Abstract
In vitro studies have demonstrated that water-soluble, nontoxic phosphoramidates of azidothymidine (zidovudine [AZT]) have significant and specific anti-human immunodeficiency virus and anticancer activity. Although polar, these compounds are internalized and processed to the corresponding nucleoside monophosphates. Eight methyl amide and methyl ester phosphoramidate monoesters composed of d- or l-phenylalanine or tryptophan and AZT were synthesized. The plasma stability and protein binding studies were carried out in vitro. Then in vivo pharmacokinetic evaluations of six of the compounds were conducted. Sprague-Dawley rats received each compound by intravenous bolus dose, and serial blood and urine samples were collected. AZT and phosphoramidate concentrations in plasma and urine were quantitated by high-performance liquid chromatography with UV or fluorescence detection. Pharmacokinetic parameters were calculated by standard noncompartmental means. The plasma half-lives of the phosphoramidates were 10- to 20-fold longer than the half-life of AZT. Although the renal clearances of the phosphoramidates were similar to AZT, their total body clearances were significantly greater than that of AZT. The 3- to 15-fold-larger volume of distribution (Vss) for the phosphoramidates relative to AZT appeared to be dependent on the stereochemistry of the amino acid, with the largest values being associated with the l-amino acids. The increased Vss indicates a much greater tissue distribution of the phosphoramidate prodrugs than of AZT. Amino acid phosphoramidate monoesters of AZT have improved pharmacokinetic properties over AZT and significant potential as in vivo pronucleotides.
Nucleoside analogs are a major class of therapeutically effective antiviral and anticancer agents. In general, to exert their biological activity these compounds must be sequentially phosphorylated by cellular kinases to the corresponding 5′ mono-, di-, and triphosphates (2). Although largely successful, nucleoside analog-based therapies suffer from a number of drawbacks. First, a decreased nucleoside kinase activity has been suggested as a mechanism by which cellular resistance to nucleosides can emerge (1). Related to this is the observation that many nucleoside analogs are poor substrates for cellular nucleoside kinases but have the potential to be biologically active if delivered as the corresponding monophosphate. Second, the utility of the nucleosides has been limited by their association with toxic side effects, such as anemia, peripheral neuropathy, and myelosuppression (11). Third, many nucleosides have poor in vivo pharmacokinetic properties since they are rapidly eliminated by a variety of mechanisms, thus limiting their tissue distribution and exposure. In addition, the polarity and biological instability of the nucleotides themselves have obviated their direct delivery (in vitro or in vivo) to the intracellular space. The search for a more efficacious way of delivering nucleotides continues to be a high priority.
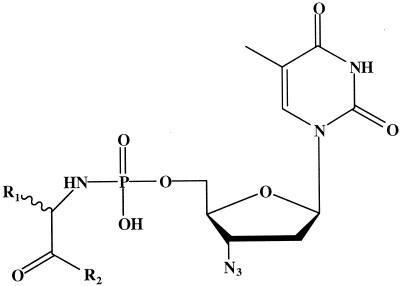
To improve the therapeutic efficacy and overcome the development of resistance against nucleoside analogs, a number of pronucleotide approaches have been developed (14). Among these strategies, the utility of nucleoside amino acid phosphoramidates has been investigated. In particular, our laboratory has shown that amino acid phosphoramidate monoesters of azidothymidine (zidovudine [AZT]) are (i) potent nontoxic antiviral and anti-breast cancer agents (3, 7, 8, 13, 15), (ii) indefinitely stable in cell medium and human plasma (13), and (iii) converted intracellularly to the corresponding triphosphates (3). Therefore, before examining their in vivo biological activity, the in vivo pharmacokinetics for a model set of AZT phosphoramidates were evaluated. Eight compounds (designated 1a, 1b, 2a, etc., through 4b) (Table 1) were selected for investigation due to their substantial in vitro antiviral or anticancer activity, and the influences of the amino acid side chain, the amide/ester moiety, and stereochemistry on in vivo pharmacokinetics were delineated.
TABLE 1.
Chemical structures of amino acid phosphoramidate monoester prodrugs of AZT
MATERIALS AND METHODS
Chemicals and reagents.
AZT, all AZT prodrugs (1a to 4b), and the free acids of the prodrugs (hydrolysis products of the ester prodrugs) were synthesized in Carston R. Wagner's laboratory at the University of Minnesota (3, 7). Rat plasma was obtained from Harlan Bioproducts for Sciences (Indianapolis, Ind.). Human plasma was purchased from the Biological Specialty Corporation (Colmar, Pa.). The human plasma was not heat inactivated. β-Glucuronidase (type IX-A, from Escherichia coli) was obtained from Sigma (St. Louis, Mo.). Acetonitrile and methanol (MeOH) (Fisher, Fair Lawn, N.J.) were high-performance liquid chromatography (HPLC) grade. All other reagents were of the highest available purity.
