To the Editor:
Subtelomeric screening and FISH analysis of a 13-year-old girl with severe mental retardation, intrauterine growth retardation, microcephaly, facial dysmorphisms, hypoplastic kidney, and short hands and feet but without congenital diaphragmatic hernia (CDH [MIM 142340]) allowed us to find a de novo deletion in 15q26.1–26.2.
That region, as shown by Klaassens et al. (2005) in a study published in the May issue of the Journal, contains a candidate region for CDH, a condition that occurrs in ∼1 of 3,000 newborns and is associated with a 30%–60% mortality rate, with significant morbidity among survivors (Harrison et al. 1994; Nobuhara et al. 1996). The etiology of this condition is barely known and, in most cases, is considered idiopathic, whereas ∼15% of patients with CDH show chromosomal abnormalities. Recently, Biggio et al. (2004) reported on a child with a 15q26.1 deletion showing CDH, coarctation of the aorta, and dysmorphic features, suggesting this region as the possible candidate locus for CDH. Furthermore, the authors proposed myocyte-specific enhancer factor–2A (MEF2A [MIM 600660]) as a candidate gene for CDH, coding for a protein playing a critical role in the control of muscle differentiation and development. Klaassens et al. (2005) found 7% numerical and 5% structural chromosome abnormalities in 200 CDH patients. The most frequent chromosome abnormality was 15q deletion. Eventually, they determined the size of the deletions in seven patients with CDH. They incorporated data from two patients with terminal 15q deletions without CDH, and data from one patient with a small 15q interstitial deletion and CDH. A minimal deletion region, spanning ∼5 Mb at chromosome bands 15q26.1–15q26.2, has been suggested by these authors. Two of the known genes of this region, namely, NR2F2 (MIM 107773) and CDH2 (MIM 602119), were considered to be the best candidates for CDH.
To better define the deletion in our patient, FISH experiments were carried out with a set of linearly ordered BACs selected by human NCBI Map Viewer (build 35.1) and provided by the Sanger Institute. This analysis showed that the BAC RP11-386M24, localized to chromosome band 15q26.1 (∼9.0 Mb from the end of the chromosome), was the closest to the telomere that hybridized on both chromosomes in all examined metaphases. The immediately more centromeric CTD—2313J17 BAC showed signals of different intensities on the 15q telomeres, suggesting that the breakpoint lay within this BAC, whereas the overlapping RP11-437B10 BAC and all the distally placed BACs showed no hybridization signal (data not shown).
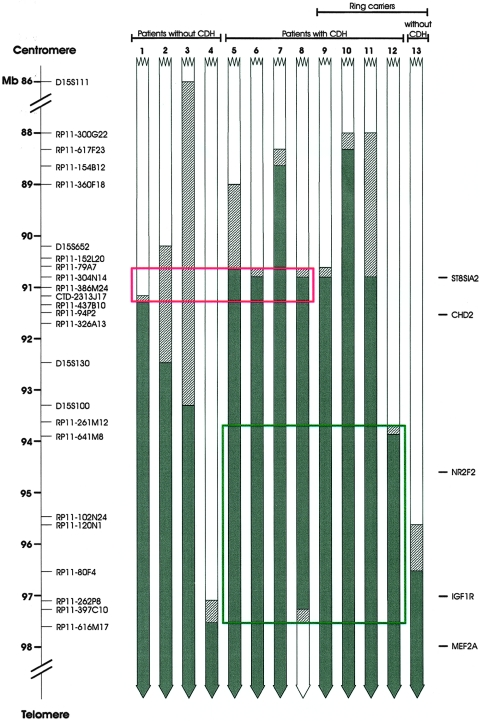
We thus compared our results with the most significant previously characterized 15q deletions, including ring chromosome 15, unbalanced translocations, and pure 15qter monosomies, either associated with the CDH phenotype or not. As shown in figure 1, no clear critical region can be drawn from these data, essentially because case 12 with CDH carried a ring (15) resulting in a smaller deletion than cases 1, 2, and 3, without CDH. At least two hypotheses can be made to explain these contradictory data. First, it is possible that haploinsufficiency of the CDH locus has a reduced penetrance and that data from patients without CDH could be useless in establishing the critical region. If this is true, the candidate region is restricted to ∼3.5 Mb (fig. 1) and includes the NR2F2 gene, but its telomeric limit is more distal than that defined by Klaassens et al. (2005), which was derived from a deletion of a patient without CDH (fig. 1, case 13). On the other hand, drawing genotype-phenotype relationships may be difficult in ring carriers because of the potential instability of ring chromosomes that can be associated with gain or loss of genetic material in other tissues (Tümer et al. 2004). If we omit ring cases from the analysis, then the critical region would be narrowed to a 0.7-Mb genomic portion (fig. 1). The NR2F2 gene, in this case, would be located outside this putative critical region.
Figure 1.
