Abstract
To determine the epidemiologic features and clinical outcomes of bloodstream infections caused by extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae isolates, cases of bacteremia caused by these organisms in children were analyzed retrospectively. Among the 157 blood isolates recovered from 1993 to 1998 at the Seoul National University Children's Hospital, the prevalence of ESBL production was 17.9% among the E. coli isolates and 52.9% among the K. pneumoniae isolates. The commonest ESBLs were SHV-2a and TEM-52. A novel ESBL, TEM-88, was identified. Pulsed-field gel electrophoresis analysis of the ESBL-producing organisms showed extensive diversity in clonality. The medical records of 142 episodes were reviewed. The risk factors for bloodstream infection with ESBL-producing organisms were prior hospitalization, prior use of oxyimino-cephalosporins, and admission to an intensive care unit within the previous month. There was no difference in clinical severity between patients infected with ESBL-producing strains (the ESBL group) and those infected with ESBL-nonproducing strains (the non-ESBL group) at the time of presentation. However, the overall fatality rate for the ESBL group was significantly higher than that for the non-ESBL group: 12 of 45 (26.7%) versus 5 of 87 (5.7%) (P = 0.001). In a subset analysis of patients treated with extended-spectrum cephalosporins with or without an aminoglycoside, favorable response rates were significantly higher in the non-ESBL group at the 3rd day (6 of 17 versus 33 of 51; P = 0.035), the 5th day (6 of 17 versus 36 of 50; P < 0.05), and the end of therapy (9 of 17 versus 47 of 50; P < 0.001). In conclusion, the ESBL production of the infecting organisms has a significant impact on the clinical course and survival of pediatric patients with bacteremia caused by E. coli and K. pneumoniae.
Escherichia coli and Klebsiella pneumoniae are leading causes of serious infections in neonates, neutropenic cancer patients, and other patients with underlying diseases. These bacteria had been uniformly susceptible to oxyimino-β-lactam antimicrobials. However, since the initial description of extended-spectrum β-lactamase (ESBL) production by K. pneumoniae strains in 1983 (18) and E. coli strains in 1987 (3), strains of E. coli and K. pneumoniae that are resistant to broad-spectrum cephalosporins are increasingly being recognized (6, 14). There have been many reports of outbreaks caused by these organisms in cancer centers, pediatric and geriatric wards, and hospitalized nursing home patients. However, epidemiologic descriptions of bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae are limited (32, 36), and clinical data regarding treatment are further limited (2, 31, 35, 36). At present, carbapenems are recommended for the treatment of infections caused by ESBL-producing organisms. However, this recommendation is primarily based on the in vitro effect (12), the results of animal experiments (33), and only very limited clinical data.
As clinical isolates of the family Enterobacteriaceae had been collected in our institute, we could analyze them for the production of ESBLs and investigate the clinical characteristics of the bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae isolates and their clinical responses to treatment by reviewing the medical records retrospectively. Thus, the objectives of this study were to analyze the risk factors, clinical outcomes, and clinical responses to treatment of bacteremia caused by ESBL-producing E. coli and K. pneumoniae in children and to investigate the prevalence and the types of their ESBLs.
(This study was presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 2001, Chicago, Ill. [Y.K. Kim, H. Pai, H. J. Lee, S. E. Park, E. H. Choi, J. H. Kim, and E. C. Kim, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-1242, 2001].)
MATERIALS AND METHODS
Bacterial strains and patients.
This study evaluated a total of 157 consecutive episodes of bacteremia, 89 caused by E. coli and 68 caused by K. pneumoniae, in children aged 0 to 17 years that were diagnosed during a 5-year period from November 1993 through December 1998 at the Seoul National University Children's Hospital. The institute is a teaching hospital with >300 pediatric beds and is located in the center of Seoul, Korea. During the study period, all strains of E. coli and K. pneumoniae isolated from blood were collected and stored at −70°C. The species of the strains were determined by standard methods (34). Of the stored isolates of E. coli and K. pneumoniae from 183 episodes of bacteremia, those responsible for 157 episodes were successfully recovered. Only one isolate from each episode was included in the microbiological analysis.
Among the 157 episodes of bacteremia, 142 episodes (81 caused by E. coli and 61 caused by K. pneumoniae) were included in the clinical analysis; 13 episodes for which medical records were unavailable (3 infections with ESBL-producing strains and 10 infections with ESBL-nonproducing strains) and 2 episodes of infection with an E. coli AmpC hyperproducer were excluded.
Clinical analysis. (i) Definitions.
Fever was defined as an axillary temperature of ≥38.0°C. The primary focus of infection was defined as a culture-positive site and/or a clinically evident site of infection concomitant with bacteremia. Bacteremia was considered nosocomial if it developed following 72 h of hospitalization or if a patient had been hospitalized within the previous 2 weeks (25). Coinfection was defined as the isolation of organisms in addition to E. coli or K. pneumoniae from the same initial blood culture or clinical or laboratory evidence of viral infection at the time of isolation of E. coli or K. pneumoniae. Superinfection was defined as the isolation of other organisms, either bacteria or viruses, or the development of clinical evidence of viral infections before control of the initial episode. Prior antibiotic use was defined as at least 24 h of therapy within the 30 days before the isolation of the organism.
Patients who had malignant diseases, premature babies, and patients receiving steroid therapy were classified as immunocompromised; the other patients were considered immunocompetent. Neutropenia was defined as an absolute neutrophil count <500/mm3 at the onset of bacteremia. Disease severity was estimated by the presence of the following variables: shock, hypothermia (body temperature, <36.0°C), renal insufficiency (a serum creatinine level of >2.0 mg/dl or a requirement for dialysis), hepatic dysfunction (a serum bilirubin concentration of >2.5 mg/dl or increased aspartate aminotransferase or alanine aminotransferase levels more than twice the normal levels), respiratory difficulty (a partial arterial O2 pressure of <60 mm Hg, a partial arterial CO2 pressure of >50 mm Hg, or a need for ventilatory assistance), or neurological dysfunction (change in consciousness level). Septic shock was defined as sepsis associated with evidence of organ hypoperfusion and a systolic blood pressure <90 or >30 mm Hg less than the baseline value or a requirement for the use of vasopressors to maintain blood pressure.
The antimicrobial therapy was presumptively considered appropriate if the causative organism was susceptible to at least one of the prescribed antimicrobials by an in vitro test. Only those who received presumptively appropriate antimicrobials for at least 24 h were included in the analysis for response to antimicrobial therapy. A response to therapy was considered favorable if the child became afebrile for the subsequent 48 h or more, initial signs and symptoms abated, and no complications developed; otherwise, the response was considered unfavorable. Fatal cases in which the cause of death was not infection were excluded in determination of the therapeutic outcome.
(ii) Treatment regimens.
Initial antibiotic therapy and subsequent modifications were supervised by the attending physician; for >90% of the patients, that person was one of the authors.
Neutropenic cancer patients were initially treated with an empirical regimen that consisted of a narrow-spectrum cephalosporin or an antistaphylococcal penicillin, an antipseudomonal penicillin (without a β-lactamase inhibitor), and gentamicin. After 3 to 4 days, the antibiotics were modified depending on the clinical response and/or the susceptibility of the isolated organism. At the time of management of the patients, antimicrobial susceptibility tests were performed for individual isolates with a commercial system (bioMérieux Vitek Inc., Hazelwood, Mo.) or by a disk diffusion test; the isolates were not screened for production of ESBLs. When there was a favorable response, the initial antibiotics were continued for most of the patients. If the blood culture grew E. coli or K. pneumoniae and the response was unfavorable, the narrow-spectrum cephalosporin and an antipseudomonal penicillin were replaced by an extended-spectrum cephalosporin (cefotaxime or ceftriaxone) or imipenem, depending on the in vitro susceptibility test result (first modification). Gentamicin was also changed to amikacin, if necessary. Three to 4 days after the first modification, the regimens for the unresponsive patients were reevaluated; for most patients, the modified regimens were continued as long as the organism was susceptible to the regimen, but for deteriorating patients, cephalosporins were changed to imipenem, even though the causative organism was susceptible to the cephalosporin being used (second modification). Some patients who were in critical condition at presentation received regimens that included extended-spectrum cephalosporins or imipenem as the initial empirical regimen.
