Abstract
Idiopathic hemihypertrophy (IH) is a congenital overgrowth syndrome associated with an increased risk of embryonal cancers in childhood. A related developmental disorder is Beckwith-Wiedemann syndrome (BWS), which increases risk for embryonal cancers, including Wilms tumor. Constitutional epigenetic alterations associated with BWS have been well characterized and include epigenetic alterations of imprinted genes on 11p15. The frequency of hypermethylation of H19 in children with IH and Wilms tumor, 20% (3/15), was significantly lower than the frequency in children with BWS and Wilms tumor, 79% (11/14; P = .0028). These results indicate that children with IH and Wilms tumor have different constitutional epigenotypes from those of children with BWS and Wilms tumor.
Idiopathic hemihypertrophy (IH [MIM 235000]) is a congenital overgrowth syndrome associated with an increased risk of embryonal cancers of childhood, including Wilms tumor (Hoyme et al. 1998). A related congenital overgrowth and cancer-predisposition syndrome is Beckwith-Wiedemann syndrome (BWS [MIM #130650]). BWS is also associated with hemihyperplasia and embryonal cancers of childhood, including Wilms tumor (DeBaun and Tucker 1998); however, there are many other manifestations, such as macroglossia, abdominal-wall defects (omphalocele, diastasis recti, or umbilical hernia), and neonatal hypoglycemia. In children with BWS, Wilms tumor is primarily associated with constitutive hypermethylation of the H19 promoter and loss of imprinting of IGF2; in a case-cohort study of children with BWS, constitutional hypermethylation of H19 was associated with a fourfold greater risk of embryonal cancers than that in children with other methylation abnormalities (DeBaun et al. 2002). In contrast to BWS, only uniparental disomy (UPD) of 11p15 has been associated with cancer in children with IH. Despite the similarities between these two syndromes, limited molecular data exist as to whether BWS and IH represent phenotype variations of the same genotype or, in fact, are two separate syndromes with different genotypes. To test the hypothesis that children with BWS and IH have the same genotype with variable expression, we performed genotype-phenotype studies in children identified as having IH or BWS and Wilms tumor from the National Wilms Tumor Study (NWTS).
Patients in the study were registered in NWTS 3 and 4; the design of NWTS has been reported elsewhere (Breslow et al. 1988). At the time of enrollment, registering physicians were requested to indicate the presence or absence of specific conditions, including BWS and IH. No systematically collected details about the clinical features of IH and BWS in the patients were available. A pediatric oncologist with expertise in evaluating children with overgrowth syndromes reviewed all records. All classifications of either IH or BWS were done prior to and independent of genetic analyses.
Patients with either BWS or IH and fresh frozen tissues submitted to the NWTS Wilms tumor bank were identified and represent the sampling frame. Frozen aliquots of all these samples were then selected for analysis. Control samples of Wilms tumors from nonsyndromic patients were similarly obtained.
Genomic DNA was prepared from snap-frozen normal kidney tissue, tumor tissue, and peripheral blood lymphocytes by standard proteinase K digestion and phenol extraction (Cui et al. 1998). H19 and LIT1 methylation and UPD analysis by microsatellite marker typing were done as described elsewhere (DeBaun et al. 2002). Loss of heterozygosity (LOH) analysis was performed similar to UPD analysis by use of FAM-labeled primer pairs, detection on a model 377 automated fluorescent DNA sequencer (Applied Biosystems), and analysis with Genescan and Genotyper software (Applied Biosystems). Examination of the chromatograms from samples with UPD revealed a small peak (<20% of the expected size), corresponding to the lost allele and thereby indicating mosaicism for UPD. Patients in whose DNA an allele was reduced in the tumor tissue compared with the normal tissue were considered to have LOH. The threshold for LOH by visual chromatogram inspection was 50%.
Abnormal methylation of H19 in kidney was defined as a methylation index >0.74 (mean + 2 SD of six kidney samples from control Wilms tumors with intralobar nephrogenic rests, the histological subtype of Wilms tumor not associated with epigenetic alterations of H19 and IGF2 [Ravenel et al. 2001]). Abnormal methylation of H19 in lymphocytes was defined as a methylation index >0.63 (mean + 2 SD of 15 normal individuals). Abnormal methylation of LIT1 in kidney and lymphocytes was defined as a methylation index <0.39 (mean − 2 SD of six kidney samples from control Wilms tumors and of 15 normal individuals, respectively). Fisher’s exact test was used to determine whether hypermethylation of H19 was associated with IH and BWS. A P value <.05 was considered statistically significant.