Analytical.
An HPLC system (Waters Associates, Milford, Mass.) was used for all quantitation and was equipped with a Waters model 600E multisolvent delivery system and controller, a Waters model 700 Satellite WISP autosampler, and Waters Millennium software (version 2.0). A Shimadzu (Columbia, Md.) model SPD10A UV detector or a Shimadzu model RF530 fluorescence detector was used. A Waters Spherisorb ODS2 analytical column (5μ, 4.6 by 250 mm), and a 3-cm ODS2 guard column was used. Details of the development and validation of analytical methods are reported elsewhere (12).
Stability study with rat and human plasma.
Stock solutions of prodrugs (1a to 4b) in 54 mM phosphate buffer (pH 7.4) were spiked into blank rat plasma to obtain the desired concentrations (2, 8, 10, 20, or 40 μM) and then were prewarmed at 37°C for 5 min. Samples of 100 μl (in triplicate) were withdrawn at various intervals up to 4 h for studies with the ester prodrugs or up to 24 h for the amide prodrugs. Plasma proteins were precipitated, and the supernatant was removed and evaporated to dryness. The residue was reconstituted in distilled water for HPLC analysis (12). The same procedure was followed with human plasma except that the incubations were extended to at least 4 days.
Both the disappearance of prodrugs and the formation of the free acids (if formed) were measured. The degradation rate constants were calculated by fitting the concentration remaining versus incubation time to a monoexponential equation to obtain k, the first-order degradation rate constant. The degradation half-lives (t1/2) of the prodrugs were determined from the rate constants (t1/2 = 0.693/k).
Analysis of variance (ANOVA) was performed on the degradation half-lives with concentration, amino acid side chain, and stereochemistry as main effects. Since multiple comparisons were carried out, a significance level of 0.025 was used.
Plasma protein binding study.
The protein binding of AZT and the AZT prodrugs (1a to 4a, 1b, and 3b) in rat plasma were determined by the method of ultrafiltration and compared to that of AZT itself. Centrifree Micropartition centrifuge tubes (Amicon, Bedford, Mass.) were used. The technique had been reported previously (10) and was adapted with minor modification. Stock solutions of AZT or prodrugs in 54 mM phosphate buffer of pH 7.4 were spiked into blank rat plasma to yield final concentrations of 2, 10, and 40 μM. The samples were prepared in triplicate. The fraction unbound in plasma (fu) was calculated according to the following equation: fu = [U/(1−NSB)]/T, where U was the free concentration, T was the total concentration, and NSB was a correction factor for nonspecific binding to the filter membrane of the Centrifree device (12). ANOVA was performed on the fu with concentration, ester/amide moiety, stereochemistry, and amino acid side chain as main effects. A P value of less than 0.05 was considered to be significant.
Pharmacokinetic study.
Normal female Sprague-Dawley rats (Harlan) weighing from 250 to 280 g were used in the pharmacokinetic study of the prodrugs (1a through 3b). The animal study was conducted in accordance with guidelines set forth in the University of Minnesota Animal Care and Use Manual. Three or four rats were used for each compound and each received a single intravenous (i.v.) bolus dose of 190 μmol/kg of body weight. In some rats, a crossover experiment with AZT (38 μmol/kg) was performed. The treatment order was chosen randomly, and the length of the washout period was determined by the half-life of the compound administered in the first phase.
Animals were allowed to acclimate to the facility for at least 3 days before the surgery. Femoral vein and artery cannulation was performed 24 to 48 h prior to the administration of the compounds (12). After the surgery, animals were kept in metabolic cages with food and water available ad libitum in a room with a 12-h light-dark cycle.
Dosing solutions were prepared by dissolving the compound of interest in sterile normal saline. On the day of study, 1 ml of the dosing solution was infused into the femoral vein over 1 min. Blood samples of 200 μl were drawn from the femoral arterial cannula at 0.25, 0.5, 0.75, 1, 2, 4, 8, 12, and 24 h postdosing. The blood volume was replaced with an equal volume of normal saline. Blood samples of 200 μl were placed into microcentrifuge tubes containing heparin and were immediately centrifuged at 13,000 × g for 2 min. The plasma was transferred to a labeled microcentrifuge tube and immediately frozen on dry ice. Urine samples were collected from the metabolism cage at 2, 4, 6, 8, 12, 20, and 24 h postdose. The volume was recorded, and then the urine was transferred into 8-ml plastic tubes and frozen on dry ice. Blank plasma and urine samples had also been collected before the study. Both plasma and urine samples were stored at −80°C before analysis.
Determination of pharmacokinetic parameters.
