Abstract
Propolis, a resinous bee product, has been shown to inhibit the growth of oral microorganisms and the activity of bacterium-derived glucosyltransferases (GTFs). Several compounds, mainly polyphenolics, have been identified in this natural product. The present study evaluated the effects of distinct chemical groups found in propolis on the activity of GTF enzymes in solution and on the surface of saliva-coated hydroxyapatite (sHA) beads. Thirty compounds, including flavonoids, cinnamic acid derivatives, and terpenoids, were tested for the ability to inhibit GTFs B, C, and D from Streptococcus mutans and GTF from S. sanguinis (GTF Ss). Flavones and flavonols were potent inhibitors of GTF activity in solution; lesser effects were noted on insolubilized enzymes. Apigenin, a 4′,5,7-trihydroxyflavone, was the most effective inhibitor of GTFs, both in solution (90.5 to 95% inhibition at a concentration of 135 μg/ml) and on the surface of sHA beads (30 to 60% at 135 μg/ml). Antibacterial activity was determined by using MICs, minimum bactericidal concentrations (MBCs), and time-kill studies. Flavanones and some dihydroflavonols, as well as the sesquiterpene tt-farnesol, inhibited the growth of S. mutans and S. sobrinus; tt-farnesol was the most effective antibacterial compound (MICs of 14 to 28 μg/ml and MBCs of 56 to 112 μg/ml). tt-Farnesol (56 to 112 μg/ml) produced a 3-log-fold reduction in the bacterial population after 4 h of incubation. Cinnamic acid derivatives had negligible biological activities. Several of the compounds identified in propolis inhibit GTF activities and bacterial growth. Apigenin is a novel and potent inhibitor of GTF activity, and tt-farnesol was found to be an effective antibacterial agent.
Glucosyltransferase (GTF; EC 2.4.1.5) enzymes produced by Streptococcus mutans have been recognized as virulence factors in the pathogenesis of dental caries (13, 45, 54). GTF enzymes catalyze the formation of soluble and insoluble α-linked glucans from sucrose and contribute significantly to the dental plaque matrix polysaccharide composition (41). Dental plaque is essentially a biofilm. Glucans promote the adherence and accumulation of cariogenic streptococci on the tooth surface and play an essential role in the development of pathogenic dental plaque related to caries-forming activity (19, 44, 54). S. mutans produces at least three GTFs: GTF B, which synthesizes a polymer of mostly insoluble α1,3-linked glucan; GTF C, which synthesizes a mixture of insoluble α1,3-linked glucan and soluble α1,6-linked glucan; and GTF D, which synthesizes α1,6-linked soluble glucan (2, 20, 21). An additional GTF enzyme from S. sanguinis (GTF Ss) may also be involved in the development of dental plaque (39, 50). S. sanguinis colonizes the tooth surface early in plaque formation, and its GTF catalyzes predominantly α1,6-linked soluble glucan (9). Enzymatically active GTFs are present in the soluble fraction of whole human saliva and are also incorporated into the salivary pellicle that is formed on the tooth surface (41, 42). Furthermore, the GTFs incorporated into an experimental pellicle demonstrate distinct physical and kinetic properties compared to the same enzymes in solution; GTFs C and D express enhanced enzymatic activity (43, 47, 51). A large proportion of the glucans synthesized by these surface-adsorbed GTFs is retained on the pellicle and may provide binding sites for S. mutans, contributing to the in situ formation of dental plaque (43, 44, 49). Therefore, inhibition of GTFs both in solution and adsorbed to the pellicle of tooth surface is one of the strategies by which to prevent dental caries and other plaque-related diseases.
Propolis, a resinous substance collected by Apis mellifera bees from various plant sources and mixed with secreted beeswax, is a multifunctional material used by bees in the construction, maintenance, and protection of their hives (8, 17). Propolis is a nontoxic natural product with multiple pharmacological effects and a complex chemical composition (8, 17). Several compounds have been identified in propolis, and three distinct chemical groups have been reported to be present: (i) flavonoid aglycones, (ii) cinnamic acid derivatives, and (iii) terpenoids (3, 4, 40, 46). Flavonoids have been considered the main biologically active compounds in propolis (1, 6, 17). Propolis exhibits a wide range of biological activities, including antimicrobial, antiinflammatory, anesthetic, and cytostatic properties (8, 17). Previously, we have demonstrated that two chemically distinct types of propolis from Brazil inhibited the activity of GTFs and the growth of S. mutans in vitro (28, 29, 30). Furthermore, topical application of propolis twice daily (27) or its inclusion in drinking water available ad libitum (25) reduced the incidence of dental caries in rats. Nevertheless, information on the biological properties of specific compounds, which could be useful for prevention of oral diseases, is sparse. Because propolis has been shown to reduce the incidence of dental caries in rats, the purpose of the present study was to explore the effects of several compounds identified in propolis on the activity of individual, purified GTF enzymes and on the growth of S. mutans.
MATERIALS AND METHODS
Test compounds.