We received samples from 24 patients with IH and Wilms tumor. On the basis of chart review, we excluded the diagnosis of IH for a total of 7 of the 24 patients. Six excluded patients had been initially classified as having IH but also had one or two of the cardinal features of BWS: macroglossia and macrosomia (n=1), macroglossia and umbilical hernia (n=1), umbilical hernia alone (n=1), or macrosomia alone (n=3). These patients were excluded because of the ambiguity of the clinical diagnosis and because they had not previously received a diagnosis of BWS. One patient was excluded for suspected neurofibromatosis because of café au lait pigmentation. The remaining 17 patients received the diagnosis of IH.
We received samples from 16 patients with BWS and Wilms tumor. When available, records of congenital anomalies and birth weight were assessed to confirm the diagnosis (table 1). Gestational ages were not available to determine whether the patient had macrosomia, defined as birth weight ⩾90th percentile.
Table 1.
Clinical Features of Patients with BWS
| Patient | BirthWeighta,b(kg) | Congenital Anomalies Noted in Chartb |
| BWS1 | 4.5 | BWS |
| BWS2 | 4.7 | BWS, somatic overgrowth |
| BWS3 | 3.9 | BWS |
| BWS4 | 4.3 | BWS, macroglossia, hemihypertrophy, adrenal adenoma |
| BWS5 | 4.7 | BWS |
| BWS6 | 3.2 | BWS, hemihypertrophy |
| BWS7 | 2.4 | BWS, omphalocele |
| BWS8 | 3.5 | BWS, macroglossia, hemihypertrophy, umbilical hernia, cryptorchidism |
| BWS9 | NA | BWS, hemihypertrophy |
| BWS10 | NA | Accessory renal artery |
| BWS11 | NA | None |
| BWS12 | NA | Accessory renal artery |
| BWS13 | NA | Accessory renal artery, 4th and 5th anterior rib anomalies |
| BWS14 | NA | Hemihypertrophy |
| BWS15 | NA | NA |
| BWS16 | NA | NA |
Gestational ages were not available; however, at 40 wk gestational age, the 90th percentile for birth weight is ∼3.9 kg, and the 50th percentile is ∼3.4 kg (Oken et al. 2003).
NA = not available.
We tested normal kidney tissue for methylation status at H19 and LIT1 and typed the normal and tumor tissues for microsatellite markers at 11p15 to identify UPD or LOH (figs. 1 and 2 and tables 2 and 3). If no normal tissue was available, we analyzed the methylation and microsatellites of the tumor tissue. We accepted the tumor data as representative of the state of the normal tissue if the tumor had the half methylation pattern that occurs in normal tissues and showed retention of heterozygosity by microsatellite analysis (patients IH10, IH13, IH14, and IH15). The assumption is that if tumor tissue from a particular sample shows normal methylation of H19 or heterozygosity, the corresponding normal tissue will not show hypermethylation or homozygosity, respectively. This assumption was reasonable and necessary because, for four samples, only tumor tissue was available. We tested this assumption in six patients (patients BWS1, BWS2, BWS3, BWS10, BWS11, and BWS16) by examining the methylation status of paired tumors and normal kidneys, which all showed abnormal methylation. In all cases, both the tumor tissue and the normal kidney tissue showed abnormal methylation. These data support the concept that normal methylation of H19 in the tumor does not occur when there is abnormal H19 methylation in the normal tissue. There is no mechanism for acquisition of heterozygosity at multiple microsatellite markers, other than microsatellite instability, which is rare in Wilms tumor (Mason et al. 2000), and the pattern of heterozygosity observed was not that seen in microsatellite instability—that is, with narrowly spaced alleles. We excluded patients in whom the tumor was the only available sample and had abnormal methylation (patients IH16 and BWS13), because we could not determine whether the normal tissue had normal or abnormal methylation. When available, we also tested patient blood genomic DNA to provide convincing evidence of UPD and LOH and tested parental blood genomic DNA to identify the parental origin of microsatellite alleles. For one patient, although kidney tissue was not available, patient and parental blood genomic DNA was available for analysis (patient BWS9). The finding of UPD in the patient’s blood sample made the testing of other tissues unnecessary.