The individual pharmacokinetic parameters were calculated for each individual rat, and then the mean values were obtained by averaging the individual data. Noncompartmental methods were used to obtain the estimates of all the pharmacokinetic parameters (5).
Calculations for the pharmacokinetic parameters for the prodrugs.
AUCtot is the total area under the plasma concentration-time curve from time zero to infinity. The AUC0→last was estimated by the linear trapezoidal rule from time zero to the time of the last quantifiable concentration (tlast). From tlast to infinity, AUClast→∝ was estimated by dividing the last measurable plasma concentration in the terminal phase (Clast) by the terminal elimination rate constant (λ). One of two methods was used to obtain the estimate of λ. In cases where there were enough data points above the limit of quantitation in the terminal phase of the plasma concentration-time curve, λ was estimated by linear regression of the terminal monoexponential portion of the log concentration-time profile. In the cases where the plasma concentrations were below the limit of quantification in the terminal phase, urinary excretion data were used to estimate the terminal rate constant. KaleidaGraph (version 3.08d; Synergy Software) was used for the regression analysis.
Elimination half-life was determined by equation 1:
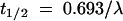 |
(1) |
The total body clearance of the prodrug was determined by dividing the dose of the prodrug (D) by the total AUC:
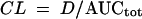 |
(2) |
The volume of distribution at steady state (Vss) was calculated with the use of the area under the moment curve (AUMC) and mean residence time (MRT) calculations (5).
The renal clearance (CLR) was calculated by the following equation:
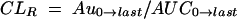 |
(3) |
where Au0→last is the total amount of the prodrug excreted in the urine from time zero to tlast. The fraction of the total dose excreted in the urine as the unchanged prodrug (fe) was calculated by the following equation:
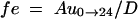 |
(4) |
In theory, Au0→∝ should be used to calculate fe, so the value of fe obtained from equation 4 was somewhat underestimated. However, such an underestimation was negligible since most of the urinary excretion occurred in the first 24 h.
Calculations for the pharmacokinetic parameters of metabolites from prodrugs.
The AUC0→last of any metabolite [AUC0→last(m)] could be calculated by the trapezoidal rule. If the metabolite was found in urine as well, then the renal clearance of that metabolite, CLR (m), was estimated by equation 3. Since AZT was identified in both plasma samples and urine samples after prodrug administration, the renal clearance of AZT (CLR (AZT)) in each individual rat was also obtained.
The fraction of the dose of prodrug that was converted to AZT (fm(AZT)) was calculated with the use of the total amount of AZT collected in the urine after administration of the prodrug, Au0→∞ (AZT). The total amount of AZT formed from prodrug is equal to the dose of the prodrug (D) multiplied by fm(AZT):
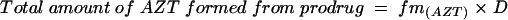 |
(5) |
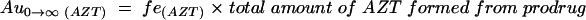 |
(6) |
Combining equations 5 and 6 gives
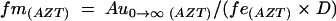 |
(7) |
where fe(AZT) is the ratio of the renal clearance of AZT and its total body clearance. This value of fe(AZT) was obtained from the crossover study when AZT was administered. Au0→∝ (AZT) was estimated for the calculation of fm(AZT) under the assumption that the excretion of AZT formed from the prodrug was essentially complete within 24 h. The underestimation of fm(AZT) by this approximation is negligible.
The mean values and standard deviations of all the pharmacokinetic parameters for each prodrug were calculated, and comparison among prodrugs and AZT was conducted by one-way ANOVA. A P value of less than 0.05 was considered to be statistically significant. The effects of the stereochemistry, the ester/amide moiety, and the amino acid side chain on the pharmacokinetic parameters were also determined with ANOVA.
RESULTS
Stability of AZT prodrugs in rat and human plasma.
In order to determine their stability in biological media, the rate of degradation of eight AZT phosphoramidates in rat and human plasma was investigated. No significant hydrolysis of the P-N or P-O bonds for the phosphoramidates was observed. However, for the methylester derivatives (1a through 4a), significant conversion to the respective free acid derivatives was observed in rat plasma. No other breakdown products were observed, and mass balance was obtained by summing the parent drug remaining and the acid appearing at each time point (12). The rate of methyl ester hydrolysis appeared to be amino acid side chain dependent (Table 2). In general, longer half-lives were observed for the tryptophan derivatives than for the phenylalanine derivatives. A marked species-dependent metabolism for the methylester derivatives was also observed. Methylester derivatives (1a through 4a) were stable in human plasma for >4 days but were slowly hydrolyzed in rat plasma. This difference is likely due to the higher level of carboxylesterase activity found in rodent plasma than in human plasma (9). The enzymatic basis for the observed hydrolysis is supported by observations that the hydrolysis of 3a in rat plasma was concentration dependent and that the methyl esters were indefinitely stable in the incubation buffer at pH 7.4 (12). In addition, the process appeared to be stereoselective since a threefold increase in stability was observed for the d-stereoisomer, 2a, over the l-stereoisomer, 1a. The stereochemical preference for the d-isomer, however, was not observed with the phenylalanine methylester derivatives. The methylester 3a, an l-isomer, was found to be twofold more stable to hydrolysis than its d-isomer counterpart (4a). In contrast to the methylesters, all of the methylamide derivatives were stable in rat and human plasma for a minimum of 1 and 4 days, respectively.
