-pseudoglioma syndrome, a disorder affecting skeletal strength and vision, is assigned to chromosome region 11q12-13. Am J Hum Genet 59:146–151 [PMC free article] [PubMed] [Google Scholar]
- Hartikka H, Makitie O, Mannikko M, Doria AS, Daneman A, Cole WG, Ala-Kokko L, Sochett EB (2005) Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res 20:783–789 [DOI] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO (2004) Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 19:2033–2040 [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Lee L, Zhang L, Wefer S, Brown K, DeRossi C, Wines ME, Rosenquist T, Holdener BC (2003) Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112:355–367 [DOI] [PubMed] [Google Scholar]
- Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF (2004) Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am J Hum Genet 75:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP (1994) Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264:1604–1608 [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR (2001) Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science 293:2256–2259 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science 275:1784–1787 [DOI] [PubMed] [Google Scholar]
- Lev D, Binson I, Foldes AJ, Watemberg N, Lerman-Sagie T (2003) Decreased bone density in carriers and patients of an Israeli family with the osteoporosis-pseudoglioma syndrome. Isr Med Assoc J 5:419–421 [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, et al (2002) A mutation in the LDL receptor–related protein 5 gene results in the autosomal dominant high–bone-mass trait. Am J Hum Genet 70:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT (2003) Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem 278:11331–11336 [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Furuta I, Watanabe Y, Tsukamoto K, Tomita H, Tsujihata M, Ohta T, Kishino T, Matsumoto N, Minakami H, Niikawa N, Yoshiura K (2004) LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J Hum Genet 49:80–86 [DOI] [PubMed] [Google Scholar]
- Patel PH, Loeb LA (2000) DNA polymerase active site is highly mutable: evolutionary consequences. Proc Natl Acad Sci USA 97:5095–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407:535–538 [DOI] [PubMed] [Google Scholar]
- Qin M, Hayashi H, Oshima K, Tahira T, Hayashi K, Kondo H (2005) Complexity of the genotype-phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Hum Mutat 26:104–112 [DOI] [PubMed] [Google Scholar]
- Richter M, Gottanka J, May CA, Welge-Lussen U, Berger W, Lutjen-Drecoll E (1998) Retinal vasculature changes in Norrie disease mice. Invest Ophthalmol Vis Sci 39:2450–2457 [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME (2002) Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32:326–330 [DOI] [PubMed] [Google Scholar]
- Sims KB (2004) NDP-related retinopathies. GeneReviews (http://www.geneclinics.org/profiles/norrie/details.html) (accessed September 12, 2005)
- Somer H, Palotie A, Somer M, Hoikka V, Peltonen L (1988) Osteoporosis-pseudoglioma syndrome: clinical, morphological, and biochemical studies. J Med Genet 25:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steichen-Gersdorf E, Gassner I, Unsinn K, Sperl W (1997) Persistent hyperplastic primary vitreous in a family with osteoporosis-pseudoglioma syndrome. Clin Dysmorphol 6:171–176 [PubMed] [Google Scholar]
- Superti-Furga A, Steinmann B, Perfumo F (1986) Osteoporosis-pseudoglioma or osteogenesis imperfecta? Clin Genet 29:184–185 [DOI] [PubMed] [Google Scholar]
- Swoboda W, Grill F (1988) The osteoporosis pseudoglioma syndrome: update and report on two affected siblings. Pediatr Radiol 18:399–404 [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530–535 [DOI] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GCM, Downey LM, Zhang K, Inglehearn CF (2004) Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet 74:721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Bénichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, de Vernejoul M-C, Bollerslev J, Van Hul W (2003) Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 72:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883–895 [DOI] [PubMed] [Google Scholar]
- Zacharin M, Cundy T (2000) Osteoporosis pseudoglioma syndrome: treatment of spinal osteoporosis with intravenous bisphosphonates. J Pediatr 137:410–415 [DOI] [PubMed] [Google Scholar]