The doses and dosing intervals of the individual antimicrobial agents were as follows: cefazolin, 30 mg/kg of body weight every 8 h; nafcillin, 50 mg/kg every 6 h; carbenicillin, 150 mg/kg every 6 h; gentamicin, 2.5 mg/kg every 6 h; amikacin, 7.5 mg/kg every 8 h; cefotaxime, 50 mg/kg every 6 h; ceftriaxone, 50 mg/kg every 12 h; imipenem, 25 mg/kg every 6 h.
For premature infants and neonates, the initial regimen was a combination of ampicillin and an aminoglycoside or ampicillin and cefotaxime. The other patients were initially treated with a narrow-spectrum cephalosporin and an aminoglycoside. For both groups of patients, the initial regimens were subsequently modified, depending on the clinical response and/or the results of susceptibility tests, in a manner similar to that for the neutropenic cancer patients.
(iii) Review of medical records.
The demographic and clinical data collected for each patient included sex; age; immune status; acquisition of polymicrobial bacteremia; underlying disease; a history of hospitalization within the previous month; admission to an intensive care unit within the previous month; previous use of antimicrobial therapy, especially extended-spectrum cephalosporins; use of a mechanical ventilator within the previous month; the presence of an indwelling catheter; the associated focal infection; parameters for determination of disease severity; response after the introduction of presumptively appropriate antimicrobials; and the final outcome of bacteremia.
(iv) Statistical analysis.
Patient characteristics and outcome measures were compared by univariate analysis by the χ2 test or Fisher's exact test or by the Mann-Whitney U test, as deemed appropriate. The significance level was 0.05. Stepwise logistic regression models determined significant predictors and interactions. The final model included confounding variables significant at a two-tailed P value of ≤0.05 and their interactions. The Student t test was used for comparison of the mean duration from the onset of sepsis to the time of administration of adequate antimicrobials. The SAS (version 6.12) and SPSS (version 10.0) software packages were used for analysis.
Microbiologic analysis. (i) Antimicrobial susceptibility tests.
MICs were determined by the agar dilution method, as described by the National Committee for Clinical Laboratory Standards (NCCLS) (23). The following antimicrobial agents were tested: cefpodoxime (Sankyo, Tokyo, Japan), ceftazidime (Hanmi Pharmaceutical Co., Seoul, Korea), cefotaxime (Hanmi Pharmaceutical Co.), aztreonam (Bristol-Myers Squibb, Syracuse, N.Y.), cefotetan (Yamanouchi Pharmaceutical Co., Tokyo, Japan), piperacillin (Wyeth-Ayerst, Pearl River, N.Y.), piperacillin-tazobactam (Wyeth-Ayerst), imipenem (Merck & Co., Elkton, Tex.), gentamicin (Young Jin Pharmaceutical Co., Seoul, Korea), and amikacin (Yuhan-Cyanamid, Seoul, Korea).
(ii) Screening and confirmatory tests for ESBL-producing strains.
Strains for which the cefpodoxime, ceftazidime, cefotaxime, or aztreonam MIC was ≥2 μg/ml were subjected to confirmatory tests by the double-disk synergy test (15) and/or the phenotypic confirmatory test for the production of ESBL recommended by NCCLS (23).
(iii) Analytical IEF and enzyme inhibition assay.
Isoelectric focusing (IEF) was performed with sonicated extracts by the method of Mathew et al. (20) by using a Mini IEF cell system (Bio-Rad Laboratories, Inc., Hercules, Calif.). Enzyme activities were detected by overlaying the gel with 0.5 mM nitrocefin and comparing the active bands with those for reference enzymes. An inhibition assay was performed by overlaying the gels with 0.5 mM nitrocefin (Glaxo-Wellcome Co., London, United Kingdom) with and without 0.3 mM cloxacillin or 0.3 mM clavulanic acid in 0.1 M phosphate buffer (pH 7.0) (8).
(iv) Transfer of resistance.
Logarithmic-phase cells of each isolate were mated with similar cultures of E. coli J53 Azir on Trypticase soy agar plates (Becton Dickinson Microbiology System, Cockeysville, Md.). After overnight incubation, transconjugants were selected on Trypticase soy agar containing 100 μg of sodium azide (Sigma, St. Louis, Mo.) per ml and 2 or 10 μg of ceftazidime or 64 μg of cefoxitin per ml (13).
(v) LCR for discrimination of SHV ESBL.
Clinical isolates or their transconjugants suspected of carrying β-lactamases of the SHV family were subjected to the ligase chain reaction (LCR) for preliminary discrimination among SHV-1, SHV-2, SHV-2a, SHV-3, SHV-4, SHV-5, and SHV-12, as recently described by the authors (17).
(vi) TEM or SHV gene sequencing.
The TEM- or SHV-related genes from clinical isolates or their transconjugants were amplified by PCR and sequenced with a dideoxy termination cycle sequencing kit (Perkin-Elmer Cetus, Norwalk, Conn.), as described previously (16, 19).
(vii) PCR for the genes of β-lactamases other than TEM or SHV.
CMY-1-related, PSE-related, and OXA-1-related genes were amplified as described previously (16, 29).
(viii) PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed with a CHEF Mapper XA system (Bio-Rad Laboratories, Inc.), as described previously (9). The chromosomal DNA was digested with XbaI (New England BioLabs Inc., Beverly, Mass.) at 37°C for 6 h. DNA was electrophoresed in 1% chromosomal-grade agarose (Bio-Rad Laboratories, Inc.). The electrophoretic conditions were as follows; initial switch time, 2.16 s; final switch time, 54.17 s; run time, 22 h; angle, 120°; gradient, 6.0 V/cm; temperature, 14°C; ramping factor, linear. The PFGE patterns were analyzed with the computer software Molecular Analyst (Bio-Rad Laboratories, Inc.). The PFGE patterns were compared by the unweighted pair group method with arithmetic averages with the Dice coefficient of similarity.
RESULTS
Risk factors for infection by ESBL-producing strains.
Among the 142 episodes of bacteremia that were analyzed, 49 episodes were caused by ESBL-producing E. coli (15 episodes) or K. pneumoniae (34 episodes) isolates (the ESBL group) and 93 episodes were caused by E. coli (66 episodes) or K. pneumoniae (27 episodes) isolates that did not produce an ESBL (the non-ESBL group). The demographic and clinical characteristics for the two groups are given in Table 1. Eighty-four (59%) patients were on anticancer chemotherapy, and all of them were neutropenic at the onset of bacteremia; 6 patients were on adrenocorticotropin or steroid therapy due to infantile spasm or nephrotic syndrome; and 12 patients were premature infants. Seven patients were full-term infants in the newborn period. Twenty-six children had a variety of underlying chronic diseases including biliary atresia, bronchopulmonary dysplasia, congenital heart diseases, congenital muscular diseases, and others. Six of the patients had no predisposing factors; four cases of bacteremia were associated with urinary tract infection, and two were associated with acute appendicitis.
TABLE 1.
Demographic and clinical features at the time of presentation of children with bacteremia due to either ESBL-producing or ESBL-nonproducing E. coli or K. pneumoniae
| Characteristic | ESBL group (n = 49) | Non-ESBL group (n = 93) | P valuea |
|---|---|---|---|
| Sex (no. of males: no. of females) | 27:22 | 54:39 | 0.79 |
| Mean ± standard deviation age (yr) | 4.58 ± 5.02 | 7.17 ± 5.97 | 0.01 |
| Status or underlying disease (no. of episodes) | |||
| Hematological malignancy | 22 | 44 | 0.77 |
| Solid tumor | 9 | 10 | 0.21 |
| Steroid user | 1 | 5 | 0.66 |
| Preterm | 6 | 6 | 0.24 |
| Neonate | 3 | 4 | 0.45 |
| Othersb | 8 | 18 | 0.66 |
| Nonec | 0 | 6 | 0.09 |
| Focus of infection (no. [%] of episodes) | 13 (27) | 39 (42) | 0.07 |
| Pneumonia | 6 | 7 | 0.35 |
| Peritonitis | 1 | 5 | 0.66 |
| Urinary tract infection | 3 | 8 | 0.75 |
| Abscess | 1 | 4 | 0.72 |
| Cellulitis | 0 | 5 | 0.72 |
| Ascending cholangitis | 2 | 6 | 0.72 |
| Meningitis/acute otitis media/septic knee/typhlitis | 0 | 1/1/1/1 | |
| Presenting condition at sepsis onset or presentationd | |||
| Shock | 6/42 | 8/87 | 0.384 |
| Hypothermia | 2/42 | 2/87 | 0.596 |
| Renal insufficiency | 1/42 | 5/86 | 0.663 |
| Hepatic dysfunction | 9/41 | 25/86 | 0.397 |
| Neurological dysfunction | 1/41 | 1/87 | 0.540 |
| Respiratory difficulty in case of pneumonia | 0/6 | 0/7 |
Association was evaluated by Pearson's χ2 test or Fisher's exact test for all parameters except age, which was compared by the Student t test.