TABLE 2.
Stability and protein binding of AZT prodrugs in plasma
| Compound | Concn (μM) | Mean (SD) of stability half-life (h) in rat plasma | Stability in human plasma | Mean (SD)f of rat plasma fu |
|---|---|---|---|---|
| Ester prodrugs (Table 1 designation) | ||||
| l-Trp-AZT-OMe (1a) | 20 | 2.11 (0.35)a, b | 10% degraded after 4 days | 0.292 (0.062) |
| 8 | 2.09 (0.25) | 10% degraded after 4 days | ||
| l-Phe-AZT-OMe (3a) | 40 | 4.12 (0.17)a, b, c | 7% degraded after 4 days | 0.155 (0.029)g |
| 10 | 1.71 (0.04)a, c | 7% degraded after 4 days | ||
| d-Trp-AZT-OMe (2a) | 20 | 7.48 (0.49)a, b | Stabled | 0.155 (0.009) |
| d-Phe-AZT-OMe (4a) | 20 | 0.81 (0.1)a, b | Stabled | 0.064 (0.010) |
| Amide prodrugs | ||||
| l-Trp-AZT-NMe (1b) | 20 | Stablee | Stabled | 0.899 (0.108) |
| l-Phe-AZT-NMe (3b) | 20 | Stablee | Stabled | 0.934 (0.054) |
| d-Trp-AZT-NMe (2b) | 20 | Stablee | Stabled | ND |
| d-Phe-AZT-NMe (4b) | 20 | Stablee | Stabled | ND |
| AZT | NDh | ND | 0.832 (0.115)i |
Stability statistically different from that of its stereoisomer at a significance level of 0.025.
Stability statistically different from the prodrug with a different amino acid side chain at a significance level of 0.025.
Stability statistically different at different concentrations of the prodrugs at a significance level of 0.025.
No degradation for at least 4 days.
No degradation for at least 24 h.
The effects of the ester/amide moiety, stereochemistry, and amino acid side chain on plasma protein binding were found to be significant (P < 0.05, ANOVA); n = 9 unless otherwise noted.
n = 6.
ND, not determined.
n = 12.
Plasma protein binding of AZT prodrugs.
The values of fu for each prodrug and AZT are summarized in Table 2. Since there was no significant effect of concentration on protein binding for any of the prodrugs or AZT, the data were combined and a grand mean and standard deviation were reported. As reported in the literature (6), AZT was found to be approximately 80% unbound in rat plasma. At concentrations ranging from 2 to 40 μM, the ester-containing phosphoramidates (1a to 4a) were found to be 70 to 94% bound to plasma protein. The phenylalanine esters were more highly protein bound than the tryptophan derivatives; the l-phosphoramidates (1a and 3a) were in general less protein bound than the d-phosphoramidates (2a and 4a). In contrast to the ester prodrugs, only 6.6 to 10% of the amide-containing phosphoramidates were found to be bound to plasma proteins.
Pharmacokinetics of AZT prodrugs after a single i.v. bolus dose administration.
Ester prodrugs.
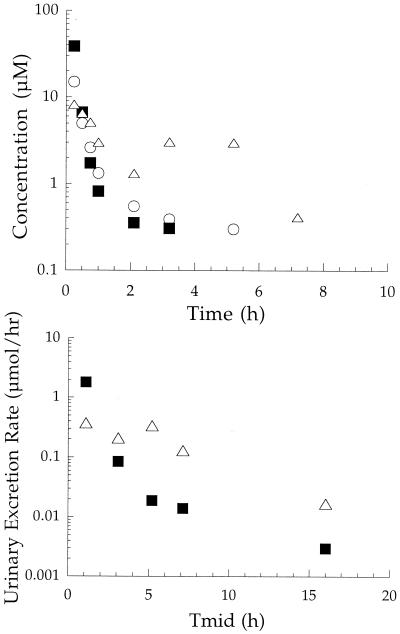
Typical plasma concentration-time and urinary excretion rate profiles of l-Trp-AZT-OMe (1a), after dosing an individual animal with 190 μmol/kg, are shown in Fig. 1. The pharmacokinetic parameter values for l-Trp-AZT-OMe (1a) are summarized in Table 3. Prodrug 1a was found in both plasma and urine samples. The plasma concentration of the prodrug declined in an apparent biexponential manner with a mean terminal half-life of 4.39 h. In urine, 16.3% of the total dose of 1a was recovered unchanged. As expected from the in vitro plasma stability study, the metabolism of 1a to its free acid was observed. The formation of the free acid was relatively rapid, but it was not excreted in the urine. However, AZT was found in both plasma and urine samples. On average, the conversion of 1a to AZT accounted for 19.1% of the total dose. The AZT plasma concentration-time profiles displayed a second peak in most animals.
