Abstract
In this study, our objective was to determine the steady-state intrapulmonary concentrations and pharmacokinetic parameters of orally administered linezolid in healthy volunteers. Linezolid (600 mg every 12 h for a total of five doses) was administered orally to 25 healthy adult male subjects. Each subgroup contained five subjects, who underwent bronchoscopy and bronchoalveolar lavage (BAL) 4, 8, 12, 24, or 48 h after administration of the last dose. Blood was obtained for drug assay prior to administration of the first dose and fifth dose and at the completion of bronchoscopy and BAL. Standardized bronchoscopy was performed without systemic sedation. The volume of epithelial lining fluid (ELF) recovered was calculated by the urea dilution method, and the total number of alveolar cells (AC) was counted in a hemocytometer after cytocentrifugation. Linezolid was measured in plasma by a high-pressure liquid chromatography (HPLC) technique and in BAL specimens and AC by a combined HPLC-mass spectrometry technique. Areas under the concentration-time curves (AUCs) for linezolid in plasma, ELF, and AC were derived by noncompartmental analysis. Half-lives for linezolid in plasma, ELF, and AC were calculated from the elimination rate constants derived from a monoexponential fit of the means of the observed concentrations at each time point. Concentrations (means ± standard deviations) in plasma, ELF, and AC, respectively, were 7.3 ± 4.9, 64.3 ± 33.1, and 2.2 ± 0.6 μg/ml at the 4-h BAL time point and 7.6 ± 1.7, 24.3 ± 13.3, and 1.4 ± 1.3 μg/ml at the 12-h BAL time point. Linezolid concentrations in plasma, ELF, and AC declined monoexponentially, with half-lives of 6.9, 7.0, and 5.7 h, respectively. For a MIC of 4, the 12-h plasma AUC/MIC and maximum concentration/MIC ratios were 34.6 and 3.9, respectively, and the percentage of time the drug remained above the MIC for the 12-h dosing interval was 100%; the corresponding ratios in ELF were 120 and 16.1, respectively, and the percentage of time the drug remained above the MIC was 100%. The long plasma and intrapulmonary linezolid half-lives and the percentage of time spent above the MIC of 100% of the dosing interval provide a pharmacokinetic rationale for drug administration every 12 h and indicate that linezolid is likely to be an effective agent for the treatment of pulmonary infections.
Linezolid is an approved antibiotic that is active against antibiotic-susceptible and antibiotic-resistant gram-positive pathogens such as Streptococcus pneumoniae, beta-hemolytic streptococci, Staphylococcus aureus, and Enterococcus spp. (6, 17, 19, 20, 23; A. P. Borek, G. A. Peterson, and G. A. Noskin, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., p. 185, 2000). Although not approved for the treatment of tuberculosis, linezolid is active, in vitro and in a murine model, against Mycobacterium tuberculosis (14, 28) and rapidly growing mycobacteria (7, 25). Bioavailability approaches 100% after oral administration. The recommended dose for bacterial respiratory infection in adults is 600 mg every 12 h. In humans, the half-life (t1/2) at the elimination phase is approximately 5.5 h, the mean maximum concentration of the drug in plasma (Cmax) at steady state ranges from 15 to 21 μg/ml, and the time to maximum concentration after oral dosing is 1.0 to 1.5 h (15, 16). Linezolid is approved for the treatment of nosocomial and community-acquired pneumonia, but the in vivo penetration of linezolid into pulmonary alveolar cells (AC) and pulmonary epithelial lining fluid (ELF) in humans has not been reported.
Researchers (1-5, 8-13) have developed techniques for the measurement, in vivo, of the concentrations of drugs in ELF and AC. The purpose of this study was to determine the steady-state intrapulmonary concentrations and intrapulmonary pharmacokinetic parameters of orally administered linezolid in healthy volunteers.
MATERIALS AND METHODS
Study design and subjects.