The compounds used in this study are classified into three groups: (i) flavonoids (flavonols, flavones, flavanones, and dihydroflavonols), (ii) cinnamic acid derivatives, and (iii) terpenoids. The flavonols (kaempferide, kaempferol, galangin, isorhamnetin, rhamnetin, myricetin, fisetin, and rutin), flavones (apigenin, acacetin, baicalein, chrysin, luteolin, and tectochrysin), flavanones (pinocembrin, sakuranetin, and isosakuranetin), cinnamic acid derivatives (ferulic acid, p-coumaric acid, and caffeic acid), and terpenoids (tt-farnesol, β-caryophyllene, terpineol, and syringaldehyde) were all obtained from Extrasynthese Co., Genay, France. Protocatechuic acid, vanillin, and benzoic acid were obtained from Sigma-Aldrich Co. The dihydroflavonols, pinobanksin, pinobanksin-7-methyl ether, and pinobanksin-3-acetate, were kindly provided by E. Wollenweber (Darmstadt, Germany). All of the chemical compounds were dissolved in dimethyl sulfoxide (DMSO)-ethanol (1:4, vol/vol) or ethanol (99.9%; high-performance liquid chromatography grade) just prior to performance of the assays. Appropriate solvent controls were always included. Table 1 summarizes all of the test compounds used in this study and their chemical structures.
TABLE 1.
Chemical structures of compounds used in this study
This compound was not identified in A. mellifera propolis.
Me, methyl; Ac, acetyl.
Bacterial strains.
The bacterial strains used for the production of GTFs were (i) Streptococcus milleri KSB8, which harbors the gtfB gene from S. mutans GS-5 (for GTF B production); (ii) S. milleri NH5, which contains the gtfD gene S. mutans GS-5 (for GTF D); (iii) S. mutans WHB410 (53), in which the genes for GTFs B and D and fructosyltransferase were deleted (for GTF C); and (iv) S. sanguinis 10904. For susceptibility and time-kill assays, the bacterial strains used were S. mutans GS-5, S. mutans UA159, and S. sobrinus 6715. The cultures were stored at −80°C in brain heart infusion or tryptic soy broth (TSB) containing 20% glycerol. The S. milleri constructs were a kind gift from Howard K. Kuramitsu (State University of New York, Buffalo), and S. mutans UA159 was obtained from Robert E. Marquis (University of Rochester, Rochester, N.Y.).
GTF enzymes.
All of the purification procedures were carried out with buffers containing the protease inhibitor phenylmethylsulfonyl fluoride (PMSF; final concentration, 1 mM) and NaN3 (final concentration, 0.02%), which was added as a preservative. Neither of the reagents had any adverse effects on enzyme activity or stability.
GTFs B, D, and Ss were obtained from culture supernatants and purified to near homogeneity by hydroxyapatite column chromatography as described by Venkitaraman et al. (51) and Vacca-Smith et al. (50). For GTF C isolation from S. mutans WHB410 (53), cell pellets were harvested from low-molecular-weight broth (2.5% Tryptone, 1.5% yeast extract, 0.3% glucose, 0.1% fructose, and 0.1% sorbitol, which had been ultrafiltered through a membrane with a 10-kDa molecular size cutoff) cultures of S. mutans WHB410 (ftf gtfD gtfB mutant derivative of S. mutans UA130) grown in dialysis tubing (43). The cells were washed twice in 20 mM potassium phosphate buffer, pH 7.5, containing 1 mM PMSF and 0.02% sodium azide. The cells were then resuspended in 30 ml of 50 mM potassium phosphate buffer, pH 7.5, containing 0.1% Triton X-100, 2 M urea, 500 mM NaCl, 0.02% sodium azide, and 1 mM PMSF and incubated at 25°C for 2 h with gentle agitation. The cell suspension was centrifuged at 10,000 × g for 15 min at 4oC. The supernatant, which was used as the source of GTF C, was carefully collected and dialyzed against 50 mM potassium buffer, pH 7.5, containing 1 mM PMSF and 0.02% sodium azide. The dialyzed preparation was purified by hydroxyapatite column chromatography as detailed by Venkitaraman et al. (51).
The purity of the enzyme preparations was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a Hoefer Mighty Small SE245 system (Hoefer Scientific Instruments, San Francisco, Calif.) and silver staining (36). Prestained standards were purchased from Bio-Rad Laboratories. Protein concentration was determined by the method of Lowry et al. (33), and bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) was used to construct standard curves.
GTF activity was measured by the incorporation of [14C]glucose from labeled sucrose (NEN Research Products, Boston, Mass.) into glucans (16, 51). The GTF enzyme added to each sample for all assays was equivalent to the amount required to incorporate 1.0 to 1.5 μmol of glucose over the 4-h reaction (1.0 to 1.5 U).
Assays of activity of GTF in solution and adsorbed onto a saliva-coated hydroxyapatite (sHA) surface.
For solution assays, purified GTFs B, C, D, and Ss were mixed with a twofold dilution series of the test compounds (concentrations ranging from 125 to 500 μM) and incubated with a [14C]glucose-sucrose substrate (0.2 μCi/ml; 200.0 mmol of sucrose per liter, 40 μmol of dextran 9000 per liter, and 0.02% sodium azide in adsorption buffer consisting of 50 mM KCl, 1.0 mM KPO4, 1.0 mM CaCl2, and 0.1 mM MgCl2 [pH 6.5]) to a final concentration of 100 mmol of sucrose per liter (200-ml final volume). For the control, the same reaction was carried out with ethanol-DMSO (final concentrations of 7.5 and 1.25%, vol/vol) or ethanol (final concentration of 5%, vol/vol) replacing the test agent solutions. The samples were incubated at 37°C with rocking for 4 h. After incubation, ice-cold ethanol (1.0 ml) was added and the samples were stored for 18 h at 4°C for precipitation of glucans. Radiolabeled glucan was determined by scintillation counting (16, 51).
