Abstract
Ca2+ channel blockers like nifedipine have been shown to increase the oral bioavailability of β-lactam antibiotics, such as cefixime, in humans. The molecular mode of action of Ca2+ channel blockers on β-lactam absorption, however, has not yet been defined. Using the Caco-2 human intestinal epithelial cell line, we assessed whether alterations in intracellular free Ca2+ ion (Ca2+in) concentrations by Ca2+ channel blockers or by Ca2+ ionophores affect [14C]cefixime absorption. Reduction of Ca2+in levels by Ca2+ channel blockers (nifedipine, verapamil, diltiazem, or bepridil) at concentrations of 100 μM led to 35 to 50% increases in the cellular uptake of 1 mM [14C]cefixime. Increases in Ca2+in levels by Ca2+ ionophores, on the other hand, led to 40% reductions in [14C]cefixime absorption. Nifedipine increased the Vmax of cefixime transport by 67%, whereas the Km of cefixime transport remained unaffected. By measuring the pH in Caco-2 cells loaded with the pH-sensitive fluorescent dye 2′,7′-bis(2-carboxyethyl)-5-(6)-carboxyfluorescein, we show that cefixime transport mediated by the intestinal H+-coupled peptide transporter PEPT1 leads to intracellular acidification. This acid load was reduced by nifedipine, although the Ca2+ channel blocker increased the level of H+ and cefixime cotransport. Increases in Ca2+in levels by ionomycin enhanced the decline in intracellular pH induced by cefixime alone, although ionomycin reduced the level of H+ and cefixime cotransport. In conclusion, our studies demonstrate that alterations of Ca2+in levels, e.g., by Ca2+ channel blockers, affect pH regulatory systems, such as apical Na+ and H+ exchange, and thereby alter the H+ gradient that serves as the driving force for uptake of β-lactams into intestinal epithelial cells.
The β-lactam antibiotic group encompasses the penicillins, cephalosporins, carbapenems, and monobactams (13). The high intestinal absorption rates of orally active β-lactams are mainly explained by the fact that they serve as substrates for the intestinal peptide transporter PEPT1 (10). PEPT1 is located in the apical membranes of intestinal epithelial cells (23) and the Caco-2 human intestinal epithelial cell line (39). The peptide transporter translocates di- and tripeptides (17, 33) as well as a variety of peptidomimetic compounds, including selected immunostimulants (25, 28), angiotensin-converting-enzyme inhibitors (7, 31), and β-lactam antibiotics (36, 43), in a proton-coupled electrogenic mode (1).
The affinities of β-lactams for PEPT1 show large variabilities, depending on the structure of the individual antibiotic (10, 42). Moreover, it was shown that different β-lactams can display completely different pH dependencies for transport into Caco-2 cells or into oocytes expressing heterologously cloned mammalian PEPT1. The optimum pH for transport of zwitterionic β-lactams such as cefadroxil is 6.5, whereas the dianionic cefixime is effectively transported only at pH values <6.0 (42). Since the intestinal epithelium has a fairly stable surface pH compartment (microclimate pH) with a pH that is >6.25 and <6.75 (22), interactions of β-lactams with PEPT1 in the normal gut and thus their in vivo absorption rates may be defined by the pH at the membrane surface. This might explain why cefixime, which is efficiently transported by PEPT1 in vitro at pH values below 6.0, shows a comparably low oral availability in vivo (3, 15). Other factors contributing to the absorption of β-lactams via PEPT1 may include regulation of transport capacity by protein kinase C (PKC)- and insulin-dependent pathways (9, 29). Understanding of those mechanisms responsible for alterations in the transport kinetics of PEPT1 could be important for specific enhancement of the absorption of drugs with low oral availabilities, such as cefixime, which is usually used for the treatment of respiratory tract infections (37). As it has been shown that rates of absorption of cefixime are increased by 30% when the Ca2+ channel blocker nifedipine is coadministered with cefixime in humans (14), we were interested in studying the underlying mechanisms responsible for this increase. Although it had been suggested that neurohormonal regulation may be involved in mediating the effects of nifedipine on cefixime transport (18), we hypothesized that the alterations in intracellular free calcium ion (Ca2+in) concentrations caused by nifedipine could enhance PEPT1 transport activity for cefixime.
We therefore altered the Ca2+in concentration in Caco-2 cells, a human intestinal epithelial cell line that expresses PEPT1, with either Ca2+ channel blockers or Ca2+ ionophores and measured the effects on the apical influx of [14-C]cefixime. Moreover, by use of intracellular pH (pHin) measurements, we assessed whether alterations in Ca2+in levels secondarily affected pHin and could thereby cause changes in the proton motive driving force for cefixime uptake.
MATERIALS AND METHODS
Cell culture.