This was a prospective, nonblind study of plasma and intrapulmonary linezolid concentrations at steady state. All subjects gave written informed consent and were required to be 18 years of age or older and have a body mass index from 18 to 29 (Nutrition and Your Health: Dietary Guidelines for Americans, 2000, U.S. Department of Agriculture and U.S. Department of Health and Human Services [http://www.health.gov/dietaryguidelines/dga2000/DIETGD.PDF]). The evaluation included a medical history, a physical examination, and baseline laboratory testing that included a complete blood count with differential; a platelet count; urinalysis with urine drug screening; and determinations of the time to the production of prothrombin, the time to the partial production of thromboplastin, and levels of urea nitrogen in the blood, serum creatinine, aspartate aminotransferase, alanine aminotransferase, gamma glutaryltransferase, alkaline phosphatase, total bilirubin, glucose, total protein, albumin, uric acid, and alcohol in the blood. Except for the blood alcohol and urine drug screenings, the evaluation was repeated following bronchoscopy. Women were excluded from the study because of a requirement to monitor female subjects to time of delivery or abortion if they became pregnant during the study. We excluded subjects who had a history of clinically significant disease, had clinically significant abnormal findings at the screening physical examination (including laboratory tests), were intolerant to linezolid or lidocaine, had a positive drug screen, had a history of smoking within the previous 3 years, were required to take chronic medications other than self-prescribed vitamins, or were receiving any investigational drug within 30 days prior to the start of the study. Twenty-five subjects were assigned to one of five groups of five subjects each according to the time of bronchoscopy: 4, 8, 12, 24, and 48 h following the last dose. The 4-h time period was chosen to approximate the peak Cmax of linezolid in the lungs; the 8-h time period was chosen as an approximate midpoint between the Cmax (4 h) and the minimum concentration (Cmin) of linezolid within the lungs (12 h) before the next dose in a 12-h dosing regimen. The 24- and 48-h time points were selected to examine the possibility of a long intrapulmonary t1/2.
Linezolid was administered orally at a dose of 600 mg every 12 h for a total of five doses. The first and last doses of study medication were administered under direct supervision in either the Clinical Trials Center or the Infectious Diseases Research Unit at the University of California, San Francisco. Subjects were observed for adverse effects for 1 h after the first dose. Subsequent doses were taken according to verbal and written instructions and documented by the subjects in written diaries. Abnormal laboratory tests that were detected on the repeat evaluation were repeated until results were normal or near normal.
Bronchoscopy and BAL.
Standardized bronchoscopy and bronchoalveolar lavage (BAL) (8-13) were performed in the Clinical Trials Center at 4, 8, 12, 24, or 48 h after the administration of the last dose. Blood pressure, heart rate, respiratory rate, and temperature were recorded prior to, and at the completion of, bronchoscopy and as clinically indicated following the procedure. Nasal oxygen was administered throughout the procedure, and fingertip oximetry was monitored in all subjects.
In preparation for the bronchoscopy, the subjects were given a solution of 4% topical lidocaine as a gargle that was then followed by a 4% topical lidocaine spray. Pledgets soaked with 4% topical lidocaine were then applied to each side of the posterior pharynx, followed by the application of 1% topical lidocaine more distally. Systemic sedation was not used.
A fiber-optic bronchoscope (Pentax FB-18BS) was inserted in the right middle lobe. Four 50-ml aliquots of normal saline were instilled, and each was immediately aspirated into a trap. The average duration of the bronchoscopy was 4 min. The specimens were kept on ice until they were frozen. The first aspirate was discarded. The second, third, and fourth aspirates were pooled (pooled BAL specimen). The volume of the pooled BAL specimen was measured and recorded. Measured aliquots of the pooled BAL specimen were sent to a clinical laboratory for a cell count and differential analysis. A known volume of the pooled BAL fluid was immediately spun at 400 × g for 5 min in a refrigerated centrifuge. The supernatant and the cells were separated and frozen at −70°C until assay. A small aliquot of the supernatant was frozen separately for urea assay.
Blood samples.
Blood was obtained for drug assay prior to administration of the first dose and fifth dose and at the completion of bronchoscopy and BAL.
Specimen handling.
Blood samples were kept on ice until centrifugation. The plasma was separated and then frozen at −70°C until assay. All plasma and BAL specimens were shipped on dry ice to Pharmacia & Upjohn (Kalamazoo, Mich.) for drug analysis. Immediately prior to analysis, the cell pellets were resuspended in 1.0 ml of normal saline solution and sonicated for 15 min.
Linezolid assay.
Assays of linezolid in plasma, BAL, and AC were performed by the Pharmacia & Upjohn Company and are reported as follows.
(i) Linezolid assay of human plasma.
Quantitation of linezolid in human plasma specimens was conducted by a sensitive and selective high-performance liquid chromatographic method that was validated at AvTech Laboratories (Kalamazoo, Mich.) (R. A. Johnson, D. E. Haan, C. A. James, and N. K. Hopkins, abstract from the Annu. Meet. Am. Assoc. Pharm. Sci., Pharm. Res. 14:S374, 1997). Briefly, plasma specimens (0.500 ml) were spiked with an internal standard (IS) and extracted with solid-phase extraction cartridges. After evaporation of the organic material, the residue was reconstituted and injected onto a chromatography system consisting of a reversed-phase analytical column (Zorbax RX-8; Dupont). The mobile phase was composed of trifluoroacetic acid-tetrahydrofuran-methanol-water, with the detection wavelength set at 251 nm. Calibration standard (CS) responses were linear over the range of 0.010 to 20.0 μg/ml by a weighted (1/concentration) linear least-squares regression analysis. The lower limit of quantitation was 0.0100 μg/ml.