For surface assays, the GTFs were adsorbed to hydroxyapatite beads coated with clarified whole saliva (free of GTF activity) as detailed elsewhere (30, 43, 51). The sHA beads were exposed to sufficient enzyme to saturate the surface as determined experimentally. Following adsorption of the enzyme, the beads were washed three times with buffer to remove the loosely bound material and exposed to 300 μl of the twofold dilution series of test (or control) compounds for 30 min at the concentrations described above. The beads were washed and exposed to 300 μl of [14C]glucose-sucrose substrate (final concentration, 100.0 mmol of sucrose per liter). The radiolabeled glucan formed was collected and quantified by scintillation counting (16, 51). All of the solution and surface assays were done in quadruplicate in at least three different experiments.
For the compound with the highest activity, inhibition curves (concentration versus activity; concentrations ranged from 62.5 μM to 1 mM) for all of the GTFs were plotted and 50% inhibitory concentrations (IC50s; the concentrations of test compounds required to inhibit enzymatic activity by 50%) were calculated from regression lines (10). For this assay, a one-way layout experimental design was used in a four by two by seven (enzyme by state by dose) factorial scheme. An analysis of variance was carried out, and qualitative treatments were compared by using Tukey's test at a 5% level of significance (P < 0.05). A nonlinear regression was applied in order to evaluate the effects of different concentrations.
Susceptibility testing.
The MIC and minimum bactericidal concentration (MBC) of each test compound were determined in accordance with the NCCLS guidelines (37, 38) and Koo et al. (29). The broth microdilution and macrodilution methods (in TSB) were used for the antibacterial tests. The starting inoculum was 5 × 105 CFU/ml, and the concentrations of test compounds ranged from 15.6 to 500 μM (twofold dilutions). The MICs and MBCs were determined in quadruplicate in at least three different experiments.
Time-kill assays.
For the compound with the highest level of antibacterial activity, time-kill studies were performed by the broth macrodilution method (37). The starting inoculum of S. mutans GS-5 and UA159 and S. sobrinus 6715 was 1 × 106 to 5 × 106 CFU/ml. The final concentration of the antibacterial agent was four times the MIC (or MBC). Tubes containing the microorganisms and the test compound in TSB were incubated in 5% CO2 at 37°C; samples were removed for determination of viable counts at 0 and 30 min and 1, 2, 4, 8, and 24 h. Serial dilutions (10−1 to 10−4) were prepared in sterile 0.9% sodium chloride solution. The diluted sample (50 μl) was plated onto tryptic soy agar with a spiral plater (Autoplate model 3000; Spiral Biotech, Inc., Bethesda, Md.). The plates were incubated in 5% CO2 at 37°C for 48 h, when the number of colonies was determined. Killing curves were constructed by plotting the log10 CFU per milliliter versus time over 24 h. All of the assays were done in quadruplicate on at least three occasions. A bactericidal effect was defined as a ≥3-log10 decrease in the number of CFU per milliliter from the original inoculum.
The potential for drug carryover to produce falsely low viability counts was minimized by dilution of inocula and plating of small volumes of diluted samples (50 μl). In addition, no evidence of drug carryover was detected at the lowest dilution used for plating (10−1).
RESULTS
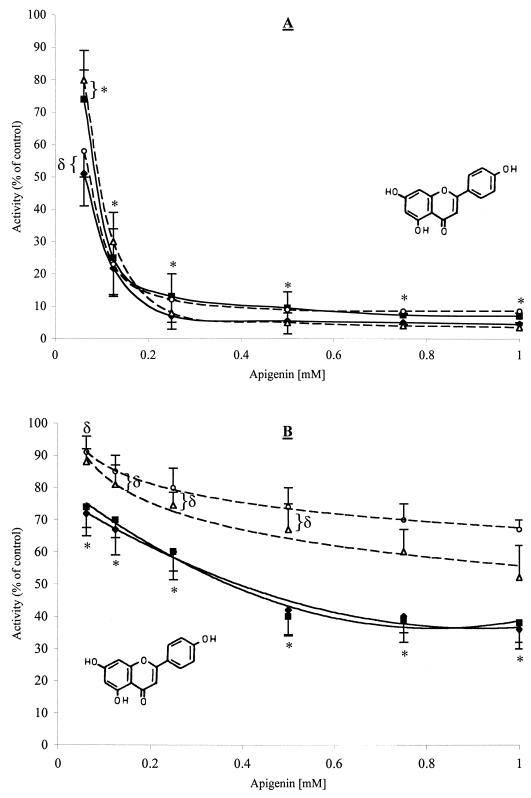
The effects of the most active compounds on the activity of GTFs are shown in Tables 2 and 3. In general, flavonols and flavones reduced the activity of all of the enzymes tested in solution (40 to 95% inhibition) and on a surface (15 to 60% inhibition) at a concentration of 500 μM. Apigenin (a 4′,5,7-trihydroxyflavone) displayed the most potent inhibition of GTF activity. Apigenin inhibited 90.5 to 95% of the activity of all of the GTFs tested in solution at a concentration as low as 500 μM (135 μg/ml). The inhibitory effect of apigenin on surface-adsorbed enzymes was not as potent as that observed when the same enzymes were in solution. Nevertheless, it was an effective inhibitor (30 to 60% inhibition at a concentration of 500 μM). The inhibitory effects of apigenin on GTFs are illustrated in Fig. 1. Apigenin reduced the activity of all of the enzymes in solution in direct proportion to the amount added in the reaction test (r2 values ranging from 0.92 to 0.99). The IC50s of apigenin for the GTFs in solution were 58 μM (16 μg/ml) to 98 μM (26 μg/ml). The IC50s for surface-adsorbed enzymes were noticeably higher; the IC50s for GTFs B and C were 478 μM (128 μg/ml) and 458 μM (122 μg/ml); those for GTFs D and Ss were >1 mM. It is conceivable that an IC50 of this agent would not be achieved for surface-adsorbed GTFs D and Ss. Surface-adsorbed GTFs B and C were inhibited significantly more at all of the concentrations tested than were GTFs D and Ss (P < 0.05). The flavone baicalein and the flavonols myricetin and rhamnetin also proved to be effective inhibitors of GTFs in solution (70 to 90% at 500 μM) and adsorbed on an sHA surface (19 to 40% at 500 μM).