Graphical representation of 15q deletions in patients with and without CDH. The most significant BAC clones analyzed are shown on the left. Solid boxes represent deleted regions, hatched boxes indicate the uncertainty of the breakpoints, and open boxes reveal the normal chromosomal regions. The green rectangle includes the narrowed candidate CDH region, taking into account only cases with CDH, whereas the red rectangle indicates the critical region resulting when the ring cases are omitted from the analysis. Patient 1, our case; patient 2, Tönnies et al. 2001; patient 3, Rogan et al. 1996 (patient K); patients 4–11 and 13, Klaassens et al. 2005 (cases 9, 7, 6, 4, 1, 2, 3, 5, and 8, respectively); patient 12, Tümer et al. 2004 (case 1).
The ST8 alpha-N-acetyl-neuraminide alpha-2, 8-sialyltransferase 2 gene (ST8SIA2 [MIM 602546]) is the unique known gene in this region and encodes for a type II membrane protein that catalyzes the transfer of sialic acid from CMP-sialic acid to the neural cell adhesion molecules (NCAMs) (Ong et al. 1998). The ST8SIA2 gene is expressed in many tissues during development (Angata et al. 1997). Evidence suggests that polysialylated NCAMs promote cell migration and, thus, they are thought to play a critical role in development. More specifically, it has been shown that, during diaphragmatic morphogenesis, the expression of polysialylated NCAMs is tightly modulated along each stage of myogenesis (Allan and Greer 1998).
Finally, although it is less likely, we cannot exclude the possibility that both the mentioned hypotheses are true. In this case, the critical region would be represented by the extent of the deletion in patient 8 (fig. 1).
Additional findings are needed to refine the search for a CDH gene in 15q chromosome. However, it seems likely that NR2F2 and ST8SIA2 are the best candidates.
Acknowledgments
This study was supported by the Italian Ministry of Health.
Web Resources
URLs for data presented herein are as follows:
- NCBI Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- Allan DW, Greer JJ (1998) Polysialylated NCAM expression during motor axon outgrowth and myogenesis in the fetal rat. J Comp Neurol 391:275-292 [DOI] [PubMed] [Google Scholar]
- Angata K, Nakayama J, Fredette B, Chong K, Ranscht B, Fukuda M (1997) Human STX polysialyltransferase forms the embryonic form of the neural cell adhesion molecule: tissue-specific expression, neurite outgrowth, and chromosomal localization in comparison with another polysialyltransferase, PST. J Biol Chem 272:7182–7190 10.1074/jbc.272.11.7182 [DOI] [PubMed] [Google Scholar]
- Biggio JR, Descartes MD, Carroll AJ, Holt RL (2004) Congenital diaphragmatic hernia: is 15q26.1–26.2 a candidate locus? Am J Med Genet A 126:183–185 10.1002/ajmg.a.20464 [DOI] [PubMed] [Google Scholar]
- Harrison MR, Adzick NS, Estes JM, Howell LJ (1994) A prospective study of the outcome for fetuses with diaphragmatic hernia. JAMA 271:382–384 10.1001/jama.271.5.382 [DOI] [PubMed] [Google Scholar]
- Klaassens M, van Dooren M, Eussen HJ, Douben H, den Dekker AT, Lee C, Donahoe PK, Galjaard RJ, Goemaere N, de Krijger RR, Wouters C, Wauters J, Oostra BA, Tibboel D, de Klein A (2005) Congenital diaphragmatic hernia and chromosome 15q26: determination of a candidate region by use of fluorescence in situ hybridization and array-based comparative genomic hybridization. Am J Hum Genet 76:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuhara KK, Lund DP, Mitchell J, Kharasch V, Wilson JM (1996) Long-term outlook for survivors of congenital diaphragmatic hernia. Clin Perinatol 23:873–887 [PubMed] [Google Scholar]
- Ong E, Nakayama J, Angata K, Reyes L, Katsuyama T, Arai Y, Fukuda M (1998) Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology 8:415–424 10.1093/glycob/8.4.415 [DOI] [PubMed] [Google Scholar]
- Rogan PK, Seip JR, Driscoll DJ, Papenhausen PR, Johnson VP, Raskin S, Woodward AL, Butler MG (1996) Distinct 15q genotypes in Russell-Silver and ring 15 syndromes. Am J Med Genet 62:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönnies H, Schulze I, Hennies H, Neumann LM, Keitzer R, Neitzel H (2001) De novo terminal deletion of chromosome 15q26.1 characterised by comparative genomic hybridisation and FISH with locus specific probes. J Med Genet 38:617–621 10.1136/jmg.38.9.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer Z, Harboe TL, Blennow E, Kalscheuer VM, Tommerup N, Brondum-Nielsen K (2004) Molecular cytogenetic characterization of ring chromosome 15 in three unrelated patients. Am J Med Genet A 130:340–344 10.1002/ajmg.a.30035 [DOI] [PubMed] [Google Scholar]