During sample analysis, the correlation coefficients were equal to 1.000. Interday accuracies of three linezolid quality control (QC) standards at concentrations of 0.0400, 4.00, and 15.0 μg/ml ranged from 100 to 102%, with precision reported to be 5.7%.
(ii) Assay of linezolid in BAL fluid and the cell pellet.
Quantitation of linezolid in BAL fluid and in the resulting cell pellet was also conducted by a sensitive and selective high-performance liquid chromatography system that was coupled with a triple-quadrupole mass spectrometer (API 365; PE Sciex) (18). This method was used to quantitate linezolid in the BAL fluid and cell pellets because it requires very small sample volumes and has a lower limit of quantitation of 1.0 ng/ml.
Previously frozen samples were thawed, and a 0.100-ml aliquot was spiked with a deuterated IS, [D3]PNU-100766, prior to extraction. After the organic phase was separated and evaporated, the residue was reconstituted and transferred to injection vials. Sample introduction was performed with a heated nebulizer (atmospheric pressure chemical ionization). Detection was by selected reaction monitoring of the product ion at an m/z of 296 (molecular ion at an m/z of 338) for linezolid and of the product ion at an m/z of 297 (molecular ion at an m/z of 341) for the IS.
Plasma calibration curves and QCs were used to quantitate amounts of drug in BAL fluid and cell pellets after a brief cross-validation demonstrated that the accuracy of the quantitations and relative standard deviations (SDs) for these samples were within 3% and less than 5%, respectively. CS responses were linear over the range of 1.00 to 250 ng/ml. Correlation coefficients were all ≥0.999. Clinical samples whose linezolid results exceeded the CS range were diluted with blank human plasma prior to sample extraction and were reassayed. Interday accuracies, monitored with three linezolid QC standards at concentrations of 17.5, 70.0, and 175 ng/ml, ranged from 104 to 106%, with a precision of ≤6.5%.
Specimens were received in dry ice and stored at −20°C until assay. Linezolid is a stable molecule in aqueous solutions, even at room temperature. The stability of linezolid in plasma stored at −20°C has been documented to be up to 1 year. Aqueous solutions of linezolid prepared for intravenous administration are stable for 24 months at room temperature (Ian Welshman [Pharmacia & Upjohn], personal communication). All supporting data for plasma, BAL, and cell pellet assays and stability are on file at Pharmacia & Upjohn.
(iii) Quantitation of the volume of ELF and concentrations of antibiotics in ELF and AC.
The amount of ELF recovered was calculated by the urea dilution method as described by Rennard et al. (22) and as reported from other previous pulmonary pharmacokinetic studies (8-13). The concentration of urea in serum was analyzed by the clinical laboratory at the University of California, San Francisco, by a coupled urease-glutamate dehydrogenase enzymatic method modified by Boehringer Mannheim Corporation (Indianapolis, Ind.) (24). Measurements were made at a fixed time interval, permitting automated analysis with a model BM 747 analyzer (Boehringer Mannheim). Urea was measured in BAL fluid supernatant by a modified enzymatic assay (kit UV-66 for measurement of urea nitrogen in the blood; Sigma, St. Louis, Mo.) as previously reported (8-13). The assay was linear (R2 = 0.99) for concentrations of urea in BAL fluid from 0.047 to 0.750 mg/dl. Controls were included with every run, and if values were not within 10% of the known value, the standard curve, controls, and specimen assays were repeated.
The volume of ELF in BAL fluid was derived from the following relationship: VELF = VBAL × (UreaBAL/Ureaser), where VELF is the volume of ELF in the BAL sample, VBAL is the volume of aspirated BAL fluid, UreaBAL is the concentration of urea in BAL fluid, and Ureaser is the concentration of urea in serum.
The concentration of antibiotic in the ELF (ABXELF) was derived from the following relationship: ABXEFL = ABXBAL × (VBAL/VELF), where ABXBAL is the measured concentration of antibiotic in BAL fluid.
The volume of AC collected in the pellet suspension was determined from the cell count in the BAL fluid. Cells were counted in a hemocytometer with a lower detection limit of 106 cells/liter. The number of cells in 1.0 ml of pellet suspension was calculated to be equal to the number of cells in 1.0 ml of BAL fluid times 30. Because of cell loss during centrifugation, the actual number of cells recovered may be lower than the number counted and the antibiotic concentration may be approximately 20% higher than what we calculated (26). Differential cell counting was performed after spinning the specimen in a cytocentrifuge. The volume of AC in the pellet suspension was determined with a mean macrophage cell volume of 2.42 μl/106 cells (4).