TABLE 2.
Effects of a selected flavone and a selected flavonol on the activities of streptococcal GTFs in solution and adsorbed onto an sHA surfacea
| Compound and concn (μM) | Mean % inhibition (SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| GTF B
|
GTF C
|
GTF D
|
GTF Ss
|
|||||
| Solution | Surface | Solution | Surface | Solution | Surface | Solution | Surface | |
| Apigenin (flavone) | ||||||||
| 125 | 78.3 (4.8) | 31.8 (8.8) | 77.5 (4.2) | 30.0 (6.4) | 70.8 (4.9) | 17.0 (8.5) | 83.2 (6.1) | 13.8 (2.6) |
| 250 | 92.8 (4.2) | 40.0 (6.3) | 87.5 (3.2) | 42.1 (8.2) | 90.2 (3.6) | 25.5 (3.9) | 91.2 (4.6) | 20.2 (5.4) |
| 500 | 94.3 (3.7) | 55.5 (8.4) | 90.5 (3.8) | 60.5 (5.7) | 95.0 (2.6) | 33.0 (7.9) | 92.7 (3.9) | 30.0 (2.6) |
| Kaempferol (flavonol) | ||||||||
| 125 | 32.4 (5.6) | 9.9 (6.7) | 41.9 (3.2) | 24.2 (4.2) | 39.1 (1.8) | 20.4 (3.3) | 37.6 (5.7) | 9.7 (6.3) |
| 250 | 80.6 (3.5) | 27.8 (2.4) | 82.2 (2.7) | 25.8 (6.7) | 76.7 (5.8) | 23.5 (7.9) | 43.1 (3.3) | 8.8 (3.7) |
| 500 | 90.4 (1.2) | 40.2 (4.5) | 90.2 (2.1) | 35.2 (5.3) | 86.7 (4.2) | 28.5 (5.5) | 88.9 (3.7) | 19.2 (7.2) |
Percent inhibition was calculated by considering the control to have maximum GTF activity (100%).
FIG. 1.
Effects of apigenin on the activities of streptococcal GTFs in solution (A) and adsorbed onto an sHA surface (B). Symbols: ♦, GTF B; ▪, GTF C; ▵, GTF D; ○, GTF Ss. The data shown are mean values ± standard deviations. Percent inhibition was calculated by considering the control (DMSO-ethanol; final concentrations of 7.5 and 1.25% [vol/vol], respectively) to contain maximum GTF activity. At each concentration of apigenin, means labeled with symbols (∗ and δ) are not significantly different from each other at P < 0.05.
The flavanones, the dihydroflavonols, and some of the terpenoids (tt-farnesol and β-caryophyllene) tested in this study showed moderate inhibitory effects (8 to 45% for GTFs in solution and 7 to 24% for GTFs on a surface at a concentration of 500 μM); the cinnamic acid derivatives showed negligible effects on GTF enzymes. In some cases, the activity of GTFs was enhanced, e.g., by cinnamic acid derivatives and some terpenoids (e.g., protocatechuic acid and terpineol).
The MICs and MBCs of the test compounds for S. mutans GS-5 and UA159 and S. sobrinus 6715 are shown in Table 4. Some of the flavanones and dihydroflavonols, as well as tt-farnesol (a terpenoid), displayed antibacterial activity. All of the flavanones inhibited bacterial growth; pinocembrin was the most effective of them, with an MIC of 250 μM (64 μg/ml) for all of the strains tested. Pinocembrin showed a bactericidal effect against S. sobrinus 6715 at 500 μM (128 μg/ml). The dihydroflavonol pinobanksin-3-acetate also inhibited the growth of S. sobrinus 6715 and S. mutans strains (MIC of 500 μM or 157 μg/ml). Of all of the test compounds, tt-farnesol was the most effective antibacterial agent. The MICs were 125 μM (28 μg/ml) for S. mutans strains and 62.5 μM (14 μg/ml) for S. sobrinus 6715. The MBCs were 500 μM (112 μg/ml) for S. mutans strains and 250 μM (56 μg/ml) for S. sobrinus 6715. Chlorhexidine (a positive control) yielded MICs of 1.1 to 2.2 μM (1 to 2 μg/ml) and an MBC of 8.9 μM (8 μg/ml). Flavonols, flavones, and cinnamic acid derivatives did not show any antibacterial activity at the concentrations used in this assay, with the exception of baicalein (MIC of 500 μM).
TABLE 4.