FIG. 1.
Pharmacokinetic profiles of l-Trp-AZT-OMe (1a) in plasma (top) and urine (bottom) after a single i.v. bolus dose of 190 μmol/kg to an individual rat. Symbols: ▪, l-Trp-AZT-OMe; ○, free acid; ▵, AZT. Tmid, midpoint time of urine collection.
TABLE 3.
Summary of estimated pharmacokinetic parametersa of AZT prodrugs after single i.v. bolus doses of 190 μmol/kg in rats (or 38 μmol/kg for AZT)
| Parameter | l-Trp-AZT-OMe (1a) | d-Trp-AZT-OMe (2a) | l-Phe-AZT-OMe (3a) | l-Trp-AZT-NMe (1b) | d-Trp-AZT-NMe (2b) | l-Phe-AZT-NMe (3b) | AZT |
|---|---|---|---|---|---|---|---|
| CL (l/h/kg) | 5.80 (1.58)b, c | 3.07 (0.78)b, c | NDg | 5.74 (1.44)b, c | 3.24 (0.62)b, c | ND | 1.35 (0.24) |
| VSS (l/kg) | 3.50 (1.97)c | 0.59 (0.09)c | ND | 15.6 (6.0)b, c | 3.12 (1.94)c | ND | 0.87 (0.10) |
| CLR (l/h/kg) | 0.80 (0.38) | 0.48 (0.07) | 0.53 (0.40) | 0.84 (0.15) | 0.93 (0.27) | 0.57 (0.15) | |
| fe (%)e | 16.3 (9.4)b | 16.0 (4.42)b | 11.8 (4.6)b | 16.4 (5.6)b | 26.5 (5.3) | 22.4 (5.4)b | 41.3 (11.6) |
| fm(AZT) (%)e | 19.1 (9.59) | 16.3 (3.27) | 22.2 (4.97) | 12.1 (5.4) | 13.2 (9.3) | 10.2 (2.7) | |
| t1/2 (h) | 4.39 (0.65)b | 6.67 (2.08)b | 6.93 (1.74)b, f | 11.7 (0.82)b, c, d | 4.67 (1.98)b, c | 6.59 (0.71)b, d, f | 0.46 (0.11) |
| CLR (AZT) (l/h/kg) | 0.70 (0.19) | 0.65 (0.16) | 0.86 (0.28) | 0.66 (0.31) | 0.84 (0.22) | 0.97 (0.18) | 0.57 (0.23) |
| n | 4 | 3 | 3 | 4 | 4 | 3 | 4 |
Data are reported as means (SD).
Significantly different from that of AZT (one-way ANOVA, P < 0.05).
Significant stereochemical effect (ANOVA, P < 0.05).
Significant amino acid side chain effect (ANOVA, P < 0.05).
fe, fm multiplied by 100 to give the percentage of dose eliminated by the respective pathway.
Half-life determined from urinary excretion rate plot.
ND, not determined.
The pharmacokinetics of d-Trp-AZT-OMe (2a) were found to be similar to those of l-Trp-AZT-OMe (1a) and are summarized in Table 3. The plasma concentration-time profile of 2a was best described by two-compartment kinetics with a prolonged terminal half-life of 6.67 h. Of the total dose, 16% was recovered in the urine as the intact phosphoramidate 2a, and another 16% was found to have been converted to AZT. The AUC of the free acid formed from 2a was almost one-third of that of 2a, but no free acid was observed in the urine. The total body clearance (CL) and apparent volume of distribution at steady state (Vss) of 2a were found to be smaller than those for the corresponding l-isomer, 1a (ANOVA, P < 0.05).
The pharmacokinetics of l-Phe-AZT-OMe (3a) were also similar to those of the l-tryptophan derivative, 2a, in terms of the pattern of the plasma concentration of metabolites. One hour after dosing, however, the plasma level of the prodrug was too low to be quantified. Therefore, the CL and volume of tissue distribution of 3a could not be accurately determined since the terminal phase in plasma could not be observed. The renal clearance was estimated based on the plasma and urine data obtained over the first hour. Based on urine data, the half-life of the terminal phase for 3a was estimated to be 6.93 h. The percentage of the total dose of 3a converted to AZT or excreted in the urine unchanged was not statistically different from that observed for the l-tryptophan phosphoramidate (Table 3). The free acid of 3a was not detected in the urine.