The concentration of antibiotic in AC (ABXAC) was calculated from the following relationship: ABXAC = (ABXpellet/VAC), where ABXpellet is the antibiotic concentration in the 1-ml cell suspension and VAC is the volume of AC in the 1-ml cell suspension.
Statistical analysis.
We used Prophet, version 6.0 (Division of Research Resources, National Institutes of Health, Bethesda, Md., and MarketMiner, Inc., Charlottesville, Va.), to obtain descriptive statistics, to create graphic representations, and for database management. Because the interpatient variability of plasma, ELF, and AC linezolid concentrations at each of the selected time periods was not known prior to the study, we used sample sizes (five in each group) based upon prior experience with rifapentine and sizes used in a study with a similar design (13). We estimated that at the 8-h time period (Cmax) and with similar degrees of interpatient variability among study subjects, we would be able to detect an approximately 65% difference between the means of the plasma and ELF or AC linezolid concentrations with a power of 80% and an α of 0.05. For lesser or greater degrees of interpatient variability, the differences that we would be able to detect would be smaller or larger, respectively. Noncompartmental modeling was performed with Kinetica 2000, version 4.0.1 (InnaPhase Corporation, Philadelphia, Pa.). The log-trapezoidal rule was used to compute the area under the concentration-time curve (AUC) from 0 to 12 h (AUC0-12) and from 0 to 24 h (AUC0-24) for the mean concentration-time data for the drug in plasma, ELF, and AC after the fifth dose. The drug concentration in plasma at the time of administration of the fifth dose was calculated from the mean of the 12-h plasma linezolid concentrations following the fourth dose. The ELF and AC linezolid concentrations at the time of administration of the fifth dose were calculated from the means of the corresponding 12-h concentrations following the fifth dose. The plasma, intracellular, and ELF drug concentration-time data declined monoexponentially; the means of the observed concentrations at each BAL time were used to calculate kel, the elimination rate constant. Fitting was performed by using a weighting function (1/y2), where 1/y was the reciprocal of the observed concentration. The t1/2s for linezolid in plasma, ELF, and AC were calculated using the equation t1/2 = 0.693/kel.
Analysis of variance was used to compare the concentrations in plasma, AC, and ELF and ELF recovery and AC recovery at the different time periods. Prior to performing the analysis of variance, we tested the data sets for normality (Wilk-Shapiro) and equality of variances (Levene's test). Parametric and nonparametric analyses were performed by the Newman-Keuls and Friedman tests, respectively (27). The AC/plasma and ELF/plasma drug concentration ratios were calculated by averaging the ratios for the five subjects at each collection time point. Linear regression was performed by the method of least-squares estimation. A P of <0.05 was considered significant.
RESULTS
Twenty-five subjects were enrolled in the study. Three were excluded after enrollment and were replaced with three additional subjects. One was excluded when he failed to comply with the dosing schedule, and a second subject experienced a vasovagal episode during the topical application of lidocaine. With the third subject, the BAL fluid contained red blood cells. Of the 25 subjects, 3 were Asian, 1 was black, and 21 were white. Their ages ranged from 22 to 40 years, with a mean ± SD of 30 ± 5 years. All had normal renal function, with serum creatinine levels that ranged from 0.7 to 1.1 mg/dl. The remaining screening laboratory tests were within normal limits. There were no major adverse events, and the subjects returned to their normal duties following the bronchoscopy and BAL. None experienced chest pain or elevated temperature. One subject experienced a mild cough, and four experienced self-limited lightheadedness. Transient rales and/or diminished breath sounds were present in three subjects following the procedure. Following drug administration, one subject had an elevated bilirubin level, two subjects had decreased neutrophil counts, and one subject had an elevated time to partial production of prothromboplastin, all of which returned to normal or toward normal on repeat testing.
The numbers of AC recovered from BAL specimens (means ± SDs) ranged from 7.0 × 107 ± 1.9 × 107 to 1.5 × 108 ± 1.2 × 108 (Table 1). Numbers of AC recovered were not significantly different among the five time groups (P > 0.05). The majority of the cells in all time groups were in the monocyte/macrophage class (range, 70.2% ± 17.0% to 86.6% ± 4.2%). AC recovery was not correlated with concentrations of linezolid in AC (R = 0.03; P = 0.44). The mean volume of ELF recovered from the 25 subjects ± the SD was 0.9 ± 0.5 ml, and volumes were not significantly different among the time groups (P > 0.05) (Table 1). ELF recovery was not correlated with concentrations of linezolid in ELF (R = −0.04; P = 0.97).
TABLE 1.