Caco-2 cells (passage 31; HTB 37 cells; American Type Culture Collection) were cultured and passaged in Dulbecco's modified Eagle medium (catalog no. 42430; Gibco) supplemented with 10% fetal calf serum, 2 mM glutamine, 1% minimal essential medium with nonessential amino acids (11140; Gibco), and 70 μg of gentamicin (15750; Gibco) per ml in a humidified incubator at 37°C in an atmosphere with 5% CO2. Cells between passages 40 and 65 were seeded at a density of 105 cells/well onto Transwell polycarbonate membranes (24 wells; Nunc) and were used 14 days after they reached confluency. Fresh medium was given every second day and on the day before uptake measurements. Transepithelial electrical resistance was measured with an epithelial voltmeter (World Precision Instruments), and resistances of ≥300 Ω/cm2 indicated the presence of an intact monolayer (2, 24). In order to determine whether any of the compounds tested had cytotoxic effects, the percentage of dead cells in a cell population was determined under conditions identical to those used in the transport studies. Therefore, the fluorescence of SYTOX Green that penetrates only the membranes of cells with compromised plasma membranes was measured prior to determination of the amount of fluorescence in lysed cells (20). The percentage of dead cells did not exceed 5% under any of the conditions tested, indicating that none of the compounds tested had cytotoxic effects.
Transport studies.
Flux studies with Caco-2 cells were performed in a modified Krebs buffer containing 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.0 mM MgSO4, 0.3 mM NaH2PO4, 0.3 mM KH2PO4, and 10 mM glucose, to which 10 mM HEPES (MES) was added for the pH 7.4 incubation buffer (pH adjusted with Tris) or to which 10 mM 2-(N-morpholino)ethanesulfonic acid-Tris was added for the pH 5.0 incubation buffer. For uptake, cell monolayers grown in 24-well plates were washed free of serum-containing medium and were incubated with radiolabeled cefixime in the presence or absence (control) of Ca2+ channel blockers or Ca2+ ionophores for 30 min at 37°C. After the incubation period, the cells were washed three times with ice-cold incubation buffer, scraped off with a rubber policeman after the addition of 500 μl of TEN buffer (150 mM NaCl, 40 mM Tris, 1 mM EDTA) per well, and digested with 50 μl of tissue solubilizer. Cellular accumulation of [14C]cefixime was measured by liquid scintillation spectroscopy after the addition of scintillation cocktail. The level of binding of the tracer to the cells was determined as the residual radioactivity associated with the cells in the presence of excess nonlabeled substrate. The linearity of uptake over 30 min was ascertained for all substrates and concentrations used.
pHin measurements.
For pHin measurements, the Caco-2 cell monolayers were loaded with 2′,7′-bis[2-carboxyethyl-5-(6)-carboxyfluorescein] (BCECF) by preincubating the cells with 5 μM lipophilic acetoxymethyl ester of BCECF (BCECF-AM) at 37°C for 30 min. Subsequently, the monolayers were washed with pH 7.4 buffer, and the buffers with or without substrates were changed by superfusion at the time points indicated in Fig. 4. Intracellular H+ activity was determined by measurement of the intensity of emission at 538 nm after excitation of the fluorophore at 444 nm (isosbestic point) and 490 nm (pH-sensitive wavelength) with a microtiter plate reader (Fluoroskan Ascent; Labsystems, Merlin Diagnostika, Bornheim-Hersel, Germany). The ratio of fluorescence at 444 nm/fluorescence at 490 nm was converted to pHin from a calibration curve generated by estimation of the fluorescence ratio in buffers of different pHs (8).
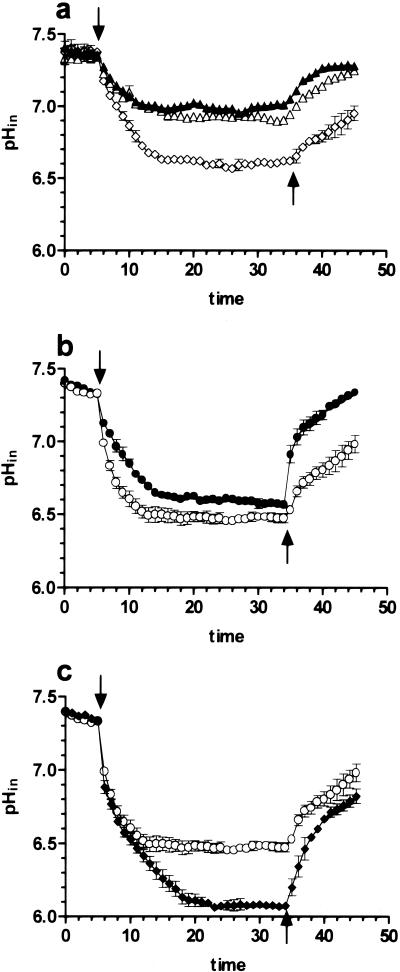
FIG. 4.