Amide prodrugs.
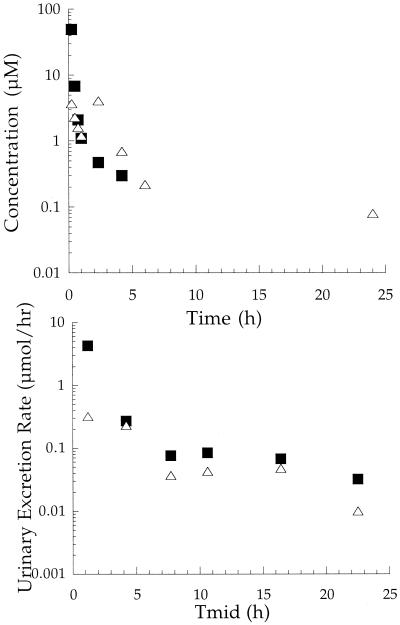
Typical plasma concentration-time and urinary excretion rate profiles of l-Trp-AZT-NMe (1b) after a dosing of an individual animal with 190 μmol/kg are shown in Fig. 2. The plasma concentration-time profile of 1b declined in an apparent biexponential manner, with a long terminal half-life of 11.7 h (Table 3), which was 2.7-fold greater than the terminal half-life for its methylester counterpart, 1a. Both 1b and AZT were found in plasma and urine samples. The conversion to AZT is likely to have occurred after the distribution of 1b into tissues and tissue space since the amide phosphoramidates were found to be indefinitely stable in plasma (Table 2). Unlike the ester prodrugs, the free acid of 1b was not found in plasma or urine samples. Out of the total dose of 1b administered, 16.4% was recovered in the urine unchanged and 12.1% had been converted to AZT. In addition, similar to what was seen with the ester phosphoramidates, the plasma concentration-time profile of AZT was characterized by a second peak.
FIG. 2.
Pharmacokinetic profiles of l-Trp-AZT-NMe (1b) in plasma (top) and urine (bottom) after a single i.v. bolus dose of 190 μmol/kg to an individual rat. Symbols: ▪, l-Trp-AZT-NMe; ▵, AZT. Tmid, midpoint time of urine collection.
After dosing, the plasma concentration-time profiles of d-Trp-AZT-NMe (2b) and d-Phe-AZT-NMe (3b) were similar to that found for l-Trp-AZT-NMe (1b). However the half-life of the terminal phase for 1b was found to be significantly longer (P < 0.05) than the terminal-phase half-lives for 2b and 3b, respectively (Table 3). Of the total dose administered, 26.5 and 22.4% of 2b and 3b, respectively, were recovered in urine; 13.2 and 10.2% of 2b and 3b, respectively, were found have been converted to AZT. Within 2 h of dosing, the plasma concentration of 3b dropped below the limit of quantification, so the CL and Vss could not be determined, but the elimination half-life could be determined from the urinary excretion rate plot.
DISCUSSION
The pharmacokinetics of phosphoramidate-based pronucleotides had not been studied in detail. Consequently, the in vitro stability and plasma protein binding and the in vivo pharmacokinetics of a set of closely related amino acid nucleoside phosphoramidate monoesters were examined in the present study.
Significant differences in the in vitro stability and protein binding behavior of the ester and amide phosphoramidates were observed. The ester phosphoramidates were hydrolyzed, presumably by plasma esterases, to their corresponding free acids and were much more highly protein bound than their amide counterparts. The degree of hydrolysis and plasma protein binding of the ester phosphoramidates were shown to be chemical structure and concentration dependent, although not in predictable ways. The ester derivatives were quite stable in human plasma (less than 10% degradation after 4 days), but their degradation in rat plasma prompted a recognition of this potential metabolic pathway in rats dosed in vivo with ester-based phosphoramidates. Amide-containing phosphoramidates were shown to be highly stable in rat and human plasma and were largely unbound to plasma proteins.
Based on the in vitro studies alone, the amide phosphoramidates had more-promising stability and protein binding profiles. Since 4a was the least stable and most highly bound prodrug, it was dropped from further consideration. In addition, its amide counterpart 4b was not studied further.