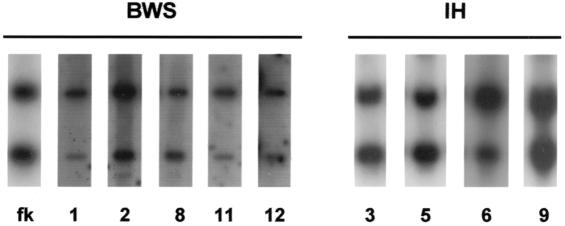
Figure 1.
Analysis of H19 methylation in patients with BWS and IH. A 1.0-kb fragment of the H19 gene was hybridized to genomic DNA digested with PstI and the methylcytosine-sensitive restriction enzyme SmaI. The upper band represents the 1.8-kb methylated fragment, and the lower band represents the 1.0-kb unmethylated digested fragment. Patient numbers corresponding to those in the tables are shown below the lanes. All DNA specimens were from the normal kidney. fk = fetal kidney.
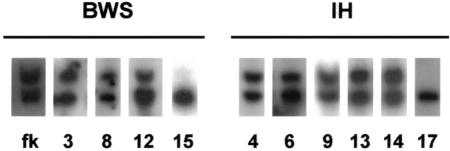
Figure 2.
Analysis of LIT1 methylation in patients with BWS and IH. An EST probe from the LIT1 gene was hybridized to genomic DNA digested with BamH1 and the methylcytosine-sensitive restriction enzyme NotI. The upper band represents the 6.0-kb unmethylated fragment, and the lower band represents the 4.2-kb unmethylated digested fragment. Patient numbers corresponding to those in the tables are shown below the lanes. All DNA specimens were from the normal kidney except samples for IH13, IH14, and IH17, which were from the Wilms tumor. fk = fetal kidney.
Table 2.
Molecular Analysis of Kidney Tissue from Patients with IH and Wilms Tumor
|
Normal Kidney Tissue |
Tumor Kidney Tissue |
||||||
| Patient | H19Methylation | LIT1Methylation | MicrosatelliteAnalysis | H19Methylation | LIT1Methylation | MicrosatelliteAnalysis | Note |
| IH1 | Normal | Normal | Normal | … | … | Normal | … |
| IH2 | Normal | Normal | Normal | … | … | Normal | … |
| IH3 | Normal | Normal | Normal | … | … | Normal | … |
| IH4 | Normal | Normal | Normal | … | … | LOH | … |
| IH5 | Normal | Normal | Normal | … | … | LOH | … |
| IH6 | Abnormal | Normal | Normal | … | … | Normal | … |
| IH7 | Abnormal | Abnormal | UPD | … | … | … | … |
| IH8 | Normal | Normal | Normal | … | … | … | … |
| IH9 | Normal | Normal | Normal | … | … | … | … |
| IH10 | … | … | … | Normal | Normal | Normal | … |
| IH11 | Normal | Normal | Normal | … | … | LOH | … |
| IH12 | Abnormal | Abnormal | UPD | … | … | … | … |
| IH13 | … | … | … | Normal | Normal | Normal | … |
| IH14 | … | … | … | Normal | Normal | Normal | … |
| IH15 | … | … | … | Normal | Normal | Normal | … |
| IH16 | … | … | … | Abnormal | Normal | Normal | Excludeda |
| IH17 | … | … | … | Abnormal | Abnormal | LOH or UPD? | Excludedb |
Patient IH16 was excluded because it was not possible to determine whether the hypermethylation of H19 in the tumor was constitutive or tumor specific.
Patient IH17 was excluded because it was not possible to determine whether multiple homozygous microsatellite markers were due to LOH or UPD.
Table 3.