Recovery of cells and ELF from BAL specimens from 25 healthy subjectsa
| BAL sampling time (h) | No. of cells/liter
|
% of cells (mean ± SD) of indicated type in BAL fluid
|
Mean ELF vol (ml) ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Polymorphonuclear leukocytes | Lymphocytes | Monocytes/macrophages | Eosinophils | Degenerated cells | ||
| 4 | 1.5 × 108 | 1.2 × 108 | 1.0 ± 0.7 | 15.2 ± 14.4 | 78.4 ± 16.7 | 0.4 ± 0.9 | 5.0 ± 6.3 | 0.8 ± 0.1 |
| 8 | 1.5 × 108 | 5.0 × 107 | 1.2 ± 2.2 | 9.0 ± 3.8 | 80.2 ± 12.2 | 0 | 9.6 ± 11.8 | 1.2 ± 0.9 |
| 12 | 9.5 × 107 | 1.9 × 107 | 1.2 ± 1.1 | 8.0 ± 1.6 | 86.6 ± 4.2 | 0.2 ± 0.4 | 4.0 ± 4.0 | 0.8 ± 0.3 |
| 24 | 1.1 × 108 | 2.8 × 107 | 1.0 ± 1.0 | 25.2 ± 19.9 | 70.2 ± 17.0 | 0.6 ± 0.9 | 3.0 ± 5.2 | 0.7 ± 0.3 |
| 48 | 7.0 × 107 | 1.9 × 107 | 1.4 ± 1.9 | 12.4 ± 2.4 | 81.2 ± 10.4 | 0 | 5.0 ± 11.2 | 0.7 ± 0.3 |
No significant differences among the groups for cell recovery, differential cell count, or the volume of ELF were found (P > 0.05). Comparison testing was not applied to zero values.
At 12 h following the fourth dose (prior to the administration of the fifth dose), the concentrations of linezolid in plasma (means ± SDs) ranged from 6.0 ± 3.3 to 7.6 ± 1.7 μg/ml and were not significantly different among the five time groups (P > 0.05) (Table 2). There was no correlation (R = −0.07; P = 0.74) between the weights of the subjects and the concentrations of linezolid at 12 h following the fourth dose.
TABLE 2.
Linezolid concentrations in plasma, ELF, and ACa
| BAL sampling time (h) | Concn in plasma 12 h after the fourth dose (μg/ml)b | Concn (μg/ml) at the indicated BAL sampling time in:
|
||
|---|---|---|---|---|
| Plasma | AC | ELFc | ||
| 4 | 7.3 ± 4.9 (2.3-14.2) | 15.5 ± 4.9 (8.9-22) | 2.2 ± 0.6 (1.7-3.1) | 64.3 ± 33.1 (43.2-123)d |
| 8 | 7.0 ± 2.6 (4.2-10.6) | 8.9 ± 3.2 (5.1-13) | 1.5 ± 2.0 (0.5-5.0) | 31.4 ± 33.0 (8.3-89.2) |
| 12 | 7.6 ± 1.7 (6.0-9.6)e | 10.2 ± 2.3 (6.8-12.6) | 1.4 ± 1.3 (0.5-3.6) | 24.3 ± 13.3 (10.2-45.9) |
| 24 | 7.6 ± 1.1 (6.2-9.1) | 1.8 ± 0.6 (0.9-2.4) | 0.2 ± 0.1 (0-0.3) | 7.6 ± 6.0 (1.5-17) |
| 48 | 6.0 ± 3.3 (2.8-10.4) | 0.2 ± 0.2 (0.02-0.5) | BLQf | 0.7 ± 0.8 (0-2) |
Data are means ± 1 SD; ranges are given in parentheses.
There were no significant differences among the plasma linezolid concentrations at 12 h after the fourth dose (P > 0.05).
ELF drug concentrations at 4, 8, 12, and 24 h were significantly greater than AC drug concentrations at the same times (P < 0.05).
Concentrations in ELF at 4 h were significantly greater than those at 8, 12, 24, and 48 h (P < 0.05).
n = 4.
BLQ, below level of quantitation.