pHin measurements in Caco-2 cells obtained by use of the pH-sensitive fluorophore BCECF. Cells were superfused from the apical side with pH 7.4 buffer for 5 min. Subsequently, the cells were perfused for 30 min with pH 5.0 buffer (↓) containing either buffer alone (▵) or buffer with 100 μM nifedipine (▴), 10 μM ionomycin (◊) (a), 5 mM cefixime (○) (b, c), a mixture of 5 mM cefixime and 100 μM nifedipine (•) (b), or a mixture of 5 mM cefixime and 10 μM ionomycin (⧫) (c). All substrates were washed out after 35 min of incubation with buffer pH 7.4 (↑).
Chemicals and materials for cell culture.
[14C]cefixime (44.7 mCi mmol−1) and unlabeled cefixime were provided by Klinge-Pharma (Munich, Germany) and Fujisawa (Osaka, Japan). All agents affecting the intracellular Ca2+in level (the Ca2+ channel blockers nifedipine, verapamil, diltiazem, and bepridil and the Ca2+ ionophores ionomycin and A23187) were purchased from Sigma (Deisenhofen, Germany). Cell culture plates and flasks were obtained from Nunc (Wiesbaden, Germany). All other materials needed for cell culture were from Gibco (Eggenstein, Germany). Rat tail collagen R was purchased from Serva (Heidelberg, Germany).
Calculations.
All calculations (linear regression analysis as well as nonlinear regression analysis) were performed by using Prism (version 2.01; Graph PAD, Los Angeles, Calif.). Four to eight independent experiments were carried out for each variable. Data are given as means ± standard deviations. Analysis of variance between groups was performed by one-way analysis of variance, and the significance of differences between control and treated cells was determined by a nonpaired Student's t test.
RESULTS
Effects of Ca2+ channel blockers and Ca2+ ionophores on [14C]cefixime uptake.
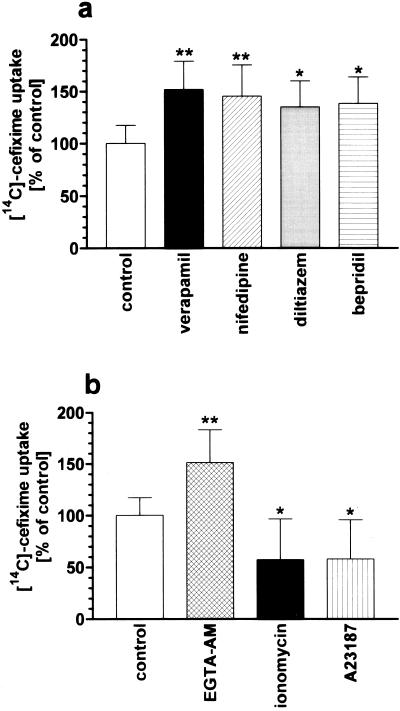
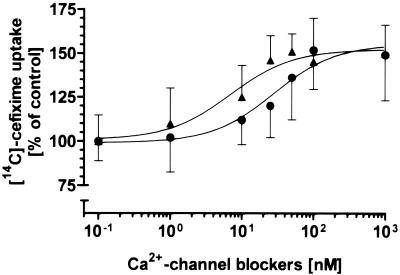
In a first series of experiments, four blockers of slow Ca2+ channels in clinical use for treatment of angina, arrhythmia, and hypertension were tested for their ability to affect [14C]cefixime transport into Caco-2 cells. The compounds selected from different chemical classes were verapamil (a phenylalkylamine), nifedipine (a dihydropyridine), diltiazem (a benzothiazepine), and bepridil (a diarylaminopropylamine). Uptake of [14C]cefixime was measured at an apical pH of 5.0 in order to provide the optimal pH for transport into Caco-2 cells (42). Each of the four Ca2+ channel blockers, used at a concentration of 100 μM in the incubation medium, increased the level of uptake of 1 mM cefixime by 35 to 50% (Fig. 1a). To demonstrate that the increase in absorption of [14C]cefixime is mediated by channel blocker effects on Ca2+in levels, a permeable Ca2+ chelator (the acetoxymethyl ester of EGTA [EGTA-AM]) capable of trapping intracellular Ca2+ ions and two Ca2+ ionophores (ionomycin and A23187) capable of increasing Ca2+in levels in cells (41) were also applied. Each Ca2+ ionophore at a concentration of 10 μM decreased the level of cefixime uptake by 40%, whereas the complexation of Ca2+ ions by EGTA-AM led to an increase in the level of cefixime uptake by 50% (Fig. 1b). Moreover, when stimulation of cefixime transport was determined as a function of the verapamil or the nifedipine concentration (Fig. 2), it was found that the 50% effective concentrations (EC50s) for the stimulation of cefixime transport are in the same range as the Ki values for inhibition of the slow-type Ca2+ channels by those blockers (6, 11).