MICs and MBCs of antibacterial compounds for S. mutans and S. sobrinus strainsa
| Test compound |
S. mutans UA159
|
S. mutans GS-5
|
S. sobrinus 6715
|
|||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| tt-Farnesol (terpenoid) | 125 | 500 | 125 | 500 | 62.5 | 250 |
| Baicalein (flavone) | 500 | >500 | 500 | >500 | 500 | >500 |
| Flavanones | ||||||
| Pinocembrin | 250 | >500 | 250 | >500 | 250 | 500 |
| Sakuranetin | >500 | >500 | >500 | >500 | 500 | >500 |
| Isosakuranetin | 500 | >500 | 500 | >500 | 250 | >500 |
| Pinobanksin-3-acetate (dihydroflavonol) | 500 | >500 | 500 | >500 | 250 | >500 |
Values are micromolar concentrations. Chlorhexidine (positive control) had MICs of 1.1 to 2.2 μM (1 to 2 μg/ml) and an MBC of 8.9 μM (8 μg/ml). Kaempferide, kaempferol, galangin, isorhamnetin, rhamnetin, fisetin, rutin, apigenin, acacetin, chrysin, luteolin, tectochrysin, ferulic acid, p-coumaric acid, caffeic acid, β-caryophyllene, terpineol, syringaldehyde, protocatechuic acid, vanillin, benzoic acid, pinobanksin, and pinobanksin-7-methyl ether had MICs greater than 500 μM.
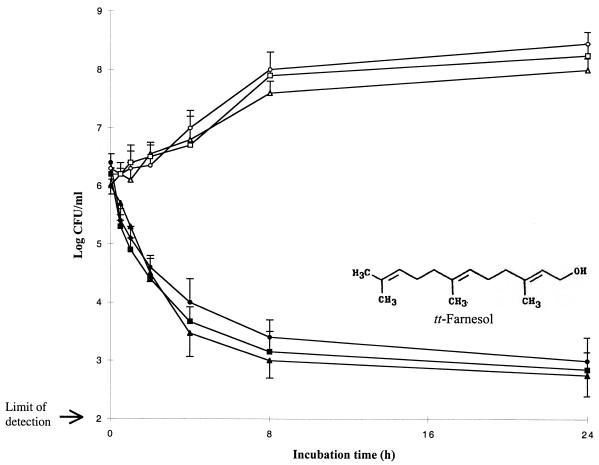
The results of the time-kill kinetic studies are summarized in Fig. 2. tt-Farnesol at four times the MIC (or MBC) rapidly reduced the viable counts of S. mutans and S. sobrinus within 30 min to 1 h of incubation (reduction of 1 log in the number of CFU per milliliter). tt-Farnesol exerted bactericidal effects (a ≥3-log decrease in the number of CFU per milliliter) on S. sobrinus 6715 and on S. mutans strains between 4 and 8 h of incubation. Chlorhexidine at 8.9 μM (MBC) displayed bactericidal effects on S. sobrinus 6715 and S. mutans strains tested after 8 h of incubation.
FIG. 2.
Time-kill curves for S. mutans strains by tt-farnesol at four times the MIC (or MBC). Symbols: ▴ and ▵, S. mutans GS-5; • and ○, S. mutans UA159; ▪ and □, S. sobrinus 6715.
DISCUSSION
Dental caries is one of the most common oral diseases worldwide (7). One approach to reducing the incidence of tooth decay is to develop therapeutic agents aimed at preventing the formation of plaque matrix (48), 30 to 40% (dry weight) of which is polysaccharide (12, 24). Indeed, most of the polysaccharide fraction of plaque is synthesized chiefly by GTFs from sucrose (31, 41), although fructan is also present (12, 24). Dental caries results from events that occur at the tooth pellicle-plaque interface; enzymatically active GTFs are present in the pellicle. Clearly, it is desirable to determine the effects of potential inhibitors on surface-adsorbed GTF.
The use of natural products has been one of the most successful strategies for the discovery of new medicines (22); 78% of new antibiotics and 61% of new antitumor drugs approved by the Food and Drug Administration or comparable entities in other countries from 1983 to 1994 were natural products or derived from natural products (11). According to Harvey (22), the access to biodiversity is fundamental to expanding the range of natural products to be used in the search for new drugs. In this context, propolis from A. mellifera, a relatively unexplored natural product, could be a valuable resource for exploration of new bioactive compounds because of the high chemodiversity of this natural substance (3, 6, 18, 27, 40). Recently, we demonstrated that propolis reduced dental caries in desalivated rats (27). Therefore, we hypothesized that there are specific compounds in propolis that can, at low concentrations, inhibit the growth of cariogenic bacteria and the activity of the GTFs, which are associated with the pathogenesis of dental caries. This was the first step toward identifying novel inhibitors of GTF enzymes and S. mutans growth.
The results obtained in the present study identified some of the compounds that may have been responsible for the previously reported effects of propolis on GTFs and bacterial growth (28, 29, 30, 40). In general, flavonoids were the most active compounds, displaying distinct biological properties; flavones and flavonols were effective GTF inhibitors, whereas flavanones and the dihydroflavonol pinobanksin-3-acetate showed antibacterial activity.
Apigenin, a 4′,5,7-trihydroxyflavone, was the most effective inhibitor of GTFs, especially GTFs B and C. Most of the known GTF inhibitors tested so far, including currently commercially available mouthwashes, failed to inhibit surface-adsorbed GTF activities effectively (48, 53). In contrast, in the present study, apigenin greatly inhibited GTF, especially GTFs B and C, irrespective of whether the enzyme was exposed before or after adsorption to a surface at concentration as low as 500 μM. This level of inhibition has not been observed previously (48, 53). The effective inhibition of GTFs B and C by apigenin may affect the pathogenic potential of dental plaque related to caries, consistent with a reduction in smooth-surface caries observed with mutants of S. mutans defective in the production of either or both GTFs (54).