Molecular Analysis of Kidney Tissue from Patients with BWS and Wilms Tumor
|
Normal Kidney Tissue |
Tumor Kidney Tissue |
||||||
| Patient | H19Methylation | LIT1Methylation | MicrosatelliteAnalysis | H19Methylation | LIT1Methylation | MicrosatelliteAnalysis | Note |
| BWS1 | Abnormal | Normal | Normal | Abnormal | Normal | Normal | … |
| BWS2 | Abnormal | Normal | Normal | Abnormal | Normal | Normal | … |
| BWS3 | Abnormal | Normal | Normal | Abnormal | Normal | Normal | … |
| BWS4 | Abnormal | Abnormal | UPD | … | … | … | … |
| BWS5 | Abnormal | Abnormal | UPD | … | … | … | … |
| BWS6 | Abnormal | Abnormal | UPD | … | … | … | … |
| BWS7 | Abnormal | Abnormal | UPD | … | … | … | … |
| BWS8 | Normal | Abnormal | Normal | … | … | LOH | … |
| BWS9 | … | … | … | … | … | … | UPD in blood |
| BWS10 | Abnormal | Normal | Normal | Abnormal | Normal | Normal | … |
| BWS11 | Abnormal | Normal | Normal | Abnormal | Normal | Normal | … |
| BWS12 | Normal | Abnormal | Normal | … | … | LOH | … |
| BWS13 | … | … | … | Abnormal | Abnormal | Normal | Excludeda |
| BWS14 | … | … | … | Abnormal | Abnormal | LOH or UPD? | Excludedb |
| BWS15 | Normal | Normal | Normal | … | … | LOH | … |
| BWS16 | Abnormal | Normal | Normal | Abnormal | Normal | Normal | … |
Patient BWS13 was excluded because it was not possible to determine whether the hypermethylation of H19 and LIT1 in the tumor was constitutive or tumor specific.
Patient BWS14 was excluded because it was not possible to determine whether multiple homozygous microsatellite markers were due to LOH or UPD.
The majority of children with IH had a normal hemimethylated pattern of H19 and LIT1. Molecular analyses of the 15 evaluable patients with IH (table 2) indicated that 12 patients had normal methylation of H19 and LIT1. Of these 12, 4 had LOH of 11p15 (patients IH3, IH4, IH5, and IH11), 6 did not have LOH (patients IH1, IH2, IH10, IH13, IH14, IH15), and 2 did not have tumor tissue available to test for LOH (patients IH8 and IH9). Three patients had an abnormal methylation pattern in H19, one patient had hypermethylation of H19 in the normal kidney tissue and retention of heterozygosity in the tumor (patient IH6), and two patients had constitutive UPD (patients IH7 and IH12).
Unlike the children with IH and Wilms tumor, the majority of children with BWS and Wilms tumor had abnormal methylation patterns of H19 or LIT1. Molecular analyses of 14 evaluable patients with BWS (table 3) indicated that 13 of them had abnormal methylation of either H19 or LIT1. Six children had hypermethylation of H19 alone, with retention of heterozygosity in the tumor (patients BWS1, BWS2, BWS3, BWS10, BWS11, and BWS16). Five patients had constitutive UPD (patients BWS4, BWS5, BWS6, BWS7, and BWS9) that included abnormal methylation in H19 and LIT1, and two patients had hypomethylation of LIT1 and LOH in the tumor (patients BWS8 and BWS12). One patient had normal methylation of H19 and LIT1 and LOH in the tumor (patient BWS15).
The frequency of hypermethylation of H19 in children with IH and Wilms tumor, 20% (3/15), was significantly lower than the frequency in children with BWS and Wilms tumor, 79% (11/14; P=.0028) (table 4). In addition, this difference between IH and BWS remained significant when patients with UPD for 11p15, which encompasses H19 along with many other genes, were excluded: hypermethylation frequencies of 67% (6/9) and 8% (1/13) for IH and BWS, respectively (P=.0066) (table 4).
Table 4.
Significant Difference in Frequency of Hypermethylation of H19 in BWS and IH
| Sample Testedand Patient Group | Hypermethylationof H19 (%) | Normal H19 (%) |
| Entire samplea: | ||
| Patients with BWS | 11/14 (79) | 3/14 (21) |
| Patients with IH | 3/15 (20) | 12/15 (80) |
| Sample excluding UPD for 11p15b: | ||
| Patients with BWS | 6/9 (67) | 3/9 (33) |
| Patients with IH | 1/13 (8) | 12/13 (92) |
P=.0028 for hypermethylation values.
P=.0066 for hypermethylation values.