FIG. 1.
Effects of selected compounds that alter the Ca2+in level on the uptake of 1 mM [14C]cefixime at pH 5.0 into Caco-2 cells. Cefixime transport was measured in the absence (control) or presence of the Ca2+ channel blockers verapamil, nifedipine, diltiazem, and bepridil (each at a concentration of 100 μM) (a) or in the absence (control) or presence of the Ca2+ ionophores ionomycin and A23187 or the Ca2+ chelator EGTA-AM (each at a concentration of 10 μM) (b). Rates of uptake into control cells were 8.9 ± 1.6 nmolcm−230 min−1. Results are expressed as the mean ± standard deviation (n = 6). ∗∗, P < 0.01; ∗, P < 0.05 versus control.
FIG. 2.
Stimulation of [14C]cefixime transport by the Ca2+ channel blockers nifedipine (▴) and verapamil (•) as a function of the blocker concentration. EC50s derived by nonlinear regression analysis were 28.3 ± 5.1 nM for verapamil and 7.0 ± 3.6 nM for nifedipine.
Effects of nifedipine on the kinetic parameters of [14C]cefixime transport.
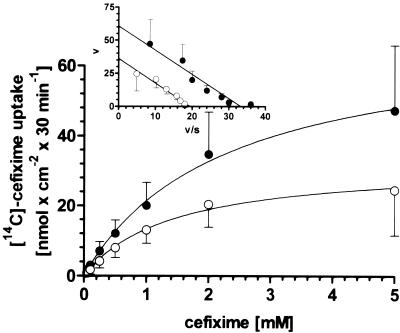
Alterations of Ca2+in levels by Ca2+ channel blockers could cause changes in the affinity of PEPT1 for cefixime or change Vmax. The kinetics of [14C]cefixime transport into Caco-2 cells determined in the absence or the presence of 100 μM nifedipine revealed that the Ca2+ channel blocker significantly increases Vmax but that it has no effects on Km (Fig. 3). Transformation of the data according to Eadie-Hofstee revealed that the maximal transport rate is enhanced by 67% in the presence of nifedipine (Fig. 3, inset).
FIG. 3.
Uptake of [14C]cefixime into Caco-2 cells as a function of substrate concentration. Transport of 0.05 to 5 mM cefixime was measured at pH 5.0 either in the absence of nifedipine (control; ○) or in the presence of 100 μM nifedipine (•). Transformation of the transport rates according to Eadie-Hofstee (inset) revealed Km and Vmax values of 1.9 ± 0.2 mM and 36.2 ± 2.7 nmol · cm−2 · 30 min−1, respectively, in control cells and 1.8 ± 0.2 mM and 60.6 ± 5.9 nmol · cm−2 · 30 min−1, respectively, in nifedipine-treated cells.
Effects of Ca2+ channel blockers and Ca2+ ionophores on the proton motive driving force for [14C]cefixime uptake.
Since it has been shown that the influx of Ca2+ across the plasma membrane into Caco-2 cells is associated with a reduction in pHin as a consequence of reduced Na+ and H+ exchange (NHE) activities (38), changes in Ca2+in levels caused by nifedipine could alter cefixime transport indirectly via modulation of NHE activities. It is known that the pH gradient across the apical membranes of Caco-2 cells limits the rate of transport of PEPT1 and that NHE activities play a crucial role in maintaining the driving force of the peptide transporter (30, 32, 34). To investigate whether Ca2+ channel blockers or Ca2+ ionophores affect pHin and thereby alter the driving force for cefixime uptake, pHin was measured when cells were superfused with nifedipine or ionomycin. We also assessed whether the Ca2+ channel blocker or the Ca2+ ionophore affects the intracellular acidification caused by cefixime and H+ cotransport via PEPT1. Superfusion of Caco-2 cells at the apical side with pH 5.0 buffer alone led to intracellular acidification that reached its equilibrium at a pHin of 7.0 (Fig. 4a). Nifedipine slightly reduced this acidification caused by exposure of cells to pH 5.0 buffer, whereas ionomycin induced a further decline in pHin to 6.6 (Fig. 4a). This reduction of pHin by ionomycin is similar to that caused by perfusion of cells with cefixime (Fig. 4b and c). When cefixime was applied on the apical side together with nifedipine or ionomycin, the Ca2+ channel blocker diminished the acidification process compared to that in response to cefixime alone (Fig. 4b), whereas ionomycin led to a further decline in pHin to 6.0 (Fig. 4c). Moreover, it was evident that the recovery of the cells from the acid load when the pH of the perfusion buffer was changed back to 7.4 was faster in the presence of nifedipine and slower in the presence of ionomycin (Fig. 4).