Apigenin is a nonmutagenic flavonoid displaying a variety of antitumor and antiinflammatory effects (32, 34); our study is the first to show apigenin as a potent inhibitor of GTF activity. The exact mechanism by which apigenin and related flavonoids act to inhibit GTF activity is unknown, although the data reported here provide some insights into the mode of their inhibitory action. It appears that inhibition of GTFs depends on the molecular structure of flavonoids and the physical state of the enzyme. Flavones and flavonols, which have an unsaturated double bond between positions C-2 and C-3 (Table 1), showed remarkable inhibition of GTF activity; in contrast, flavanones and dihydroflavonols, which lack a double bond between C-2 and C-3, exhibited only modest inhibitory activities. Results from previous studies have shown that flavones and flavonols are the main flavonoids related to inhibition of several mammalian enzymes, which suggests that a double bond between C-2 and C-3 is required for inhibitory effects (15, 52). The presence of a double bond between C-2 and C-3 may provide a site for nucleophilic addition by side chains of amino acids in GTFs. Several amino acid residues have been identified as essential for expression of the catalytic activity of GTFs, especially aspartic acid (26, 35). It is likely that the side chain of aspartic acid (CH2COOH) acts as a nucleophile and reacts with flavones and flavonols, causing GTF inhibition. The resistance displayed by surface-adsorbed GTF enzymes may be related to conformational changes the GTF undergoes during the adsorption process, consistent with differences between the adsorbed and soluble forms of the enzyme in terms of physical or kinetic properties and the products synthesized (31, 43, 51). Further research is needed to elucidate the mechanistic details of GTF inhibition by these groups of flavonoids.
Several compounds from propolis inhibited S. mutans growth. However, none of them was as potent as chlorhexidine, which is a clinically proven antimicrobial. Of the compounds tested, tt-farnesol was the most effective antibacterial agent and produced a rapid decrease in S. mutans viable counts. This observation is in agreement with previous reports on the antimicrobial activity of farnesol and related compounds in a variety of other taxa (5, 14). Terpenes such as farnesol have been reported to disrupt membrane function, ultimately reducing cell viability (5). It is noteworthy that streptococci treated with high concentrations of tt-farnesol (>10 mM) produced membrane disruption visible in a phase-contrast microscope; whether the streptococcal membrane is affected at the molecular level by lower concentrations of tt-farnesol (e.g., 0.5 mM) needs to be further elucidated. Recently, farnesol has been shown to be an antifungal agent, displaying quorum-sensing molecule activity (23). We are currently evaluating the antibacterial effect of tt-farnesol on sessile bacterial cells or biofilms.
Our data support the hypothesis that the biological activity of propolis, such as caries prevention (27), is related to the effects of several compounds, as suggested by Amoros et al. (1) and Bonhevì et al. (6), rather than a single compound. Apigenin and tt-farnesol are active compounds in propolis. It is conceivable that propolis compounds can be exploited commercially. The concentrations used in our study may be readily achievable in the mouth through topical application. Although the toxicology of these compounds was not studied here, there is no evidence in the literature that apigenin or tt-farnesol has any potential cellular toxicity or hemolytic effects.
In summary, we have described two new potential anticaries-antiplaque agents, apigenin and tt-farnesol, each of which exhibits distinct biological activities. Since some of the biological activities of these compounds have been clearly shown, further studies involving animal models are worth performing to evaluate their effects in vivo. Propolis appears to be a promising source of new agents that may prevent dental caries and other oral diseases.
TABLE 3.
Effects of a selected flavanone and a selected dihydroflavonol on the activities of streptococcal GTFs in solution and adsorbed onto an sHA surfacea
| Compound and concn (μM) | Mean % inhibition (SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| GTF B
|
GTF C
|
GTF D
|
GTF Ss
|
|||||
| Solution | Surface | Solution | Surface | Solution | Surface | Solution | Surface | |
| Pinocembrin (flavanone) | ||||||||
| 125 | 3.7 (5.2) | 0.7 (3.3) | 0.0 (0.7) | 0.0 (0.0) | 1.5 (0.1) | 0.0 (0.7) | 25.7 (4.1) | 0.0 (0.2) |
| 250 | 7.2 (1.6) | 8.8 (4.3) | 8.5 (3.2) | 0.4 (0.1) | 4.8 (2.7) | 8.2 (2.7) | 32.6 (6.8) | 0.0 (4.2) |
| 500 | 15.9 (4.7) | 18.9 (5.4) | 17.2 (4.2) | 13.5 (4.9) | 14.9 (4.2) | 15.5 (6.2) | 45.7 (8.3) | 10.5 (2.7) |
| Pinobanksin-3-acetate (dihydroflavonol) | ||||||||
| 125 | 0.0 (0.8) | 9.4 (4.8) | 0.0 (0.0) | 0.0 (0.1) | 5.5 (4.5) | 0.0 (0.0) | 4.6 (5.7) | 0.0 (0.6) |
| 250 | 7.7 (3.5) | 7.3 (2.1) | 3.5 (4.2) | 0.0 (0.0) | 2.7 (3.3) | 4.5 (2.0) | 22.8 (3.6) | 0.0 (2.7) |
| 500 | 18.9 (2.8) | 10.3 (3.5) | 16.7 (4.3) | 0.0 (3.3) | 3.5 (2.1) | 10.1 (2.8) | 30.5 (7.2) | 9.9 (5.9) |
Percent inhibition was calculated by considering the control to have maximum GTF activity (100%).
Acknowledgments
We are grateful to Kathleen Scott-Anne for technical assistance.
This research was supported by U.S. Public Health Service grant DE07907 and the Basil G. Bibby Fellowship.