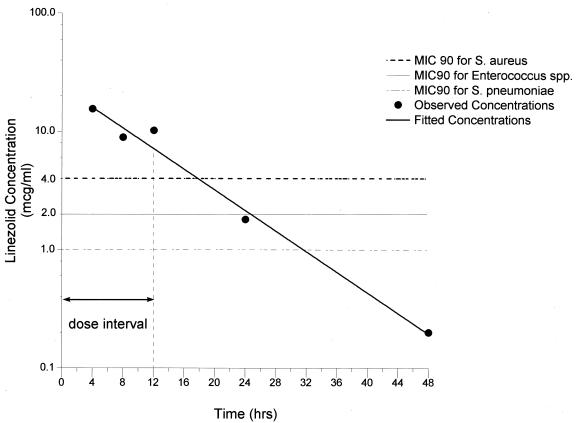
The linezolid concentrations in plasma (means ± SDs) determined at the times of bronchoscopy (4, 8, 12, 24, and 48 h after the fifth dose) ranged from 15.5 ± 24.2 μg/ml (Cmax in plasma) to 0.2 ± 0.2 μg/ml (Cmin in plasma) and declined monoexponentially (R = −0.99) with a t1/2 of 6.9 h. The mean plasma drug concentrations, SDs, and minima and maxima for each time point are summarized in Table 2. The AUC0-12 and AUC0-24 for plasma following the fifth dose were 132.2 and 204.2 μg·h/ml, respectively (Fig. 1 and Table 2). The MIC of linezolid at which 90% of the strains were inhibited (MIC90) for antibiotic-susceptible and -resistant gram-positive organisms is ≤4.0 μg/ml (6, 19, 20, 23; Borek et al., 40th ICAAC). Using this value, we calculated Cmax/MIC90, AUC0-12/MIC90, and AUC0-24/MIC90 ratios for plasma samples during the 24-h period following the last dose to be 3.9, 33.1, and 51.1, respectively. The percentage of time linezolid remained above the MIC90 in plasma was 100% of the 12-h dosing interval (Fig. 1). With organisms for which MICs are 2.0 μg/ml, e.g., S. pneumoniae, beta-hemolytic streptococci, and Enterococcus, these ratios would be considerably greater and the percentage of time the drug remained above the MIC90 would be 100% of the dosing interval.
FIG. 1.
Mean concentrations of linezolid in plasma at the times of bronchoscopy. SDs and the range for each time period are given in Table 2. The MIC90s for S. aureus, Enterococcus spp., and S. pneumoniae are included for comparison.
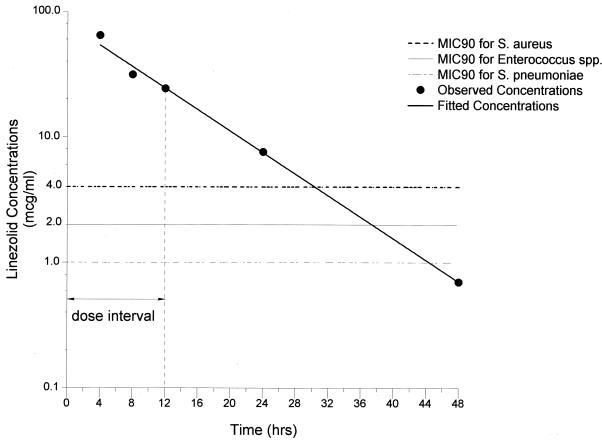
The linezolid concentrations in ELF (means ± SDs) determined at the time of bronchoscopy (4, 8, 12, 24, and 48 h following the fifth dose) ranged from 64.3 ± 33.1 μg/ml at 4 h (Cmax in ELF) to 0.7 ± 0.8 μg/ml at 48 h (Cmin in ELF). The mean linezolid concentrations in ELF, SDs, and minima and maxima for each time point are summarized in Table 2. As in plasma, the mean linezolid concentrations in ELF declined monoexponentially (R = −1.0), with a t1/2 of 7.0 h. The AUC0-12 and AUC0-24 for ELF were 480 and 672 μg·h/ml, respectively (Fig. 2 and Table 2). Using the MIC90 of 4 μg/ml (see above), we calculated Cmax/MIC90, AUC0-12/MIC90, and AUC0-24/MIC90 ratios for ELF during the 24-h period following the last dose to be 16.1, 120, and 168, respectively. The ratios of drug concentrations in ELF to those in plasma at 4, 8, 12, 24, and 48 h were 4.2 ± 1.4, 3.1 ± 2.2, 2.4 ± 1.2, 3.9 ± 2.3, and 2.3 ± 1.6, respectively, and were not significantly different among the five time periods (P > 0.05). The percentage of time linezolid remained above the MIC in ELF was 100% of the 12-h dosing interval (Fig. 2). For S. pneumoniae, beta-hemolytic streptococci, and Enterococcus (MIC90, 2 μg/ml), the ratios of the concentrations of drug in ELF to the MICs would be considerably greater and the percentage of time the drug remained above the MIC90 in ELF would be 100% of the dosing interval.
FIG. 2.
Mean concentrations of linezolid in eELF. SDs and the range for each time period are given in Table 2. The MIC90s for S. aureus, Enterococcus spp., and S. pneumoniae are included for comparison.