The higher proportion of abnormal methylation of H19 in patients with BWS and Wilms tumor corroborated our previous results in the BWS Registry and was expected (DeBaun et al. 2002). Given the significant overlap between BWS and IH, we did not expect the low frequency of H19 methylation abnormalities in the patients with IH and Wilms tumor.
Several possibilities exist as to why the children with IH and Wilms tumor did not have the expected epigenetic abnormalities. Perhaps children with IH and Wilms tumor are genetically different from children with IH and without Wilms tumor. Alternatively, patients with IH may have a different genotype that acts in a common causal pathway and results in a similar phenotype to that of BWS. The rare cases of IH with UPD of 11p15.5, described here and by others (Grundy et al. 1991), suggest that there might be another gene on 11p15.5 included in the disomic region that is responsible for the phenotype.
An unexpected finding was LOH of 11p15.5 in Wilms tumors from patients with BWS. Specifically, three tumors from patients with BWS showed LOH; two arose in patients with hypomethylation of LIT1. In addition, four tumors from patients with IH were determined to have LOH, all arising in patients without any known epigenetic alteration. These data are consistent with a model we have proposed in which epigenetic alterations (hypermethylation of LIT1, in this case) represent the first “hit” in Knudson’s two-hit model, and subsequent genetic changes represent the second hit (Feinberg and Tycko 2004). Knudson’s hypothesis does not require allelism for the two hits, and, by our modified Knudson model, epigenetic alterations can lead to an expanded population of precursor cells that are targets for subsequent oncogenetic activation, thereby increasing the risk of malignancy in the presence of constitutional epigenetic alterations in BWS.
In summary, children with IH and Wilms tumor do not have the constitutional hypermethylation of H19 that is associated with cancer, unlike those with BWS, in which cancer is associated with constitutional hypermethylation of H19.
Acknowledgments
Tumor and related tissues were provided by the National Wilms Tumor Study Group (NWTSG) and the Cooperative Human Tissue Network, Pediatric Division. Funding was provided by the Robert Woods Johnson Foundation (to M.R.D.), by National Institutes of Health grant CA65438 (to A.P.F.), and in part by United States Public Health Service grants CA 42326 and CA 54498 (to NWTSG).
Web Resources
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.gov/Omim/ (for BWS and IH) [PubMed]
References
- Breslow N, Beckwith JB, Ciol M, Sharples K (1988) Age distribution of Wilms’ tumor: report from the National Wilms’ Tumor Study. Cancer Res 48:1653–1657 [PubMed] [Google Scholar]
- Cui H, Horon IL, Ohlsson R, Hamilton SR, Feinberg AP (1998) Loss of imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nat Med 4:1276–1280 10.1038/3260 [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, Feinberg AP (2002) Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet 70:604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Tucker MA (1998) Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr 132:398–400 [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Tycko B (2004) The history of cancer epigenetics. Nat Rev Cancer 4:143–153 [DOI] [PubMed] [Google Scholar]
- Grundy P, Telzerow P, Paterson MC, Haber D, Berman B, Li F, Garber J (1991) Chromosome 11 uniparental isodisomy predisposing to embryonal neoplasms. Lancet 338:1079–1080 10.1016/0140-6736(91)91937-P [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Seaver LH, Jones KL, Procopio F, Crooks W, Feingold M (1998) Isolated hemihyperplasia (hemihypertrophy): report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet 79:274–278 [DOI] [PubMed] [Google Scholar]
- Mason JE, Goodfellow PJ, Grundy PE, Skinner MA (2000) 16q Loss of heterozygosity and microsatellite instability in Wilms’ tumor. J Pediatr Surg 35:891–896 10.1053/jpsu.2000.6911 [DOI] [PubMed] [Google Scholar]
- Oken K, Kleinman KP, Rich-Edwards J, Gillman MW (2003) A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 3:6 10.1186/1471-2431-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenel JD, Broman KW, Perlman EJ, Niemitz EL, Jayawardena TM, Bell DW, Haber DA, Uejima H, Feinberg AP (2001) Loss of imprinting of insulin-like growth factor-II (IGF2) gene in distinguishing specific biologic subtypes of Wilms tumor. J Natl Cancer Inst 93:1698–1703 10.1093/jnci/93.22.1698 [DOI] [PubMed] [Google Scholar]