REFERENCES
- 1. Amoros, M., C. M. Simões, L. Girre, F. Sauvager, and M. Cormier. 1992. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 55:1732-1740. [DOI] [PubMed] [Google Scholar]
- 2. Aoki, H., T. Shiroza, M. Hayakawa, S. Sato, and H. K. Kuramitsu. 1986. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect. Immun. 53:587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankova, V., R. Christov, A. Kujumgiev, M. C. Marcucci, and S. Popov. 1995. Chemical composition and antibacterial activity of Brazilian propolis. Z. Naturforsch. 50c:167-172. [DOI] [PubMed] [Google Scholar]
- 4.Banskota, A. H., Y. Tezuka, J. K. Prasain, K. Matsushige, I. Saiki, and S. Kadota. 1998. Chemical constituents of Brazilian propolis and their cytotoxic activities. J. Nat. Prod. 61:896-900. [DOI] [PubMed] [Google Scholar]
- 5.Bard, M., M. R. Albrecht, N. Gupta, C. J. Guynn, and W. Stillwell. 1988. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids 23:534-538. [DOI] [PubMed] [Google Scholar]
- 6.Bonhevì, J. S., F. V. Coll, and R. E. Jordà. 1994. The composition, active components and bacteriostatic activity of propolis in dietetics. J. Am. Oil Chem. Soc. 71:529-532. [Google Scholar]
- 7.Bowen, W. H. 1999. Wither or whither caries research? Caries Res. 33:1-3. [DOI] [PubMed] [Google Scholar]
- 8.Burdock, G. A. 1998. Review of the biological properties and toxicity of propolis. Food Chem. Toxicol. 36:341-363. [DOI] [PubMed] [Google Scholar]
- 9.Ceska, M., K. Granath, B. Norman, and B. Guggenheim. 1972. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem. Scand. 26:2223-2230. [DOI] [PubMed] [Google Scholar]
- 10.Copeland, R. A. 2000. Enzymes: a practical introduction to structure, mechanism, and data analysis, 2nd ed. Wiley-VCH, Inc., New York, N.Y.
- 11.Cragg, G. M., D. J. Newman, and K. M. Snader. 1997. Natural products in drug discovery and development. J. Nat. Prod. 60:52-60. [DOI] [PubMed] [Google Scholar]
- 12.Critchley, P. 1969. The breakdown of the carbohydrate and protein matrix of dental plaque. Caries Res. 3:249-265. [DOI] [PubMed] [Google Scholar]
- 13.De Stoppelaar, J. D., K. G. König, A. J. M. Plasschaert, and J. S. van der Hoeven. 1971. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch. Oral Biol. 16:971-975. [DOI] [PubMed] [Google Scholar]
- 14.Dionigi, C. P., D. F. Millie, and P. B. Johnsen. 1991. Effects of farnesol and the off-flavor derivative geosmin on Streptomyces tendae. Appl. Environ. Microbiol. 57:3429-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton, E. A., U. K. Walle, A. J. Lewis, T. Hudson, A. A. Wilson, and T. Walle. 1996. Flavonoids, potent inhibitors of the human p-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab. Dispos. 24:232-237. [PubMed] [Google Scholar]
- 16.Germaine, G. R., C. F. Schachtele, and A. M. Chludzinski. 1974. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J. Dent. Res. 53:1355-1360. [DOI] [PubMed] [Google Scholar]
- 17.Ghisalberti, E. L. 1979. Propolis: a review. Bee World 60:59-84. [Google Scholar]
- 18.Greenaway, W., T. Scaysbrook, and F. R. Whatley. 1990. The composition and plant origins of propolis: a report of work at Oxford. Bee World 71:107-118. [Google Scholar]
- 19.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanada, N., and H. K. Kuramitsu. 1988. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect. Immun. 56:1999-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanada, N., and H. K. Kuramitsu. 1989. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect. Immun. 57:2079-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey, A. 2000. Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 5:294-300. [DOI] [PubMed] [Google Scholar]
- 23.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotz, P., B. Guggenheim, and R. Schmid. 1972. Carbohydrates in pooled dental plaque. Caries Res. 6:103-121. [DOI] [PubMed] [Google Scholar]
- 25.Ikeno, K., T. Ikeno, and C. Miyazawa. 1991. Effects of propolis on dental caries in rats. Caries Res. 25:347-351. [DOI] [PubMed] [Google Scholar]
- 26.Kato, C., Y. Nakano, M. Lis, and H. K. Kuramitsu. 1992. Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem. Biophys. Res. Commun. 189:1184-1188. [DOI] [PubMed] [Google Scholar]
- 27.Koo, H., P. L. Rosalen, J. A. Cury, Y. K. Park, M. Ikegaki, and A. Sattler. 1999. Effect of Apis mellifera propolis from two Brazilian regions on caries development in desalivated rats. Caries Res. 33:393-400. [DOI] [PubMed] [Google Scholar]
- 28.Koo, H., B. P. F. A. Gomes, P. L. Rosalen, G. M. B. Ambrosano, Y. K. Park, and J. A. Cury. 2000. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch. Oral Biol. 45:141-148. [DOI] [PubMed] [Google Scholar]
- 29.Koo, H., P. L. Rosalen, J. A. Cury, G. M. B. Ambrosano, R. M. Murata, R. Yatsuda, M. Ikegaki, S. M. Alencar, and Y. K. Park. 2000. Effect of a new variety of Apis mellifera propolis on mutans streptococci. Curr. Microbiol. 41:192-196. [DOI] [PubMed] [Google Scholar]