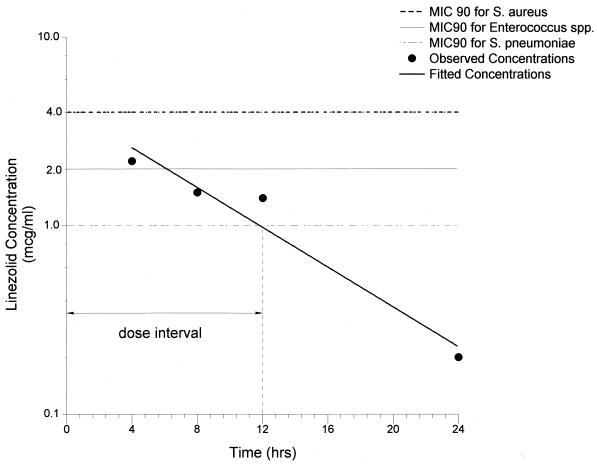
The linezolid concentrations in AC (means ± SDs) determined at the time of bronchoscopy (4, 8, 12, 24, and 48 h following the fifth dose) ranged from 2.2 ± 0.6 μg/ml at 4 h (Cmax in AC) to undetectable levels at 48 h (Cmin in AC). The mean concentrations in AC, SDs, and minima and maxima for each time point are summarized in Table 2. As in plasma and ELF, the mean linezolid concentrations in AC also declined monoexponentially (R = 0.97), with a t1/2 of 5.7 h. The AUC0-12 and AUC0-24 for AC were 20.4 and 30.0 μg · h/ml (Fig. 3 and Table 2). Using the upper end of the range of MICs of linezolid for M. tuberculosis (0.5 to 2.0 μg/ml), we calculated Cmax/MIC, AUC0-12/MIC, and AUC0-24/MIC ratios for AC to be 1.1, 10.2, and 15.0, respectively.
FIG. 3.
Mean concentrations of linezolid in AC. SDs and the range for each time period are given in Table 2. The MIC90s for S. aureus, Enterococcus spp., and S. pneumoniae are included for comparison.
The ratios of drug concentrations in AC to those in plasma (means ± SDs) at 4, 8, 12, and 24 h were 0.15 ± 0.05, 0.15 ± 0.14, 0.13 ± 0.10, and 0.11 ± 0.07, respectively, and were not significantly different among the four time periods (P > 0.05) from 4 to 24 h. Linezolid was not detectable in AC in any of the five subjects at the 48-h time period.
DISCUSSION
Linezolid is active against penicillin-sensitive and penicillin-resistant S. pneumoniae (MIC90 = 1 μg/ml), viridans group streptococci (MIC90 = 2 μg/ml), beta-hemolytic streptococci (MIC90 = 2 μg/ml), and other antibiotic-resistant organisms such as methicillin-resistant S. aureus (MIC90 = 4 μg/ml), methicillin-resistant Staphylococcus epidermidis (MIC90 = 2 μg/ml), and vancomycin-resistant enterococci (MIC90 = 2 μg/ml) (6, 19, 20, 23; Borek et al., 40th ICAAC). It is also active, but not approved for treatment, against M. tuberculosis (MIC range, 0.5 to 2 μg/ml) (14, 28) and rapidly growing mycobacteria, such as Mycobacterium chelonae (MIC90 range, 4 to 16 μg/ml) (7, 25).
AUC/MIC ratios of ≥100 and percentages of time linezolid remained above the MIC in plasma of ≥85% have been associated with optimal efficacy for linezolid treatment of gram-positive infections in humans (C. R. Rayner, A. Forrest, A. K. Meagher, M. C. Birmingham, and J. J. Schentag, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., p. 29, 2000). In a rat model of pneumococcal pneumonia, the percentage of time linezolid remained above a MIC of greater than 45% of the dosing interval was the strongest predictor of outcome (K. M. Olsen, L. C. Preheim, and M. J. Gentry-Nielsen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., p. 34, 2000). In this study, the approximate 7-h plasma and ELF linezolid t1/2s and the percentages of time linezolid remained above its MIC in plasma of 100% of the dosing interval observed indicate that linezolid is likely to be effective for the treatment of linezolid-susceptible bacterial infections and support the 600-mg twice daily dosing regimen for the treatment of methicillin-resistant S. aureus, S. pneumoniae, and vancomycin-resistant-enterococcal infections. Whether the ratios in plasma that are predictive of a successful outcome are related to the high ratios of the concentration in ELF to the MIC observed in this study is unknown, and further animal model and human clinical investigations are warranted.
At all time periods, linezolid concentrations in AC were less than those observed in plasma and ELF, suggesting that the drug was excluded or rapidly removed from this compartment. We have reported a similar partitioning for pyrazinamide (10). The physiological basis for this differential penetration is unknown, as is the significance of intracellular linezolid concentrations in relation to the treatment of tuberculosis or diseases caused by other intracellular pathogens. It is plausible, but not proven, that in vivo intracellular drug concentrations that exceed the MIC for target pathogens are desirable in pharmacodynamics.
In summary, our data indicate that linezolid in a twice-daily dosing regimen is likely to be effective for the treatment of respiratory infection due to gram-positive pathogens, as has been demonstrated in clinical trials (21).