Others include biliary atresia (n = 8 episodes), chronic renal diseases (n = 3); chronic encephalopathy (n = 3); congenital neuromuscular diseases (n = 2); bronchopulmonary dysplasia (n = 2); chronic hepatitis (n = 2); failure to thrive (n = 2), and one episode each of congenital heart disease, Kasabach-Meritt syndrome, intestinal pseudo-obstruction with central venous catheter, and toxic shock syndrome.
These six patients were associated with urinary tract infections (4 cases) or acute appendicitis (2 cases).
The values represent the number of episodes with the indicated condition/total number of patients evaluated.
The mean age of the children infected with ESBL-producing strains was significantly lower than that of children infected with ESBL-nonproducing strains (4.58 ± 5.02 years [standard deviation] versus 7.17 ± 5.97 years [P = 0.01 by the Student t test]; range, 2 days to 17.4 years versus 0 days to 17.8 years).
Twenty-seven percent (13 of 49) of the patients in the ESBL group and 42% (39 of 93) of the patients in the non-ESBL group had concomitant focal infections such as pneumonia, urinary tract infections, or ascending cholangitis; but the frequencies of these focal infections were not significantly different between the two groups either overall or for each category of focal infection. The frequency of shock or organ dysfunction at the time of presentation was not different between the groups.
The risk factors for bacteremia caused by ESBL-producing strains of E. coli or K. pneumoniae determined by univariate analysis included hospitalization, care in an intensive care unit, ventilator care, antibiotic use within the preceding 30 days, the presence of a central venous access catheter, and the development of breakthrough bacteremia during antibiotic therapy (Table 2). Exposure to extended-spectrum cephalosporins was a risk factor for infection with ESBL-producing strains, while other antimicrobial agents did not increase the risk. Forty-five (92%) of the 49 episodes in the ESBL group were nosocomial in origin, as were 59 (63%) of 93 episodes in the non-ESBL group. Among the remaining four patients infected with ESBL-producing strains, two patients had been hospitalized 3 or 4 weeks before the onset of bacteremia, one had visited a hospital every week for chemotherapy, and the other was born in the hospital 30 days before the onset of sepsis. Nosocomial infection was a statistically significant risk factor for infections with ESBL-producing strains (P = 0.001). Among the patients with nosocomially acquired cases of infection, however, the mean duration of hospitalization was not significantly different between the ESBL group and the non-ESBL group (P = 0.683).
TABLE 2.
Analysis of risk factors for bloodstream infections caused by ESBL-producing versus ESBL-nonproducing E. coli or K. pneumoniae
| Risk factor | No. (%) of episodes
|
P valuea | |
|---|---|---|---|
| ESBL group (n = 49) | Non-ESBL group (n = 93) | ||
| Hospitalization within previous 1 mo | 36 (73) | 42 (45) | 0.001b |
| Use of any antibiotics within previous 1 mo | 35 (71) | 24 (26) | 0.001 |
| Use of extended-spectrum cephalosporin within previous 1 mo | 20 (41) | 6 (6) | 0.001c |
| Use of any antibiotics other than extended-spectrum cephalosporins within previous 1 mo | 15 (31) | 18 (19) | 0.131 |
| Infection during use of antibiotics | 13 (27) | 4 (4) | 0.001 |
| Nosocomial acquisitiond | 45 (92) | 59 (63) | 0.001 |
| Care in an intensive care unit within previous 1 mo | 12 (24) | 3 (3) | 0.001e |
| Ventilator use in previous 1 mo | 6 (12) | 1 (1) | 0.003 |
| Presence of an indwelling central venous catheter | 20 (41) | 19 (20) | 0.009 |
| Neutropenia | 31 (63) | 56 (60) | 0.7 |
Logistic regression analysis showed significant interactions between acquisition of sepsis by ESBL-producing strains and three variables, that is, prior hospitalization, prior use of an extended-spectrum cephalosporin, and admission to an intensive care unit within the previous month.
OR (95% CI), 4.52 (1.6 to 13.1), by linear logistic regression analysis.
OR (95% CI), 5.56 (1.9 to 16.0).
The mean numbers of days of hospitalization at the time of onset of sepsis among the episodes in patients with nosocomial infections where 31.5 ± 25.9 and 25.5 ± 24.4 days (P = 0.33) for the ESBL and non-ESBL groups, respectively.
OR (95% CI), 35.7 (6.0 to 214.1).
Logistic regression analysis showed a significant interaction between the acquisition of sepsis caused by ESBL-producing strains and three variables: prior hospitalization (odds ratio [OR], 4.52; 95% confidence interval [CI], 1.6 to 13.1), prior use of extended-spectrum cephalosporins (OR, 5.56; 95% CI, 1.9 to 16.0), and admission to an intensive care unit within the previous month (OR, 35.7; 95% CI, 6.0 to 214.1) (Table 2).
Clinical outcome of bacteremia caused by ESBL-producing E. coli or K. pneumoniae isolates.
Among the 49 patients in the ESBL group and the 93 patients in the non-ESBL group, 3 and 4 patients in the two groups, respectively, were discharged or transferred to another hospital before their infections were controlled; the discharges or transfers occurred 1 to 17 days after the development of bacteremia. Additionally, one patient in the ESBL group and two patients in the non-ESBL group died of underlying diseases. Thus, 45 patients in the ESBL group and the 87 patients in the non-ESBL group were included in the clinical outcome analysis.
The fatality rates were analyzed according to the patients' clinical characteristics such as immune status and presence of shock at the time of presentation, as well as the infecting organisms' ESBL production status (Table 3). Production of ESBL or the presence of shock at the time of presentation was associated with a significantly higher fatality rate. The overall fatality rate for the patients in the ESBL group was 26.7% (12 of 45), whereas it was 5.7% (5 of 87) for the patients in the non-ESBL group (P = 0.001 by the χ2 test), and the overall fatality rate for patients who presented with shock was 40.0% (6 of 15), whereas that for patients who presented without shock was 9.4% (11 of 117) (P = 0.001 by the χ2 test). In the analysis of subgroups classified by the presence of shock at the time of presentation, the fatality rates for the ESBL groups were consistently higher than those for the non-ESBL groups, regardless of the presence of shock at presentation: 83.3% (5 of 6) versus 11.1% (1 of 9) for patients with shock (P = 0.01 by Fisher's exact test) and 17.9% (7 of 39) versus 5.1% (4 of 78) for patients without shock (P = 0.04). However, the patients' immune status did not have significant effect on the fatality rate.
TABLE 3.
Fatality rates for episodes of bloodstream infections caused by ESBL-producing versus ESBL-nonproducing E. coli or K. pneumoniae isolates by immune status and presence of shock at presentation
| Immune status | No. of fatal episodes/total no. of episodes (%)a for the indicated status at presentation:
|
||||
|---|---|---|---|---|---|
| ESBL group
|
Non-ESBL group
|
Total | |||
| Shock positive | Shock negative | Shock positive | Shock negative | ||
| Immunocompromised | 3/4 | 6/31 | 0/8 | 4/53 | 13/96 (13.5)b |
| Immunocompetent | 2/2 | 1/8 | 1/1 | 0/25 | 4/36 (11.1)b |
| Subtotal | 5/6 (83.3)c | 7/39 (17.9)d | 1/9 (11.1)c | 4/78 (5.1)d | |
Twelve of 45 patients (26.7%) in the ESBL group and 5 of 87 patients (5.7%) in the non-ESBL group died. The total number of deaths among patients in both groups was 17 (12.9%). The P value for the overall number of fatalities for the ESBL group versus that for the non-ESBL group, 0.001.
P value (Mann-Whiney U test) for immunocompetent group versus immunocompromised group, 1.0.
P value for mortality rate for the ESBL group versus that for the non-ESBL group among patients who presented with shock, 0.01.
P value for mortality rate for the ESBL group versus that for the non-ESBL group among patients who presented without shock, 0.04.
Response to antimicrobial therapy of bacteremia caused by ESBL-producing E. coli or K. pneumoniae isolates.