- 30.Koo, H., A. M. Vacca Smith, W. H. Bowen, P. L. Rosalen, J. A. Cury, and Y. K. Park. 2000. Effects of Apis mellifera propolis on the activities of streptococcal glucosyltransferases in solution and adsorbed onto saliva-coated hydroxyapatite. Caries Res. 34:361-442. [DOI] [PubMed] [Google Scholar]
- 31.Kopec, L. K., A. M. Vacca-Smith, and W. H. Bowen. 1997. Structural aspects of glucans formed in solution and on the surface of hydroxyapatite. Glycobiology 7:929-934. [DOI] [PubMed] [Google Scholar]
- 32.Liang, Y.-C., Y.-T. Huang, S.-H. Tsai, S.-Y. Lin-Shiau, C.-F. Chen, and J.-K. Lin. 1999. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 20:1945-1952. [DOI] [PubMed] [Google Scholar]
- 33.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 34.McVean, M., H. Xiao, K. Isobe, and J. C. Pelling. 2000. Increase in wild-type p53 stability and transactional activity by the chemopreventive agent apigenin in keratinocytes. Carcinogenesis 21:633-639. [DOI] [PubMed] [Google Scholar]
- 35.Mooser, G., S. A. Hefta, R. J. Paxton, J. E. Shively, and T. D. Lee. 1991. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus α-glucosyltransferases. J. Biol. Chem. 266:8916-8922. [PubMed] [Google Scholar]
- 36.Morrisey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 37.National Committee for Clinical Laboratory Standards. 1992. Methods for determining bactericidal activity of antimicrobial agents. Tentative standard M26-T. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 38.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard. NCCLS publication no. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 39.Nyvad, B., and M. Kilian. 1987. Microbiology of the early colonization on human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:369-380. [DOI] [PubMed] [Google Scholar]
- 40.Park, Y. K., M. H. Koo, J. A. S. Abreu, M. Ikegaki, J. A. Cury, and P. L. Rosalen. 1998. Antimicrobial activity of propolis on oral microorganisms. Curr. Microbiol. 36:24-28. [DOI] [PubMed] [Google Scholar]
- 41.Rölla, G., J. E. Ciardi, K. Eggen, W. H. Bowen, and J. Afseth. 1983. Free glucosyl- and fructosyltransferase in human saliva and adsorption of these enzymes to teeth in vivo, p. 21-30. In R. J. Doyle and J. E. Ciardi (ed.), Glucosyltransferases, glucans, sucrose, and dental caries. Chemical Senses, IRL Press, Washington, D. C.
- 42.Scheie, A. A., K. H. Eggen, and G. Rölla. 1987. Glucosyltransferase activity in human in vivo formed pellicle and in whole saliva. Scand. J. Dent. Res. 95:212-215. [DOI] [PubMed] [Google Scholar]
- 43.Schilling, K. M., and W. H. Bowen. 1988. The activity of glucosyltransferase adsorbed onto saliva-coated hydroxyapatite. J. Dent. Res. 67:2-8. [DOI] [PubMed] [Google Scholar]
- 44.Schilling, K. M., and W. H. Bowen. 1992. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 60:284-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanzer, J. M., M. L. Freedman, and R. J. Fitzgerald. 1985. Virulence of mutants defective in glucosyltransferase, dextran-mediated aggregation, or dextranase activity, p. 204-211. In S. E. Mergenhagen and B. Rosan (ed.), Molecular basis of oral microbial adhesion. American Society for Microbiology, Washington, D.C.
- 46.Tazawa, S., T. Warashina, T. Noro, and T. Miyase. 1998. Studies on the constituents of Brazilian propolis. Chem. Pharm. Bull. 46:1477-1479. [Google Scholar]
- 47.Vacca-Smith, A. M., A. R. Venkitaraman, K. M. Schilling, and W. H. Bowen. 1996. Characterization of glucosyltransferase of human saliva adsorbed onto hydroxyapatite surfaces. Caries Res. 30:354-360. [DOI] [PubMed] [Google Scholar]
- 48.Vacca-Smith, A. M., and W. H. Bowen. 1997. Effects of some antiplaque agents on the activity of glucosyltransferases of Streptococcus mutans adsorbed onto saliva-coated hydroxyapatite and in solution. Biofilm J. 2. [Online.] http://www.bdt.org.br/bioline/bf.
- 49.Vacca-Smith, A. M., and W. H. Bowen. 1998. Binding properties of streptococcal glucosyltransferases for hydroxyapatite, saliva-coated hydroxyapatite, and bacterial surfaces. Arch. Oral Biol. 43:103-110. [DOI] [PubMed] [Google Scholar]
- 50.Vacca-Smith, A. M., L. Ng-Evans, D. Wunder, and W. H. Bowen. 2000. Studies concerning the glucosyltransferase of Streptococcus sanguis. Caries Res. 34:295-302. [DOI] [PubMed] [Google Scholar]
- 51.Venkitaraman, A. R., A. M. Vacca-Smith, L. K. Kopec, and W. H. Bowen. 1995. Characterization of glucosyltransferase B, GtfC, and GtfD in solution and on the surface of hydroxyapatite. J. Dent. Res. 74:1695-1701. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler, E. L., and D. L. Berry. 1986. In vitro inhibition of mouse epidermal cell lipoxygenase by flavonoids: structure-activity relationships. Carcinogenesis 7:33-36. [DOI] [PubMed] [Google Scholar]
- 53.Wunder, D., and W. H. Bowen. 1999. Action of agents on glucosyltransferases from Streptococcus mutans in solution and adsorbed to experimental pellicle. Arch. Oral Biol. 44:203-214. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita, Y., W. H. Bowen, R. A. Burne, and H. K. Kuramitsu. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 61:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]