DISCUSSION
The intestinal absorption of orally active β-lactam antibiotics is mainly determined by their affinities to the intestinal peptide transporter PEPT1 (10). Moreover, this interaction with PEPT1 occurs with quite different pH dependencies for zwitterionic and anionic β-lactams (42). We proposed previously that the zwitterionic species is the preferred transported substrate form, which could explain why the anionic compounds are transported only at low pH with an increased fraction present as a neutral species. Whereas alterations of medium pH in cell culture studies cause pronounced changes in the pH at the brush border membrane, in vivo the intestinal epithelium maintains a fairly stable surface pH compartment, with pH values being ≥6.25 (22), although the luminal pH may be as low as 5.0. At a surface pH of ≥6.25, the predominant fraction of anionic β-lactams, like cefixime, is charged and consequently shows only very low levels of interaction with PEPT1 (42). This might explain the significantly lower oral availability of cefixime in humans of ≤50% of a dose, whereas the zwitterionic compounds generally have availability rates of ≥80% (3, 21, 27).
The interesting clinical observation that the coadministration of cefixime with Ca2+ channel blockers can increase the availability of the anionic compound by 30% (14) suggested that there are mechanisms that can increase the level of cefixime uptake by PEPT1. It has been shown that this increase in transport by nifedipine also applies to the PEPT1 substrates cephalexin and amoxicillin (4, 5, 45), zwitterionic compounds that already show high intestinal absorption rates in vivo. Enhanced rates of PEPT1 transport were observed when insulin-dependent pathways were activated in Caco-2 cells, and it has been proposed that this effect is due to an increased population of PEPT1 in the membrane following translocation of preformed transporters from a cytoplasmic pool into the membrane (29). PKC-dependent pathways have also been shown to affect the Vmax of the transporter. Activation of PKC reduced the maximal velocity of PEPT1, and inhibition of PKC was able to block the Vmax-reducing effects of the PKC activator but had no effect on the maximal transport rate when the PKC inhibitor was applied alone (9).
The action of nifedipine on cefixime absorption in humans and on cephalexin absorption in rats was suggested to be due to activation of neuronal networks by the channel blocker that in turn could affect PEPT1 and increase its transport rate (4, 18). However, studies in the rat intestine in vitro that have used different maneuvers to mimic the effects of nifedipine on the enteric nervous system failed to show the effects on cefixime transport seen in vivo (4). To investigate the underlying mechanisms by which the Ca2+ channel blockers could enhance intestinal cefixime absorption at the cellular level, we used the human intestinal epithelial cell line Caco-2, which expresses PEPT1 and which has already been used for characterization of cefixime transport (42).
When cefxime uptake into Caco-2 cells was determined at optimal pH conditions (i.e., at pH 5.0), the Ca2+ channel blockers verapamil, nifedipine, bepridil, and diltiazem were all found to stimulate cefixime influx by 51, 45, 38, and 34%, respectively, suggesting a common mechanism of action. The EC50s for half-maximal stimulation of transport were 7 nM for nifedipine and 28 nM for verapamil, which are in good agreement with the corresponding Ki values for inhibition of slow Ca2+ channels reported for those blockers (6, 11). Voltage-sensitive Ca2+ channels have been divided into at least three subtypes on the basis of their conductances and sensitivities to voltage (26, 35). The channels best characterized to date are the L, N, and T subtypes. Only the L-type channel is sensitive to the dihydropyridine Ca2+ channel blockers, like nifedipine (16). Since cefixime transport is stimulated by nifedipine and its EC50 for transport effects is in the range at which L-type channel blocking occurs, one may conclude that the effects of this blocker on enhancement of cefixime transport are mediated by L-type Ca2+ channels. Recently, the main subunit of L-type voltage-dependent Ca2+ channels, which contains the ion conduction pore and the drug-binding sites, has been demonstrated to be expressed in Caco-2 cells, especially in the apical membrane (40).