In the analysis of the response to antimicrobial therapy, 27 patients (10 in the ESBL group, 17 in the non-ESBL group) among the 132 patients that were analyzed for clinical outcome were excluded. These included 10 patients with coinfection, 8 patients with superinfection, 1 patient who died within 24 h of antimicrobial therapy, and 8 patients who never received antimicrobials that were effective against the infecting organism as determined by in vitro susceptibility testing. However, one patient in the ESBL group who was discharged after more than 5 days of antibiotic therapy that was effective in vitro was also included. Thus, the response to antimicrobial therapy was evaluated for 106 patients (36 in the ESBL group and 70 in the non-ESBL group) who received antibiotics presumptively appropriate against the infecting organism, according to in vitro testing, for at least 24 h.
The numbers of favorable responses in the ESBL group were compared to those in the non-ESBL group at the 3rd day, the 5th day, and the end of treatment. The periods from the onset of bacteremia to the administration of presumptively appropriate antimicrobials ranged from 0 to 16 days, and the mean periods were not different between the two groups (1.44 ± 2.66 days for the ESBL group versus 1.10 ± 2.27 days for the non-ESBL group; P = 0.489 by the Student t test). The favorable response rate was significantly higher in the non-ESBL group at the 3rd day (30.6% [11 of 36] in the ESBL group versus 52.9% [37 of 70] in the non-ESBL group; P = 0.03), the 5th day (33.3% [12 of 36] versus 68.6% [48 of 70]; P = 0.01), and the end of therapy (69.4% [25 of 36] versus 98.6% [69 of 70]; P < 0.001).
In order to evaluate the clinical efficacies of oxyimino-cephalosporins for the treatment of bloodstream infections caused by ESBL-producing organisms, we selected the patients who received regimens that included extended-spectrum cephalosporins shown to be effective against the infecting organisms according to the results of in vitro tests. Among the 106 patients who were analyzed for their response to antimicrobial therapy, 68 patients received regimens that included extended-spectrum cephalosporins shown to be effective against the infecting organism by in vitro testing as an initial choice of therapy (28 patients; 7 in the ESBL group and 21 in the non-ESBL group) or as part of a modification of the antibiotic therapy (40 patients; 10 in the ESBL group and 30 in the non-ESBL group) for more than 5 days to allow evaluation of the efficacy of the regimen. The periods from the onset of bacteremia to the administration of cephalosporins were not different between the ESBL and the non-ESBL groups, and an aminoglycoside was coadministered to 64.7% (11 of 17) and 78.4% (40 of 51) of the patients in each group, respectively (P = 0.302). Favorable response rates were significantly higher in the non-ESBL group at the 3rd day, the 5th day, and the end of cephalosporin treatment (Table 4). Among the patients in the ESBL group, there was no correlation between the MICs of the cephalosporins used for treatment and favorable responses (data not shown). However, among the six patients in the ESBL group who received extended-spectrum cephalosporins, which were the only antibiotics effective in vitro (that is, aminoglycosides effective in vitro were not coadministered), one of one patient infected with an organism for which the cephalosporin MIC was 2 μg/ml and one of three patients infected with organisms for which the MIC was 4 μg/ml responded to treatment, but none of two patients infected with organisms for which the MIC was 8 μg/ml responded by day 3 of therapy.
TABLE 4.
Favorable response rates for patients who received regimens that included extended-spectrum cephalosporins that were shown to be effective against the infecting organism by in vitro susceptibility testing for more than 5 daysa
| Time of therapy | No. of patients with favorable response/ no. of patients evaluated (%)b
|
Pc | ||
|---|---|---|---|---|
| ESBL group | Non-ESBL group | Total | ||
| 3rd day | 6/17 (35.3) [1] | 33/51 (64.7) [0] | 39/68 (57.4) [2] | 0.035 |
| 5th day | 6/17 (35.3) [4] | 36/50 (72.0) [1] | 42/67 (62.7) [5] | 0.007 |
| End | 9/17 (52.9) [4] | 47/50 (94.0) [1] | 56/67 (83.6) [5] | <0.001 |
Effective aminoglycosides were also administered during the course of cephalosporin therapy for 64.7% of the patients in the ESBL group and 78.4% of the patients in the non-ESBL group (P = 0.302).
The values in brackets represent the number of fatal cases. The times from the onset of sepsis to the time of cephalosporin therapy were 3.17 ± 2.80 and 2.53 ± 2.83 days (mean ± standard deviation) for the ESBL and non-ESBL groups, respectively (the mean for all patients in both groups was 2.69 ± 2.75 days). The P value for the comparison of the times for the two groups was 0.401 by the Student t test.
P value by the Mann-Whitney U test.
Twenty-two patients, 15 in the ESBL group and 7 in the non-ESBL group, were given an aminoglycoside, which was the only antibiotic effective in vitro, for more than 5 days during the course of antibiotic therapy. The periods from the onset of bacteremia to the time of administration of aminoglycosides effective in vitro were not significantly different between the two groups. There was no difference in the favorable response rates between the two groups at the 3rd day and 5th day of aminoglycoside therapy, but the favorable response rate at the end of aminoglycoside therapy was significantly higher in the non-ESBL group (Table 5). These 22 patients were further analyzed by stratification of the patients according to the MICs of the aminoglycosides administered to each patient for the infecting organisms. As the patients received gentamicin or amikacin and the susceptibility breakpoint concentrations (SBPCs) of gentamicin (4 μg/ml) and amikacin (16 μg/ml) are different, we analyzed the clinical response rate according to the MIC/SBPC ratio of the aminoglycoside for the infecting organism. Eight patients in the ESBL group were infected with organisms for which the MIC/SBPC ratio was ≤1/8, and seven patients in the ESBL group were infected with organisms for which the MIC/SBPC ratio was ≥1/4 (up to 1); all patients in the non-ESBL group were infected with organisms for which the MIC/SBPC ratio was ≤1/8 (range, 1/16 to 1/8). Among the patients in the ESBL group infected with organisms for which MIC/SBPC ratios were ≤1/8, favorable response rates were not different from those for patients in the non-ESBL group at each time of evaluation. However, the favorable response rates for the patients in the ESBL group infected with organisms for which MIC/SBPC ratios were ≥1/4 were significantly lower than those for patients in the non-ESBL group at each time of evaluation (Table 5).
TABLE 5.
Favorable response rates in 22 patients who received an aminoglycoside as the only effective antibiotic against the infecting organism, as determined by in vitro susceptibility testing, for more than 5 days
| Aminoglycoside MIC/SBPC ratioa | No. of patients with favorable response/no. of patients evaluated (%)
|
|||||
|---|---|---|---|---|---|---|
| ESBL group
|
Non-ESBL group
|
|||||
| 3rd day | 5th day | End of therapy | 3rd day | 5th day | End of therapy | |
| 1/16 | 4/4 | 4/4 | 4/4 | 3/4 | 4/4 | 4/4 |
| 1/8 | 3/4 | 3/4 | 3/4 | 2/3 | 2/3 | 3/3 |
| Subtotalb | 7/8 (87.5) | 7/8 (87.5) | 7/8 (87.5) | 5/7 (71.4) | 6/7 (85.7) | 7/7 (100) |
| 1/4 | 0/1 | 0/1 | 0/1 | |||
| 1/2 | 0/1 | 0/1 | 0/1 | |||
| 1 | 0/5 | 0/5 | 1/5 | |||
| Subtotalc | 0/7 (0) | 0/7 (0) | 1/7 (14.3) | |||
| Totald | 7/15 (46.7) | 7/15 (46.7) | 8/15 (53.3) | 5/7 (71.4) | 6/7 (85.7) | 7/7 (100) |
Aminoglycoside MIC/SBPC ratio, ratio of the MIC of the aminoglycoside administered for the infecting organism to the SBPC of the aminoglycoside administered (that is, 4 μg/ml for gentmicin and 16 μg/ml for amikacin, as recommended by NCCLS [23]). The periods from the onset of bacteremia to the time of administration of aminoglycosides effective in vitro were not significantly different between the ESBL group (1.4 ± 3.07 days) and the non-ESBL group (0.57 ± 0.53 days) (P = 0.085).
The differences in favorable response rates between the ESBL group infected with organisms for which the aminoglycoside MIC/SBPC ratios were ≤1/8 and the non-ESBL group were not statistically significant at the 3rd day (P = 0.45), the 5th day (P = 0.92), and the end of aminoglycoside therapy (P = 0.35).
The differences in favorable response rates between the ESBL group infected with organisms for which the aminoglycoside MIC/SBPC ratios were ≥1/4 and the non-ESBL group were statistically significant at the 3rd day (P = 0.007), the 5th day (P = 0.007), and the end of amonoglycoside therapy (P = 0.002).