Pharmacokinetic studies of the phosphoramidates in rats were carried out with l-and d-tryptophan and l-phenylalanine methyl ester and methyl amide phosphoramidate monoesters of AZT (1a and b through 3a and b). A biexponential decline in plasma concentration was observed for all of the phosphoramidates after a single i.v. bolus dose. The plasma concentration-time profiles were characterized by a prolonged terminal half-life compared to that of AZT. Among the phosphoramidates studied, the longest terminal half-life (i.e., 11.7 h) was observed for the l-tryptophan methyl amide phosphoramidate of AZT (1b). About 10 to 25% of the total dose of the phosphoramidates was excreted into the urine unchanged. The absolute values of renal clearance were found to be significantly greater than the product of the free fraction of the phosphoramidates in rat plasma (fu) and the glomerular filtration rate in rats (4), suggesting that active secretion was likely to be the major mechanism for renal clearance, in addition to glomerular filtration.
Regardless of the phosphoramidate dosed, AZT was the common metabolite found in both plasma and urine samples. After a single i.v. bolus dose, 10 to 20% of a given phosphoramidate dose was converted to AZT. AZT could be generated from the prodrugs directly inside the cell through the P-O bond breakage or formed from AZT monophosphate through P-N bond cleavage (3, 8). Further experiments incorporating labeled phosphate should clarify the mechanism of AZT formation.
Although the mechanism for in vivo generation of AZT from the phosphoramidates is currently unknown, a second peak in the plasma concentration profile of AZT was observed after i.v. dosing of the rats with the phosphoramidates. The AZT phosphoramidates, while found to be highly secreted into bile in rats, do not appear to be reabsorbed intact due to low intestinal permeability (12). Consequently, degradation of the phosphoramidates of AZT in the gastrointestinal tract with subsequent AZT absorption may account for the second AZT plasma peak.
Consistent with the stability studies conducted with rat plasma, the free acids of the phosphoramidates were also found as major in vivo metabolites of the ester phosphoramidates (1a, 2a, and 3a), but not the amide phosphoramidates (1b, 2b, and 3b). The free acid metabolites of the compounds were identified in plasma samples only and not in urine. Neither were urinary glucuronides detected from β-glucuronidase incubations of urine samples (data not shown). The elimination via the bile of the phosphoramidates or metabolites is apparently a significant pathway of removal from the body (12).
Substantial differences were observed between the pharmacokinetics of AZT and the phosphoramidates (Table 3). The CL of the AZT phosphoramidates was at least three times higher than that of AZT in rats. The renal clearances of the phosphoramidates were not significantly different from that of AZT, so their nonrenal clearances were considerably greater than that of AZT. This is reflected in the significantly lower fraction of dose excreted unchanged in urine (fe). Since the phosphoramidate clearances from elimination pathways other than renal excretion were close to the hepatic blood flow rate of 3.3 liter/h/kg for rats (4), the phosphoramidates can be considered high-clearance drugs. However, the volume of distribution (Vss) of the AZT prodrugs was significantly increased compared to that of AZT, suggesting that the phosphoramidates have greater access to tissue space. In general, the increase in the Vss more than compensated for the increase in CL, resulting in significantly longer half-lives than that of AZT. This may indicate that an amino acid phosphoramidate pronucleotide strategy may be useful for the tissue delivery of nucleotides. Tissue distribution studies with radiolabeled phosphoramidates will identify the tissue space or organ into which the compounds are distributed.
The pharmacokinetic differences between the phosphoramidates and AZT were generally greater than the differences among the phosphoramidates. Among the phosphoramidates studied here, the overall pharmacokinetics of the AZT phosphoramidates were affected by chemical structure to various degrees. The methylamide phosphoramidates of AZT were more stable than the methylesters in vivo and in vitro with no free acid generated by the methyl amides. For ester phosphoramidates, the change from l-tryptophan (1a) to l-phenylalanine (3a) generally had no effects on pharmacokinetics, while replacement of the l-tryptophan (1a) with d-tryptophan (2a) significantly reduced the Vss. The high tissue distribution of the l-tryptophan derivative (1a) relative to that of the d-isomer (2a) may be partially explained by its lower plasma protein binding behavior (Table 2). A similar decrease in the Vss values was observed for the d-tryptophan amide derivative (2b) when compared to the l-tryptophan derivative (1b). The renal clearance was unaffected by the differences in the chemical structures of the phosphoramidates. In addition, the fraction of the phosphoramidate excreted unchanged in the urine and the fraction metabolized to AZT were not influenced by chemical structure.
Overall it appeared that the methylesters were less stable in vitro and in vivo. They generated a significant amount of free acid, which would decrease the intracellular delivery of the AZT monophosphate because of the additional negative charge. In general, the d-isomers had smaller volumes of distribution, presumably due to their increased plasma protein binding. This would be associated with less tissue penetration. Based on its in vitro stability and protein binding and its significantly larger Vss and longer half-life, l-Trp-AZT-NMe (1b) was selected for further evaluation (12).