That the uptake of cefixime is affected by the Ca2+ channel blockers, most likely via a reduction of Ca2+in levels, is suggested by the observation that the complexation of intracellular Ca2+ ions by EGTA-AM mimics the effects of the channel blockers on cefixime transport. The responsiveness of PEPT1-mediated cefixime transport to alterations of Ca2+in levels also occurs in the opposite direction, as shown with the Ca2+ ionophores, which reduced cefixime transport significantly due to increased Ca2+in levels. The proposed reduction of Ca2+in levels, e.g., by nifedipine, was associated with a 67% increase in the maximal transport capacity of PEPT1 but with no effects on substrate affinity. The increased transport capacity of PEPT1 may be caused by either an increased membrane transporter density or an increase in the driving force. Because previous studies on the effects of alterations of Ca2+in levels in intestinal epithelial cells (38) showed that pHin and NHE activities were changed and because PEPT1 activity depends on maintenance of the transmembrane proton gradient (30, 34, 42), we measured the intracellular pH changes occurring in Caco-2 cells during β-lactam transport and the response to Ca2+ channel blockers and ionophores. Nifedipine was clearly able to reduce the intracellular acidification induced by cefixime influx and thereby maintained a higher electrochemical proton gradient across the apical plasma membrane, allowing higher transport rates. In contrast, the Ca2+ ionophore ionomycin enhanced intracellular acidification in response to cefixime, thus reducing the transmembrane pH gradient; consequently, the level of cefixime uptake was also reduced. These effects of alterations of Ca2+in levels on pHin appear to be best explained by involvement of NHE isoform 3 (NHE-3). NHE-3 was identified functionally in the apical membrane of differentiated Caco-2 cells and was shown to be preferentially activated as a consequence of H+ influx across the apical membrane (32). Moreover, this antiporter has been shown to be inhibited by Ca2+- and PKC-dependent pathways (12, 19), and therefore, the modulation of NHE-3 activity by Ca2+ channel blockers or other compounds affecting Ca2+in concentrations in epithelial cells may thereby especially affect H+ gradient-dependent transport processes at the apical plasma membrane, such as H+-coupled transport of β-lactams. The importance of NHE activities for absorption of β-lactams has also been established in vivo by demonstrating that NHE inhibition significantly reduces the rate of amoxicillin absorption in humans (44).
In conclusion, our studies demonstrate that an increase in the Ca2+in level in Caco-2 cells is associated with a decrease in PEPT1-mediated cefixime transport, whereas a decrease in the Ca2+in level enhances the maximum transport capacity for this β-lactam antibiotic. This establishes that the Ca2+ channel blockers can increase the level of cefixime transport not only in vivo but also in vitro in Caco-2 cells without the involvement of the enteric nervous system. The results provided here suggest that the underlying mechanism is stimulation of NHE-3 activity following a reduction in Ca2+in levels that in turn maintains a higher electrochemical driving force for PEPT1. A reduction of the intraepithelial Ca2+in level could therefore enhance the intestinal absorption of all peptidomimetic compounds that utilize PEPT1.
REFERENCES
- 1.Amasheh, S., U. Wenzel, M. Boll, D. Dorn, W.-M. Weber, W. Clauss, and H. Daniel. 1997. Transport of charged dipeptides by the intestinal H+/peptide symporter PepT1 expressed in Xenopus laevis oocytes. J. Membr. Biol. 155:247-256. [DOI] [PubMed] [Google Scholar]
- 2.Artursson, P. 1990. Epithelial transport of drugs in cell culture. I. A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79:476-482. [DOI] [PubMed] [Google Scholar]
- 3.Bergan, T. 1984. Pharmacokinetics of beta-lactam antibiotics. Scand. J. Infect. Dis. 42:83-98. [PubMed] [Google Scholar]
- 4.Berlioz, F., S. Julien, A. Tsocas, J. Chariot, C. Carbon, R. Farinotti, and C. Roze. 1999. Neural modulation of cephalexin intestinal absorption through the di- and tripeptide brush border transporter of rat jejunum in vivo. J. Pharmacol. Exp. Ther. 288:1037-1044. [PubMed] [Google Scholar]
- 5.Berlioz, F., B. Lepere-Prevot, S. Julien, A. Tsocas, C. Carbon, C. Roze, and R. Farinotti. 2000. Chronic nifedipine dosing enhances cephalexin bioavailability and intestinal absorption in conscious rats. Drug Metab. Dispos. 28:1267-1269. [PubMed] [Google Scholar]