The differences in favorable response rates between the ESBL group as a whole and the non-ESBL group were not significant at the 3rd day (P = 0.29 by the Mann-Whitney test) or the 5th day (P = 0.09) but reached statistical significance at the end of aminoglycoside therapy (P = 0.032).
Antimicrobial susceptibility and prevalence and types of ESBLs among the E. coli and K. pneumoniae isolates. (i) Antimicrobial susceptibility.
For 20 strains of E. coli and 36 strains of K. pneumoniae, cefpodoxime, ceftazidime, cefotaxime, or aztreonam MICs were ≥2 μg/ml. Of those strains, 14 and 35 strains, respectively, were positive by the double-disk synergy test or a phenotypic confirmatory test. Of the seven strains against which clavulanic acid and cefotaxime, ceftazidime, or aztreonam did not have synergistic activity, five strains were subjected to further study because the MICs of cefpodoxime, ceftazidime, and/or cefotetan were ≥2 μg/ml. Thus, a total of 18 strains of E. coli and 36 strains of K. pneumoniae were further investigated.
(ii) IEF.
Each strain produced one to four β-lactamases with a pI of 5.4, 5.7, 5.9, 7.4, 7.6, 8.0, 8.2, or 8.5 in various combinations (Table 6).
TABLE 6.
Antimicrobial susceptibilities, pIs, and types of β-lactamases for E. coli and K. pneumoniae strains isolated from children with bloodstream infections at the Seoul National University Children's Hospital, 1993 to 1998a
| Group | No. of strains | pI value(s) of β-lactamase(s)b | β-Lactamase type(s) produced | MIC range/MIC50/MIC90 (μg/ml)c
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime | Cefotaxime | Aztreonam | Cefotetan | Piperacillin | Piperacillin- tazobactamd | Gentamicin | Amikacin | ||||
| E. coli | |||||||||||
| Ec-A | 7 | 5.4, 5.9 | TEM-1 + TEM-52 | 8-128/16/32 | 4-128/16/32 | 4-64/8/16 | 0.25-8/1/2 | 128->256/256/>256 | 1-2/1/2 | 0.5->256/128/>256 | 1-4/2/2 |
| Ec-B | 3 | 5.4, 7.6 | TEM-1 + SHV-2a | 1, 2, 8 | 2, 2, 16 | 0.5, 0.5, 4 | 0.12, 0.12, 0.25 | >256, 64, 128 | 1, 1, 4 | 8, 16, 256 | 4, 4, 8 |
| Ec-C | 2 | 5.4, 5.7, 5.9 | TEM-1 + PSE-1-like + TEM-52 | 16, 8 | 8, 4 | 4, 2 | 0.25, 1 | 256, 128 | 8, 2 | 64 | 16, 64 |
| Ec-D | 2 | 5.4, 5.7, 7.6, 8.0 | TEM-1 + TEM-88 + SHV-2a + CMY-1-like | 32, 16 | 64, >32 | 4, 8 | 256, >32 | >256, >128 | 64, 8 | 2 | 16 |
| Ec-E | 1 | 5.4, 5.9, 8.0 | TEM-1 + TEM-52 + CMY-1-like | 16 | 64 | 2 | 256 | >128 | 4 | >256 | 32, >32 |
| Ec-F | 1 | 7.6 | SHV-2a | 1 | 2 | 0.5 | 0.12 | 64 | 1 | 4 | 2 |
| K. pneumoniae | |||||||||||
| Kp-A | 12 | 5.4, 5.9 | TEM-1 + TEM-52 | 8-64/32/64 | 4-64/16/32 | 4-32/8/16 | 0.12-64/0.5/1 | 128->256/128/>256 | 0.5-4/2/4 | 8->128/128/128 | 1-84/2/32 |
| Kp-B | 10 | 7.6 | SHV-2a | 0.5-32/2/4 | 2-16/4/8 | 0.5-8/1/2 | 0.003-1/0.006/0.12 | 64->256/128/256 | 0.5-4/1/1 | 0.5->256/1/16 | 1-32/2/8 |
| Kp-C | 3 | 5.4, 7.6 | TEM-1 + SHV-2a | 8, 2, 8 | 8, 2, 16 | 4, 1, 4 | 0.12, 0.12, 1 | 128, 256, >256 | 8, 4, 1 | 4, 8, 64 | 1, 8, 32 |
| Kp-D | 3 | 5.4, 8.2 | TEM-1 + SHV-12 | 1, 64, 64 | 1, 8, 16 | 4, 32, 64 | 8, 0.25, 0.25 | 256, 128, 256 | 2, 1, 4 | 256, 128, 256 | 8, 16, 16 |
| Kp-E | 2 | 5.4, 5.9 | TEM-1 + TEM-15 | 8, 8 | 16, 4 | 4, 2 | 0.5, 0.5 | >256, >256 | 2, 2 | 128, 64 | 1 |
| Kp-F | 1 | 5.9, 7.6 | TEM-52 + SHV-2a | 32 | 16 | 16 | 0.5 | >256 | 0.5 | 8 | 8 |
| Kp-G | 1 | 5.4, 5.9, 7.4 | TEM-1 + TEM-52 + OXA-1-like | 256 | 256 | 128 | 8 | >256 | 64 | 256 | 16 |
| Kp-H | 1 | 5.4, 5.9, 8.0 | TEM-1 + TEM-52 + CMY-1-like | 64 | 128 | 16 | 256 | >256 | 64 | 64 | 16 |
| Kp-I | 1 | 5.4, 5.6 | TEM-1 + TEM-88 | 64 | 16 | 32 | 1 | >128 | 8 | 8 | 64 |
| Kp-J | 1 | 5.4, 8.0 | TEM-1 + CTX-M-14 | 1 | 32 | 2 | <0.06 | >128 | 0.5 | 0.25 | 1 |
| Kp-K | 1 | 5.4, 7.6, 8.0 | TEM-1 + SHV-2a + CTX-M-14 | 4 | 16 | 4 | 0.25 | 256 | 2 | 128 | 2 |
Among the 54 strains analyzed for ESBLs, 2 strains of E. coli that were considered AmpC hyperproducers were not included in the analysis for the data presented in this table.
In addition to the chromosomal SHV-type β-lactamase of K. pneumoniae.
MICs were determined by the agar dilution method. MIC50 and MIC90, MICs at which 50 and 90% of isolates tested are inhibited, respectively. The inhibitory concentration for each strain rather than the MIC range, MIC50, and MIC90 is shown for groups with less than four isolates.
Tazobactam was used at fixed concentration of 4 μg/ml, and the concentration of piperacillin is presented in the table.
(iii) Transfer of resistance.
The conjugation experiment was performed with 10 of 16 strains of E. coli and 30 of 36 strains of K. pneumoniae by the use of selective media. As a result, resistance to ceftazidime or cefoxitin was transferred by conjugation in 10 of 10 E. coli strains and 30 of 30 K. pneumoniae strains.
(iv) LCR, PCR, and sequencing of β-lactamase genes.
The strains that produced β-lactamases with a pI of 7.6 and those that produced β-lactamases with a pI of 8.2 had SHV-2a- and SHV-12-specific mutations, respectively, as determined by a previously described LCR (17). Nucleotide sequencing of the blcSHV genes of those strains confirmed the results of LCR, and there was no additional mutation in blcSHV. All 27 strains that produced a pI 5.9 β-lactamase and 17 of 42 strains that produced a pI 5.4 β-lactamase were subjected to TEM-specific PCR and nucleotide sequencing for blaTEM. The results revealed that the β-lactamase with a pI of 5.9 was TEM-52 in 25 of 27 strains and TEM-15 in 2 strains. All 17 strains that produced a pI 5.4 β-lactamase had TEM-1 sequences. The β-lactamase of pI 5.6 in three strains was TEM-88, a new member of the TEM family of ESBLs (http://www.lahey.org/studies.htm). TEM-88 had a mutation at position 196 (GGC [glycine]→GAC [aspartate]; the numbering is according to the scheme of Ambler et al. [1]), in addition to TEM-52-specific mutations. The enzymatic characteristics of the TEM-88 β-lactamase have been published separately (28).
Three strains produced an OXA-type enzyme which was not inhibited by either clavulanic acid or cloxacillin. Two strains produced β-lactamases with a pI of 5.7, and one strain produced a pI 7.4 β-lactamase. The genes for the pI 7.4 and the pI 5.7 β-lactamases were positively amplified by OXA-1-specific and PSE-1-specific primers, respectively. Thus, the pI 7.4 and pI 5.7 enzymes were considered OXA-1-like and PSE-1-like β-lactamases respectively.