In summary, the pharmacokinetics of phosphoramidate monoesters of AZT are significantly different from those of AZT. In particular, the volume of distribution is increased, suggesting a substantial increase in tissue exposure and a prolonged biological half-life. AZT and the phosphoramidates have similar renal clearances, but nonrenal clearance pathways are more important for the phosphoramidates. Among the phosphoramidates, the most important influence of changes in chemical structure appeared to be on the volume of distribution, leading to significant changes in biological half-life. Ongoing evaluation of the in vivo antiviral and anti-breast cancer studies should help determine the relationship between the pharmacokinetics and efficacy of amino acid phosphoramidates of AZT.
Acknowledgments
This study was supported in part by a grant from Advanced Magnetics, Inc. (C.R.W.), the University of Minnesota Graduate School, and the International Student Work Opportunity Program fellowship (H.S.) from the University of Minnesota.
This work was submitted in partial fulfillment of the requirements for a Ph.D. at the University of Minnesota (H.S.).
REFERENCES
- 1.Antonelli, G., O. Turriziani, A. Verri, P. Narciso, F. Ferri, G. D'Offizi, and F. Dianzini. 1996. Long-term exposure to zidovudine affects in vitro and in vivo the efficiency of phosphorylation of thymidine kinase. AIDS Res. Hum. Retrovir. 12:223-228. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J. 1994. Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16:113-126. [DOI] [PubMed] [Google Scholar]
- 3.Chang, S.-L., G. W. Griesgraber, P. J. Southern, and C. R. Wagner. 2001. Amino acid phosphoramidate monoesters of 3′-azido-3′-deoxythymidine: the relationship between antiviral potency and cellular decomposition. J. Med. Chem. 44:223-231. [DOI] [PubMed] [Google Scholar]
- 4.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 5.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, p. 409-417, 2nd ed. Marcel Dekker, New York, N.Y.
- 6.Good, S. S., and P. de Miranda. 1992. Species differences in the metabolism and disposition of antiviral nucleoside analogues. II. Zidovudine. Antivir. Chem. Chemother. 3:65-77. [Google Scholar]
- 7.Iyer, V. V., G. W. Griesgraber, M. R. Radmer, E. J. McIntee, and C. R. Wagner. 2000. Synthesis, in vitro anti-breast cancer activity and intracellular decomposition of amino acid methyl ester and alkyl amide phosphoramidate monoesters of 3′-azido-3′-deoxythymidine (AZT). J. Med. Chem. 43:2266-2274. [DOI] [PubMed] [Google Scholar]
- 8.McIntee, E. J., R. P. Remmel, R. F. Schinazi, T. W. Abraham, and C. R. Wagner. 1997. Probing the mechanism of action and decomposition of amino acid phosphomonoester amidates of antiviral nucleoside prodrugs. J. Med. Chem. 40:3323-3331. [DOI] [PubMed] [Google Scholar]
- 9.Minagawa, T., Y. Kohno, T. Suwa, and A. Tsuji. 1995. Species differences in hydrolysis of isocarbacyclin methyl ester (TEI-9090) by blood esterases. Biochem. Pharmacol. 49:1361-1365. [DOI] [PubMed] [Google Scholar]
- 10.Roumi, M., S. Marleau, M. Boghen, M. Nilsson, P. Du Souich, and H. Ong. 1997. Hepatic extraction of hexarelin, a new peptidic GH secretagogue, in the isolated perfused rat liver. Pharm. Res. 14:1008-1013. [DOI] [PubMed] [Google Scholar]
- 11.Sommadossi, J. P. 1993. Nucleoside analogs: similarities and differences. Clin. Infect. Dis. 16:S7-S15. [DOI] [PubMed] [Google Scholar]
- 12.Song, H. 2000. Influence of chemical structure on pharmacokinetics of amino acid phosphoramidate prodrugs of AZT-monophosphate in rats. Ph.D. thesis. University of Minnesota, Minneapolis.
- 13.Wagner, C. R., S.-L. Chang, G. W. Griesgraber, H. Song, E. J. McIntee, and C. L. Zimmerman. 1999. Antiviral nucleoside drug delivery via amino acid phosphoramidates. Nucleosides Nucleotides 18:913-919. [DOI] [PubMed] [Google Scholar]
- 14.Wagner, C. R., V. V. Iyer, and E. J. McIntee. 2000. Pronucleotides: toward the in vivo delivery of antiviral and anticancer nucleotides. Med. Res. Rev. 20:417-451. [DOI] [PubMed] [Google Scholar]
- 15.Wagner, C. R., E. J. McIntee, R. F. Schinazi, and T. W. Abraham. 1995. Aromatic amino acid phosphoramidate di- and triesters of 3′-azido-3′-deoxythymidine (AZT) are non-toxic inhibitors of HIV-1 replication. Bioorg. Med. Chem. Lett. 5:1819-1824. [Google Scholar]