- 6.Bhat, M. B., S. K. Mishra, and V. Raviprakash. 1989. Differential susceptibility of cholinergic and noncholinergic neurogenic responses to calcium channel blockers and low Ca2+ medium in rat urinary bladder. Br. J. Pharmacol. 96:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boll, M., D. Markovich, W.-M. Weber, H. Korte, H. Daniel, and H. Murer. 1994. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, beta-lactam antibiotics and ACE-inhibitors. Pflugers Arch. 429:146-149. [DOI] [PubMed] [Google Scholar]
- 8.Boyarsky, G., C. Hanssen, and L. A. Clyne. 1996. Inadequacy of high K+/nigericin for calibrating BCECF. II. Intracellular pH dependence of the correction. Am. J. Physiol. 271:C1146-C1156. [DOI] [PubMed] [Google Scholar]
- 9.Brandsch, M., Y. Miyamoto, V. Ganapathy, and F. H. Leibach. 1994. Expression and protein kinase C-dependent regulation of peptide/H+ co-transport system in the Caco-2 human colon carcinoma cell line. Biochem. J. 299:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bretschneider, B., M. Brandsch, and R. Neubert. 1999. Intestinal transport of beta-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm. Res. 16:55-61. [DOI] [PubMed] [Google Scholar]
- 11.Burges, R. A., D. G. Gardiner, M. Gwilt, A. J. Higgins, K. J. Blackburn, S. F. Campbell, P. E. Cross, and J. K. Stubbs. 1987. Calcium channel blocking properties of amlodipine in vascular smooth muscle and cardiac muscle in vitro: evidence for voltage modulation of vascular dihydropyridine receptors. J. Cardiovasc. Pharmacol. 9:110-119. [PubMed] [Google Scholar]
- 12.Burns, K. D., T. Homma, M. D. Breyer, and R. C. Harris. 1991. Cytosolic acidification stimulates a calcium influx that activates Na(+)-H+-exchange in LLC-PK1. Am. J. Physiol. 261:F617-F625. [DOI] [PubMed] [Google Scholar]
- 13.Donowitz, G. R., and G. L. Mandell. 1988. Beta-lactam antibiotics (1). N. Engl. J. Med. 318:419-426. [DOI] [PubMed] [Google Scholar]
- 14.Duverne, C., A. Bouten, A. Deslandes, J. F. Westphal, J. H. Trouvin, R. Farinotti, and C. Carbon. 1992. Modification of cefixime bioavailability by nifedipine in humans: involvement of the dipeptide carrier system. Antimicrob. Agents Chemother. 36:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulkner, R. D., P. Fernandez, G. Lawrence, L. L. Sia, A. J. Falkowski, A. I. Weiss, A. Yacobi, and B. M. Silber. 1988. Absolute bioavailability of cefixime in man. J. Clin. Pharmacol. 28:700-706. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa, T., T. Yamakawa, T. Midera, T. Sagawa, Y. Mori, and T. Nukada. 1999. Selectivities of dihydropyridine derivatives in blocking Ca2+ channel subtypes expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 291:464-473. [PubMed] [Google Scholar]
- 17.Ganapathy, V., G. Burckhardt, and F. H. Leibach. 1984. Characteristics of glycylsarcosine transport in rabbit intestinal brush-border membrane vesicles. J. Biol. Chem. 259:8954-8959. [PubMed] [Google Scholar]
- 18.Harcouët, L., D. Lebrec, C. Roze, C. Carbon, and R. Farinotti. 1997. Increased intestinal absorption of cefixime by nifedipine in the rat intestinal perfusion model: evidence for a neural regulation. J. Pharmacol. Exp. Ther. 281:738-745. [PubMed] [Google Scholar]
- 19.Kandasamy, R. A., F. H. Yu, R. Harris, A. Boucher, J. W. Hanrahan, and J. Orlowski. 1995. Plasma membrane Na+/H+ exchanger isoforms (NHE-1, -2, and -3) are differentially responsive to second messenger agonists of the protein kinase A and C pathways. J. Biol. Chem. 270:29209-29216. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz, S., U. Wenzel, and H. Daniel. 1999. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 38:133-142. [DOI] [PubMed] [Google Scholar]
- 21.Leroy, A., G. Humbert, M. Godin, and J. P. Fillastre. 1982. Pharmacokinetics of cefadroxil in patients with impaired renal function. Antimicrob. Chemother. 10:39-46. [DOI] [PubMed] [Google Scholar]
- 22.McEwan, G. T., H. Daniel, C. Fett, M. N. Burgess, and M. L. Lucas. 1988. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc. R. Soc. London Ser. B Biol. Sci. 234:219-237. [DOI] [PubMed] [Google Scholar]
- 23.Ogihara, H., H. Saito, B. C. Shin, T. Terado, S. Takenoshita, Y. Nagamachi, K. Inui, and K. Takata. 1996. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem. Biophys. Res. Commun. 220:848-852. [DOI] [PubMed] [Google Scholar]
- 24.Riley, S. A., G. Warhurst, P. T. Crowe, and L. A. Turnberg. 1991. Active hexose transport across cultured human Caco-2 cells: characterisation and influence of culture conditions. Biochim. Biophys. Acta 1066:175-182. [DOI] [PubMed] [Google Scholar]
- 25.Saito, H., and K. Inui. 1993. Dipeptide transporters in apical and basolateral membranes of the human intestinal cell line Caco-2. Am. J. Physiol. 265:G289-G294. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz, A. 1992. Molecular and cellular aspects of calcium channel antagonism. Am. J. Cardiol. 70:6F-8F. [DOI] [PubMed] [Google Scholar]