Six strains produced a pI 8.0 β-lactamase. The cefotetan MICs for four strains (three strains of E. coli and one strain of K. pneumoniae) were ≥256 μg/ml, and the enzymes produced by these strains were inhibited by cloxacillin but not by clavulanic acid. All four strains were positive by a CMY-1-specific PCR and were considered to produce a CMY-1-like β-lactamase. Confirmatory tests for the production of ESBL were positive for two of the six strains (both were K. pneumoniae) that produced a β-lactamase with a pI of 8.0 and for which cefotetan MICs (<0.06 and 0.25 μg/ml, respectively) and ceftazidime MICs (1 and 4 μg/ml, respectively) were low but for which cefotaxime MICs were relatively high (16 and 32 μg/ml, respectively). These results suggest the production of CTX-M-type enzymes (4, 10). These enzymes were proved to be CTX-M-14 by cloning and nucleotide sequencing of the blc genes of these strains (27).
Two strains of E. coli produced β-lactamases with a pI of 8.5. The cefotetan MICs for these strains (1 and 8 μg/ml, respectively) were borderline, and their β-lactamases were inhibited by 0.3 mM cloxacillin but not by 0.3 mM clavulanic acid. These strains were considered AmpC enzyme hyperproducers.
(v) Prevalence and types of ESBLs.
To summarize, 16 (17.9%) of 89 strains of E. coli and 36 (52.9%) of 68 strains of K. pneumoniae produced one or more ESBLs, and 2 strains of E. coli were probable AmpC hyperproducers. The patterns of β-lactamase production, the MICs of the β-lactam antimicrobials, and the numbers of strains with each pattern are summarized in Table 6. It is of note that so many different ESBLs were isolated during the 5 years that were studied. The types (frequencies) of ESBLs detected were TEM-52 (identified in 25 strains), SHV-2a (21 strains), CMY-1-like (4 strains), TEM-88 and SHV-12 (3 strains each), and TEM-15 and CTX-M-14 (2 strains each). When the ESBLs were analyzed by year of isolation, TEM-52 and SHV-2a were identified throughout the years of the study, while other enzymes were identified during 1- or 2-year periods.
The most common ESBL produced by E. coli was TEM-52 (which was produced by 10 of the 16 ESBL-producing strains), followed by SHV-2a (which was produced by 6 strains). TEM-52 and SHV-2a were most commonly identified among the ESBL-producing K. pneumoniae strains (each ESBL was produced by 15 strains). TEM-88 and CMY-1-like ESBLs were identified in both E. coli and K. pneumoniae, while SHV-12, TEM-15, and CTX-M-14 were identified only in K. pneumoniae.
(vi) PFGE.
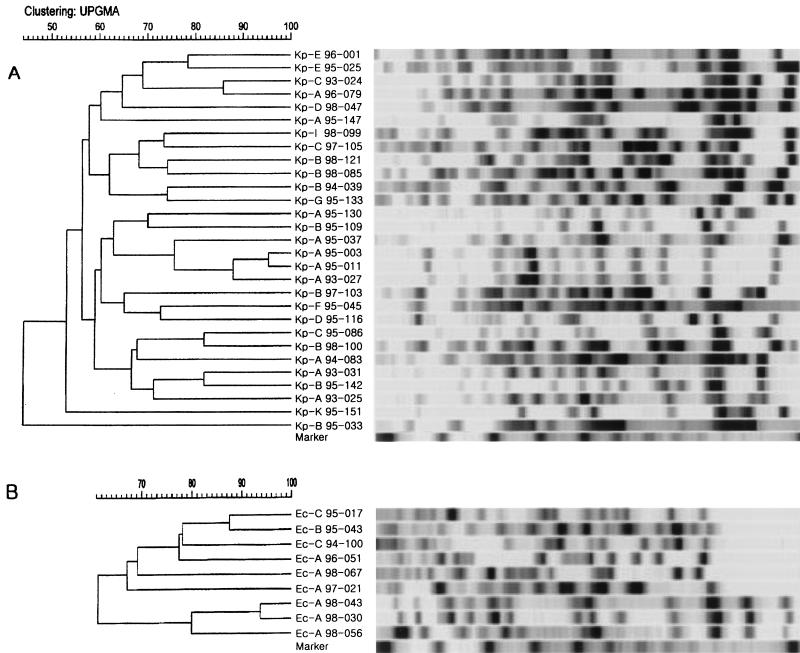
Nine strains of E. coli and 29 strains of K. pneumoniae, all of which produced ESBLs, were included in the PFGE analysis. The results of PFGE showed extensive diversity (Fig. 1). A few strains with the same β-lactamase profile(s) had similar restriction patterns (e.g., strains Ec-A 98-043 and Ec-A 98-030 and strains Kp-A 95-003 and Kp-A 95-011, as shown in Fig. 1), and these strains were temporally closely related. However, most strains showed unrelated patterns, even though they had the same β-lactamases.
FIG. 1.
Dendrograms of ESBL-producing K. pneumoniae (A) and E. coli (B) strains based on PFGE results. Twenty-nine strains of K. pneumoniae and nine strains of E. coli were included in the analysis. For PFGE analysis, the chromosomal DNA was digested with XbaI and electrophoresed in 1% agarose. The strains were clustered by the unweighted pair group method with arithmetic averages (UPGMA). The scale indicates the percentage of genetic similarity. The molecular size marker is a bacteriophage lambda ladder (Bio-Rad Laboratories, Inc.). The strain designations indicate the species (Kp, K. pneumoniae; Ec, E. coli), patterns of production of β-lactamases (A to K, as grouped in Table 6), year of isolation, and strain number.
DISCUSSION
With the widespread use of extended-spectrum cephalosporins throughout the world, strains that produce ESBLs have been detected on every inhabited continent. These enzymes are most commonly found in K. pneumoniae, but they are increasingly found in E. coli, Proteus mirabilis, and other gram-negative bacilli. The emergence and spread of ESBL-producing strains have led to questions regarding the optimal therapy for infections caused by ESBL-producing strains. Although many reports have described outbreaks of infections caused by ESBL-producing organisms, until now no randomized prospective study of the treatment of infections caused by ESBL-producing organisms has been conducted.
Most published reports on outbreaks caused by extended-spectrum cephalosporin-resistant organisms contain limited information about the therapy used for the treatment of bloodstream infections caused by ESBL-producing E. coli or K. pneumoniae, as reviewed by Schiappa et al. (35). Recently, more systematic attempts have been made to look at the clinical outcomes of bacteremia caused by ESBL-producing E. coli or K. pneumoniae strains or the therapeutic efficacies of treatments for bacteremia caused by ESBL-producing E. coli or K. pneumoniae strains.
Mehlhaff et al. (D. L. Mehlhaff, L. Breiceland, E. Tobin, R. Venezia, B. Lomaestro, M. Miller, and D. Stein, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-098, 1996) performed a matched case-control study in which they compared the rates of survival of 16 patients with bacteremia caused by ESBL-producing members of the family Enterobacteriaceae and 24 controls and investigated the relationship between ceftizoxime use without an aminoglycoside and the clinical outcome. The characteristics of the patients were not different between the ESBL and the non-ESBL groups with respect to age, McCabe score, or hospital location; but infections caused by ESBL-producing organisms were associated with significantly higher rates of mortality (P = 0.05). There was no relationship between ceftizoxime use and death among the case patients.
Schiappa et al. (35) published a case-control study of 31 cases of bacteremia caused by ESBL-producing E. coli or K. pneumoniae strains. Mean APACHE II scores (21.8 versus 13.1; P < 0.001) were significantly higher for patients with ceftazidime-resistant K. pneumoniae or E. coli bacteremia than for controls. Mortality rates were similar between the case and control patients who received an antibiotic to which the isolates were susceptible within 3 days of the identification of the bacteremia, and case patients were less likely to die if they received treatment for >1 day with an antibiotic to which the isolates was susceptible within 3 days of identification of the bacteremia (P = 0.02).