Acknowledgments
This work was carried out with funds provided by Pharmacia & Upjohn, protocol M/1260/0060.
We acknowledge the assistance of Ian Welshman, Dennis Stalker, Nancy Hopkins, and Gail Jungbluth of Pharmacia and Upjohn and Eve Benton for manuscript preparation.
REFERENCES
- 1.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob. Agents Chemother. 36:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob. Agents Chemother. 36:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, D. R., S. R. Maxwell, D. Honeybourne, J. M. Andrews, J. P. Ashby, and R. Wise. 1991. The penetration of cefpirome into the potential sites of pulmonary infection. J. Antimicrob. Chemother. 28:79-86. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, D. R., R. Wise, J. M. Andrews, J. P. Ashby, and D. Honeybourne. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3: 886-890. [PubMed] [Google Scholar]
- 5.Baldwin, D. R., R. Wise, J. M. Andrews, and D. Honeybourne. 1991. Microlavage: a technique for determining the volume of epithelial lining fluid. Thorax 46:658-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betriu, C., M. Redondo, M. L. Palau, A. Sanchez, M. Gomez, E. Culebras, A. Boloix, and J. J. Picazo. 2000. Comparative in vitro activities of linezolid, quinupristin-dalfopristin, moxifloxacin, and trovafloxacin against erythromycin-susceptible and -resistant streptococci. Antimicrob. Agents Chemother. 44:1838-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown-Elliott, B. A., R. J. Wallace, Jr., R. Blinkhorn, C. J. Crist, and L. B. Mann. 2001. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin. Infect. Dis. 33: 1433-1434. [DOI] [PubMed] [Google Scholar]
- 8.Conte, J. E., Jr., J. Golden, S. Duncan, E. McKenna, E. Lin, and E. Zurlinden. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1995. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob. Agents Chemother. 39:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte, J. E., Jr., J. A. Golden, J. Kipps, E. T. Lin, and E. Zurlinden. 2001. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob. Agents Chemother. 45:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, E. T. Lin, and E. Zurlinden. 2000. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethionamide concentrations. Antimicrob. Agents Chemother. 44:1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, E. T. Lin, and E. Zurlinden. 2000. Single-dose intrapulmonary pharmacokinetics of rifapentine in normal subjects. Antimicrob. Agents Chemother. 44:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cynamon, M. H., S. P. Klemens, C. A. Sharpe, and S. Chase. 1999. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 43:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekema, D. I., and R. N. Jones. 2000. Oxazolidinones: a review. Drugs 59:7-16. [DOI] [PubMed] [Google Scholar]
- 16.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 17.Fines, M., and R. Leclercq. 2000. Activity of linezolid against gram-positive cocci possessing genes conferring resistance to protein synthesis inhibitors. J. Antimicrob. Chemother. 45:797-802. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins, N. K., D. J. Stalker, R. A. Johnson, J. C. Gammill, and A. R. Cazers. 1999. Determination of linezolid (PNU-100766) in human plasma g isocratic HPLC-MS-MS with liquid-liquid extraction (AvTech Laboratories, Inc.). In Proceedings of the Tenth International Symposium on Pharmaceutical and Biomedical Analysis. Pharmacia & Upjohn, Kalamazoo, Mich.
- 19.Jones, R. N., D. M. Johnson, and M. E. Erwin. 1996. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob. Agents Chemother. 40:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noskin, G. A., F. Siddiqui, V. Stosor, D. Hacek, and L. R. Peterson. 1999. In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plouffe, J. F. 2000. Emerging therapies for serious gram-positive bacterial infections: a focus on linezolid. Clin. Infect. Dis. 31(Suppl. 4):S144-S149. [DOI] [PubMed] [Google Scholar]
- 22.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 23.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talke, H. S. G. E. 1965. Enzymatische Harnstoffbestimmung im Blut und Serum im optischem Test nach Warburg. Klin. Wochenschr. 43:174.. [DOI] [PubMed] [Google Scholar]
- 25.Wallace, R. J., Jr., B. A. Brown-Elliott, S. C. Ward, C. J. Crist, L. B. Mann, and R. W. Wilson. 2001. Activities of linezolid against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 45:764-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willcox, M., A. Kervitsky, L. C. Watters, and T. E. J. King. 1988. Quantification of cells recovered by bronchoalveolar lavage. Comparison of cytocentrifuge preparations with the filter method. Am. Rev. Respir. Dis. 138:74-80. [DOI] [PubMed] [Google Scholar]
- 27.Zar, J. H. 1984. Multisample hypotheses: the analysis of variance. Multiple comparisons, p. 162-205. In J. H. Zar (ed.), Biostatistical analysis. Prentice-Hall, Englewood Cliffs, N.J.
- 28.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]