- 27.Sjövall, J., G. Alvan, and D. Westerlund. 1985. Oral cyclacillin interacts with the absorption of oral ampicillin, amoxycillin, and bacampicillin. Eur. J. Clin. Pharmacol. 29:495-502. [DOI] [PubMed] [Google Scholar]
- 28.Takano, M., Y. Tomita, T. Katsura, M. Yasuhara, K. Inui, and R. Hori. 1994. Bestatin transport in rabbit intestinal brush-border membrane vesicles. Biochem. Pharmacol. 47:1089-1090. [DOI] [PubMed] [Google Scholar]
- 29.Thamotharan, M., S. Z. Bawani, X. Zhou, and S. A. Adibi. 1999. Hormonal regulation of oligopeptide transporter pept-1 in a human intestinal cell line. Am. J. Physiol. 276:C821-C826. [DOI] [PubMed] [Google Scholar]
- 30.Thwaites, D. T., C. D. Brown, B. H. Hirst, and N. L. Simmons. 1993. H(+)-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers displays distinctive characteristics. Biochim. Biophys. Acta 1151:237-245. [DOI] [PubMed] [Google Scholar]
- 31.Thwaites, D. T., M. Cavet, B. H. Hirst, and N. L. Simmons. 1995. Angiotensin-converting enzyme (ACE) inhibitor transport in human intestinal epithelial (Caco-2) cells. Br. J. Pharmacol. 114:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thwaites, D. T., D. Ford, M. Glanville, and N. L. Simmons. 1999. H(+)/solute-induced intracellular acidification leads to selective activation of apical Na(+)/H(+) exchange in human intestinal epithelial cells. J. Clin. Investig. 104:629-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thwaites, D. T., B. H. Hirst, and N. L. Simmons. 1994. Substrate specificity of the di/tripeptide transporter in human intestinal epithelia (Caco-2): identification of substrates that undergo H(+)-coupled absorption. Br. J. Pharmacol. 113:1050-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thwaites, D. T., G. T. McEwan, B. H. Hirst, and N. L. Simmons. 1993. Transepithelial dipeptide (glycylsarcosine) transport across epithelial monolayers of human Caco-2 cells is rheogenic. Pflugers Arch. 425:178-180. [DOI] [PubMed] [Google Scholar]
- 35.Tsien, R. W., D. Lipscombe, D. V. Madison, K. R. Bley, and A. P. Fox. 1988. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 11:431-438. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji, A., I. Tamai, H. Hirooka, and T. Terasaki. 1987. Beta-lactam antibiotics and transport via the dipeptide carrier system across the intestinal brush-border membrane. Biochem. Pharmacol. 36:565-567. [DOI] [PubMed] [Google Scholar]
- 37.Verghese, A., D. Roberson, J. H. Kalbfleisch, and F. Sarubbi. 1990. Randomized comparative study of cefixime versus cephalexin in acute bacterial exacerbations of chronic bronchitis. Antimicrob. Agents Chemother. 34:1041-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wali, R. K., C. L. Baum, M. J. Bolt, T. A. Brasitus, and M. D. Sitrin. 1992. 1,25-Dihydroxyvitamin D3 inhibits Na(+)-H+ exchange by stimulating membrane phosphoinositide turnover and increasing cytosolic calcium in CaCo-2 cells. Endocrinology 131:1125-1133. [DOI] [PubMed] [Google Scholar]
- 39.Walker, D., D. T. Thwaites, N. L. Simmons, H. J. Gilbert, and B. H. Hirst. 1998. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J. Physiol. (London) 507:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, X. T., Y. Nagaba, H. S. Cross, F. Wrba, L. Zhang, and S. E. Guggino. 2000. The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. Am. J. Pathol. 157:1549-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenzel, U., D. Diehl, M. Herget, S. Kuntz, and H. Daniel. 1999. Regulation of the high-affinity H+/peptide cotransporter in renal LLC-PK1 cells. J. Cell. Physiol. 178:341-348. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel, U., I. Gebert, H. Weintraut, W.-M. Weber, W. Clauss, and H. Daniel. 1996. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells. J. Pharmacol. Exp. Ther. 277:831-839. [PubMed] [Google Scholar]
- 43.Wenzel, U., D. T. Thwaites, and H. Daniel. 1995. Stereoselective uptake of beta-lactam antibiotics by the intestinal peptide transporter. Br. J. Pharmacol. 116:3021-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westphal, J. F., F. Jehl, J. M. Brogard, and C. Carbon. 1995. Amoxicillin intestinal absorption reduction by amiloride: possible role of the Na(+)-H+ exchanger. Clin. Pharmacol. Ther. 57:257-264. [DOI] [PubMed] [Google Scholar]
- 45.Westphal, J. F., J. H. Trouvin, A. Deslandes, and C. Carbon. 1990. Nifedipine enhances amoxicillin absorption kinetics and bioavailability in humans. J. Pharmacol. Exp. Ther. 255:312-317. [PubMed] [Google Scholar]