In a report of 216 patients with K. pneumoniae bacteremia, Paterson et al. (D. L. Paterson, W. C. Ko, S. Mohapatra, A. Van Gottberg, L. Mulazimoglu, J. M. Casellas, K. P. Klugnam, G. M. Trenholme, M. M. Wagener, and V. L. Yu, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-210, 1997) indicated that the mortality rate was 46% for 32 patients with bacteremia caused by ESBL-producing K. pneumoniae strains and 34% for patients with bacteremia caused by ESBL-nonproducing K. pneumoniae strains (P = 0.22). In addition, the rate of mortality for patients with bacteremia caused by ESBL-producing K. pneumoniae strains was 23% if treatment was with imipenem, whereas it was 42% when other active antibiotics were used (P = 0.07).
In a study performed in a pediatric oncology ward (2), the sepsis-related mortality rate was higher among 16 patients infected with ceftazidime-resistant K. pneumoniae strains (50.0%) than 15 patients infected with ceftazidime-susceptible K. pneumoniae strains (13.3%) (P = 0.02). Patients who did not receive antibiotics directed against ceftazidime-resistant K. pneumoniae strains within 48 h of admission were more likely to have a fatal outcome than those who did (P = 0.009).
In an analysis of 80 patients infected with ESBL-producing organisms, the overall rate of mortality was 24%, and the mortality rate was significantly lower when a carbapenem was used in the first 5 days than when a noncarbapenem was used (mortality rate, 5 versus 43%; P = 0.01) (D. L. Paterson, W. C. Ko, A. V. Gottberg, S. Mohapatra, J. M. Casellas, L. Mulazimoglu, H. Goossens, G. Trenholme, K. Klugman, L. B. Rice, R. A. Bonomo, and V. L. Yu, Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 188, 1998).
In an international multicenter study that analyzed 36 cases of serious infections due to apparently susceptible organisms producing ESBLs, including 26 cases published in the literature, 100% (4 of 4) of the patients experienced clinical failure when the MICs of the cephalosporins used for treatment were in the intermediate range and 54% (15 of 28) experienced clinical failure when the MICs of the cephalosporins were in the susceptible range. In addition, failure rates were the highest (100%; 6 of 6) in patients infected with organisms for which the MIC of the cephalosporin used was 8 μg/ml and were the lowest in patients (27%; 3 of 11) infected with organisms for which the MIC was <2 μg/ml; there was a statistically significant increase in the failure rate as the MICs rose within the susceptible range (P = 0.004) (31).
The studies described above suggest that bacteremia caused by ESBL-producing strains is associated with a higher mortality rate and that carbapenem use and early administration of appropriate antimicrobials may reduce the rate of mortality among patients with infections caused by ESBL-producing organisms. However, information regarding the efficacies of extended-spectrum cephalosporins is limited. The prognosis of bacteremia caused by a member of the family Enterobacteriaceae depends on several factors such as the underlying disease, the clinical severity at the time of administration of antimicrobials, and the antibiotic regimen. In the present series, there were no differences between the ESBL and non-ESBL groups in the frequency or types of underlying diseases, clinical severity, and the interval from the onset of sepsis to the time of administration of presumptively appropriate antibiotics. However, the overall rate of mortality was higher in the ESBL group. In an analysis of a subset of patients treated for more than 5 days with a regimen that included extended-spectrum cephalosporins, favorable response rates at the 3rd day, the 5th day, and the end of cephalosporin therapy were significantly lower in the ESBL group than in the non-ESBL group. These observations support the current recommendation that extended-spectrum cephalosporins should not be used for the treatment of bloodstream infections caused by ESBL-producing E. coli or K. pneumoniae strains, even though they are susceptible to extended-spectrum cephalosporins by in vitro susceptibility testing. However, it is of note that among seven patients described by Wong-Beringer et al. (A. Wong-Beringer, N. Lee, J. Hindler, M. Loeloff, R. Goldschmidt, L. Licata, and K. Bush, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1478, 1999) with bacteremia caused by ESBL-producing E. coli or K. pneumoniae strains, patients with favorable outcomes were infected with isolates that produced a single ESBL, whereas patients with unfavorable outcomes were infected with isolates that produced multiple ESBLs. This suggests that the production of an ESBL does not necessarily preclude successful cephalosporin therapy if the ESBL-producing organism is fully susceptible to the agent, which is also suggested by one of the reports mentioned above (31). This tendency was also observed in the present study, although the number of cases was small; one of one patient infected with an organism for which the MIC of the cephalosporin was 2 μg/ml and one of three patients infected with organisms for which the MIC was 4 μg/ml responded to treatment, but none of two patients infected with organisms for which the MIC was 8 μg/ml responded by the third day of therapy with cephalosporins.
The optimal therapy for infections caused by ESBL-producing members of the family Enterobacteriaceae has yet to be established. Therapeutic options include β-lactam-β-lactamase inhibitor combinations, cephamycin, carbapenems, fluoroquinolones, and aminoglycosides. Imipenem has been the most successful drug in many of the published reports (2, 5, 22) and is the most promising antibiotic for the treatment of infections caused by ESBL-producing organisms. It is well recognized that antibiotic-induced endotoxin release significantly contributes to mortality from sepsis. Imipenem may be superior to cephalosporins in the treatment of infections caused by gram-negative organisms due to its rapid bacteriolysis with low levels of endotoxin release, as well as its stability to hydrolysis by ESBLs (11). In the present series, three patients in the ESBL group and seven patients in the non-ESBL group received imipenem within the first 5 days of the onset of bacteremia. One patient in the ESBL group was in shock at the time of presentation. All of the imipenem-treated patients in the non-ESBL group recovered, but only one imipenem-treated patient in the ESBL group recovered. The two patients in the ESBL group who did not improve with imipenem therapy had been administered imipenem from the onset of sepsis. The patient who presented with shock at the onset of sepsis died on day 6 of therapy, and the other patient did not recover until day 14 of therapy, when the patient, whose bacteremia was uncontrolled, was transferred to another hospital. The number of patients in this series was too small to draw any meaning from the results of imipenem therapy.
Although many reports have mentioned decreased rates of mortality in patients treated with imipenem, the severity of the illness was not reflected and the numbers of patients evaluated were small in most analyses. Further investigation is needed to evaluate the efficacy of carbapenem in the treatment of infections caused by ESBL-producing organisms. It should also be noted that the emergence of imipenem resistance in Pseudomonas aeruginosa and other gram-negative bacilli is a well-described complication of frequent imipenem use.
One of the remarkable findings from this study is that infections caused by organisms for which aminoglycoside MICs are elevated had poor responses to those antimicrobials, even though the MICs were within the susceptible range. Among the patients treated with aminoglycosides and for whom aminoglycosides were the only effective antibiotic, as determined in vitro, there was a striking difference in the favorable response rates between patients infected with strains for which the aminoglycoside MIC/SBPC ratios were ≤1/8 and those infected with strains for which the MIC/SBPC ratios were ≥1/4 (Table 5). Aminoglycosides have a concentration-dependent killing ability, and peak concentration in serum/MIC ratios and/or area under the concentration-time curve/MIC ratios are the most important parameters with which the efficacies of aminoglycosides are correlated (7). Aminoglycoside MICs for ESBL-producing organisms are often elevated. It is possible that the decreased peak concentration in serum/MIC ratio with increasing MICs causes clinical failure. Our data suggest that aminoglycosides alone should not be used for the treatment of bloodstream infections caused by ESBL-producing organisms with reduced susceptibilities to aminoglycosides, even though the organisms are found to be susceptible by in vitro testing.
The unusually high prevalence of ESBL producers in the present series may partly be explained by the fact that the hospital serves as a tertiary referral center in Korea and by the fact that most of the ESBL producers were nosocomial in origin. When analyzed by year of isolation, the prevalence of ESBL producers was almost the same throughout the study period, which suggests that ESBL (especially TEM-52 and SHV-2a)-producing strains were already endemic in the hospital by 1993. The extensive diversity of the PFGE patterns of the ESBL-producing strains also indicates that ESBL-producing strains were endemic in the hospital and that this was due to the dissemination of plasmids rather than the clonal spread of resistant organisms, even though we did not analyze the plasmids.
The predominant ESBL types vary geographically (21, 24). In the United States, most hospital outbreaks have been due to TEM mutant β-lactamases produced by K. pneumoniae, particularly TEM-12, TEM-10, and TEM-26. Recent studies of hospital-associated infections in the United States have noted that SHV-4 and SHV-5 are becoming the predominant types of ESBLs found in nosocomial isolates of K. pneumoniae. In Germany, SHV-2 and SHV-5 seem to be the most predominant; and in France, SHV-3, SHV-4, and TEM-3 are more common. SHV-2 is widespread internationally (14).
The predominant ESBL types in this series were TEM-52 and SHV-2a among strains of both E. coli and K. pneumoniae. Additionally, TEM-88 and CMY-1-like ESBLs were identified in both E. coli and K. pneumoniae strains, while SHV-12, TEM-15, and CTX-M-14 were identified only in K. pneumoniae strains. In other studies that have looked at the ESBL types in Korea, TEM-52 was the only TEM-type ESBL identified, and in contrast to the present series, the most common SHV-type ESBL was SHV-12 (16, 26, 30). The identification of TEM-15 and TEM-88 in Korea makes it possible to speculate on the evolutionary sequence of TEM-type ESBLs in Korea, i.e., TEM-15 to TEM-52 and then to TEM-88 (28). We recently identified the CTX-M-14 ESBL in clinical isolates of E. coli and Shigella sonnei, in addition to that identified in K. pneumoniae included in this study (27). The ESBLs from each of these species had the same amino acid sequences, which suggests that this enzyme is disseminated in Korea.
In summary, we analyzed the clinical outcomes and responses to antibiotic therapy of patients with bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae strains in comparison with those in patients with bloodstream infections caused by strains that did not produce an ESBL. We also analyzed the types of ESBLs produced by these strains. Logistic regression analysis showed a significant interaction between the acquisition of sepsis caused by ESBL-producing strains and three variables: prior hospitalization, prior use of extended-spectrum cephalosporins, and admission to an intensive care unit within the previous month. Infections with ESBL-producing strains were associated with higher mortality rates and lower rates of favorable clinical responses to antibiotic regimens that included extended-spectrum cephalosporins with or without aminoglycosides to which the infecting organisms were susceptible by in vitro testing. Among the patients treated with an aminoglycoside and for whom an aminoglycoside was the only effective antibiotic in vitro, elevated aminoglycoside MICs were associated with lower favorable clinical responses, even though the MIC of the aminoglycoside was within the susceptible range. The most common ESBLs were SHV-2a and TEM-52, followed by SHV-12, TEM-15, CMY-1-like ESBLs, etc. A novel TEM ESBL (TEM-88) was also identified. This report represents the largest molecular epidemiologic and clinical analysis of bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae strains in children reported so far.
Acknowledgments
This work was supported by a grant (grant 04-2000-026-4) from the Seoul National University Hospital.
Yun-Kyung Kim and Hyunjoo Pai contributed equally to the study.
We thank Byung-Joo Park, Department of Preventive Medicine, Seoul National University College of Medicine (Seoul), for assistance in statistical analysis.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. J. Biochem. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariffin, H., P. Navaratnam, M. Mohamed, A. Arasu, W. A. Abdullah, C. L. Lee, and L. H. Peng. 2000. Ceftazidime-resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia. Int. J. Infect. Dis. 4:21-25. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind, A., and G. Horl. 1987. Novel R-factor-borne β-lactamase conferring resistance to cephalosporins. Infection 15:257-259. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind, A., I. Stemplinger, R. Jungwirth, S. Ernst, and J. M. Casellas. 1996. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob. Agents Chemother. 40:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingen, E. H., P. Desjardins, G. Arlet, F. Bourgeois, P. Mariani-Kurkdjian, N. Y. Lambert-Zechovsky, E. Denamur, A. Philippon, and J. Elion. 1993. Molecular epidemiology of plasmid spread among extended broad-spectrum β-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J. Clin. Microbiol. 31:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K., and G. Jacoby. 1997. Nomenclature of TEM β-lactamases. J. Antimicrob. Chemother. 39:1-3. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., S. C. Ebert. 1991. Killing and regrowth of bacterial in vitro: a review. Scand. J. Infect. Dis. 74(Suppl.):63-70. [PubMed] [Google Scholar]
- 8.Danel, F., L. M. Hall, D. Gur, and D. M. Livermore. 1997. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 41:785-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii, Y., A. Ohno, H. Taguchi, S. Imajo, M. Ishiguro, and H. Matsuzawa. 1995. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob. Agents Chemother. 39:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, J. J., and H. Kropp. 1992. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 65:1033-1041. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby, G. A. 1994. Genetics of extended-spectrum β-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 13(Suppl. 1):S2-S11. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby, G. A., and P. Han. 1996. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 34:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad spectrum β-lactamase conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J., Y. Kwon, H. Pai, J. W. Kim, and D. T. Cho. 1998. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J. Clin. Microbiol. 36:1446-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J., and H. J. Lee. 2000. Rapid discriminatory detection of genes coding for SHV β-lactamases by ligase chain reaction. Antimicrob. Agents Chemother. 44:1860-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knothe, H., P. Shah, V. Krcmery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 19.Mabilat, C., and S. Goussard. 1993. PCR detection and identification of genes for extended-spectrum β-lactamases, p. 553-559. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular biology: principles and application. American Society for Microbiology, Washington, D.C.
- 20.Mathew, A., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):S19-S45. [DOI] [PubMed] [Google Scholar]
- 22.Meyer, K. S., C. Urban, J. A. Eagan, B. J. Berger, and J. J. Rahal. 1993. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann. Intern. Med. 119:353-358. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing, 11th supplement. M100-S11, vol. 21, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Nordmann, P. 1998. Trends in β-lactam resistance among Enterobacteriaceae. Clin. Infect. Dis. 27(Suppl. 1):S100-S106. [DOI] [PubMed] [Google Scholar]
- 25.Noskin, G. A., L. R. Peterson, and J. R. Warren. 1995. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin. Infect. Dis. 20:296-301. [DOI] [PubMed] [Google Scholar]
- 26.Pai, H. 1998. The characteristics of extended-spectrum β-lactamases in Korean isolates of Enterobacteriaceae. Yonsei Med. J. 39:514-519. [DOI] [PubMed] [Google Scholar]
- 27.Pai, H., E. H. Choi, H. J. Lee, J. Y. Hong, and G. A. Jacoby. 2001. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J. Clin. Microbiol. 39:3747-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai, H., H. J. Lee, E. H. Choi, J. Kim, and G. A. Jacoby. 2001. The evolution of TEM-related extended-spectrum β-lactamases in Korea. Antimicrob. Agents Chemother. 45:3651-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai, H., J. W. Kim, J. Kim, H. Lee, K. W. Choe, and N. Gotoh. 2001. Carbapenem resistance mechanisms in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 45:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai, H., S. Lyu, J. H. Lee, J. Kim, Y. Kwon, J. W. Kim, and K. W. Choe. 1999. Survey of extended-spectrum β-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J. Clin. Microbiol. 37:1758-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson, D. L. W. C., W.-C. Ko, A. Van Gottberg, J. M. Casellas, L. Mulazimoglu, K. P. Klugnam, R. A. Bonomo, L. B. Rice,. J. G. McCormack, and V. L. Yu. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum β-lactamases: implication for the clinical microbiology laboratory. J. Clin. Microbiol. 39:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pornull, K., E. Goranseson, A. Rytting, and K. Dorubusch. 1993. Extended-spectrum β-lactamases in Escherichia coli and Klebsiella spp. in European septicemia isolates. J. Antimicrob. Chemother. 32:559-570. [DOI] [PubMed] [Google Scholar]
- 33.Rice, L. B., L. L. Carias, R. A. Bonomo, and D. M. Shlaes. 1996. Molecular genetics of resistance to both ceftazidime and β-lactam-β-lactamase inhibitor combinations in Klebsiella pneumoniae and in vivo response to β-lactam therapy. J. Infect. Dis. 173:151-158. [DOI] [PubMed] [Google Scholar]
- 34.Farmer, J. J., III. 1999. Enterobacteriaceae: introduction and identification, p. 442-458. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 35.Schiappa, D. A., M. K. Hayden, M. G. Matushek, F. N. Hashemi, J. Sullivan, K. Y. Smith, D. Miyashiro, J. P. Quinn, R. A. Weinstein, and G. M. Trenholme. 1996. Ceftazidime-resistant Escherichia coli and Klebsiella pneumoniae blood stream infection: a case-control and molecular epidemiologic investigation. J. Infect. Dis. 174:529-536. [DOI] [PubMed] [Google Scholar]
- 36.Siu, L. K., P. L. Lu, P. R. Hsueh, F. M. Lin, S. C. Chang, K. T. Luh, M. Ho, and C. Y. Lee. 1999. Bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric oncology ward: clinical features and identification of differential plasmids carrying both SHV-5 and TEM-1 genes. J. Clin. Microbiol. 37:4020-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]