Abstract
The prophylactic and therapeutic activities of two fluoroquinolones, levofloxacin and alatrofloxacin (the l-Ala-l-Ala prodrug of trovafloxacin), were compared to those of vancomycin in two different experimental models of foreign-body-associated infections caused by methicillin-resistant but quinolone-susceptible Staphylococcus aureus (MRSA) isolates. In a guinea pig model of prophylaxis, subcutaneously implanted tissue cages were infected with 103 CFU of MRSA, which was a 100% infectious dose in control animals. A single dose of 50 mg of levofloxacin per kg of body weight, administered intraperitoneally 3 h before bacterial challenge, was more efficient than vancomycin for the prevention of infections in tissue cages with MRSA inocula of 104 and 105 CFU. In a rat model used to evaluate therapy of chronic tissue cage infection caused by MRSA, the efficacies of 7-day high-dose regimens of levofloxacin (100 mg/kg once a day [q.d.] or 50 mg/kg twice a day [b.i.d.]) or alatrofloxacin (50 mg/kg q.d.) were compared to the efficacy of vancomycin (50 mg/kg b.i.d.). Active levels of levofloxacin, trovafloxacin, and vancomycin were continuously present in tissue cage fluid, with the levels exceeding the minimal bactericidal concentrations for MRSA during therapy. The q.d. and b.i.d. regimens of levofloxacin had equivalent activities and were significantly (P < 0.05) more active than alatrofloxacin or vancomycin in decreasing the viable counts of MRSA in tissue cage fluids. No quinolone-resistant mutants emerged during therapy with either fluoroquinolone. The mechanisms explaining the inferior activity of alatrofloxacin compared to the activity of levofloxacin against chronic foreign-body-associated infections by MRSA are unknown.
Antimicrobial therapy of prosthetic device infections, in particular, those due to Staphylococcus aureus, is notoriously difficult, and microbial eradication frequently requires the removal of infected materials. Several clinical and experimental studies have reported on the therapeutic values of the fluoroquinolones ciprofloxacin, ofloxacin, or pefloxacin, alone or in combination with rifampin, against serious S. aureus infections (8-10, 24, 44, 46). In particular, some very interesting results were recently reported from studies with either ciprofloxacin (44, 46) or ofloxacin (8) in combination with rifampin for the treatment of orthopedic prosthetic device-associated S. aureus infections (without prosthesis removal). Newer molecules of the quinolone family such as levofloxacin, trovafloxacin, gatifloxacin, or moxifloxacin not only demonstrate enhanced in vitro activities and broader spectra of activity against most important gram-positive bacterial pathogens, but they are also endowed with optimized pharmacokinetic and pharmacodynamic properties (for a review, see reference 22).
Unfortunately, strains of methicillin-resistant S. aureus (MRSA) (32, 33, 39) are generally resistant to ciprofloxacin and all newer fluoroquinolones, which severely limits the therapeutic armamentarium (42) for the treatment of foreign-body-associated infections. Thus, the glycopeptides vancomycin and teicoplanin, alone or in combination with rifampin (2, 17, 31), may remain the only therapies available for the treatment of severe MRSA infections. Furthermore, combination therapy does not always prevent the emergence of rifampin-resistant mutants (1, 11, 12, 39).
The aim of our experimental study was to evaluate the efficacy of levofloxacin or alatrofloxacin in either a prophylactic (40, 45) or a therapeutic (29) model of foreign-body-associated infections caused by a quinolone-susceptible strain of MRSA. Levofloxacin is the l isomer of ofloxacin and is available both in an oral dosage form and as an intravenous preparation, while alatrofloxacin is the l-Ala-l-Ala prodrug of trovafloxacin used for parenteral administration. We previously showed the usefulness of both guinea pig and rat tissue cage models for evaluating a range of different antimicrobial agents including vancomycin and teicoplanin (37), imipenem (36), and the fluoroquinolones fleroxacin (4, 29) and sparfloxacin, temafloxacin, and ciprofloxacin (5), alone or in combination with rifampin (7, 29, 37), against S. aureus foreign-body-associated infections.
(This study was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, September 2000 [Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 993, 2000].)
MATERIALS AND METHODS
Bacterial strains.
MRSA MRGR3 (5, 7, 29) was used in both animal models. It was isolated from a patient with catheter-related sepsis in 1979 and was selected for its virulence properties in animal models of tissue cage infections. Strain MRGR3 is heterogeneously resistant to methicillin but remains uniformly susceptible to all fluoroquinolones (5, 7, 29). Strain MRGR3 has additional determinants for resistance to penicillin, gentamicin, chloramphenicol, erythromycin, tetracycline, and polymyxin B.
Antimicrobial agents.
For in vitro and in vivo studies, a 5-mg/ml injectable solution of levofloxacin hemihydrate was provided by Aventis Pharma (Zürich, Switzerland). A standard powder of trovafloxacin for the in vitro studies and the l-Ala-l-Ala prodrug of trovafloxacin, alatrofloxacin (CP-116,517-27) mesylate, for the in vivo studies were provided by Pfizer Inc. (Groton, Conn.) Alatrofloxacin mesylate used for parenteral administration is rapidly hydrolyzed in serum to form trovafloxacin. For the in vivo studies, 1 g of alatrofloxacin was first solubilized in 5 ml of dimethyl sulfoxide and was then further diluted with 245 ml of 0.23% NaCl to a final concentration of 4 mg per ml. Commercially available vancomycin hydrochloride (Lilly, Giessen, Germany) was solubilized as recommended by the manufacturer.
In vitro studies.
The MICs of each agent for MRSA MRGR3 were determined in cation-adjusted Mueller-Hinton broth (MHB; Difco, Detroit, Mich.) by the standard tube macrodilution method with an average inoculum of 106 CFU/ml (30). To screen for the possible carryover effects of each antibiotic during determinations of the minimal bactericidal concentrations (MBCs), 100-μl portions were taken from all tubes with no visible growth. These were subcultured, either undiluted or diluted 10-fold in saline, on Mueller-Hinton agar (MHA; Difco) for 36 h at 37°C. The MBC was defined as the lowest concentration that killed 99.9% of the original inoculum.
Killing kinetic studies.
Sterile plastic tubes containing 1 ml of MHB with either 1 or 0.25 μg of levofloxacin or trovafloxacin per ml, respectively, were incubated with 106 CFU of MRSA MRGR3 (obtained from exponential-phase cultures) in a shaking water bath at 37°C. The number of viable organisms was determined by subculture of 50 μl of 10-fold serially diluted portions of broth on MHA after 0, 1, 3, 6, and 24 h of incubation. The bacteria were plated, and the colonies were counted with a laser colony counter (Spiral System) after 48 h of incubation at 37°C. The detection limit was 2 log10 CFU/ml. No significant carryover of antibiotics was observed by using these experimental conditions. To evaluate the impacts of tissue cage fluid proteins on the bactericidal activities of either 1 or 0.25 μg of levofloxacin or trovafloxacin per ml, respectively, the rate of elimination of strain MRGR3 from tubes containing 1 ml of a mixture of MHB and pooled sterile tissue cage fluid in a 1:1 ratio was also recorded.
To evaluate the susceptibility to trovafloxacin of MRSA MRGR3 recovered from infected tissue cage fluids, the bacteria were isolated from the tissue cage fluids by centrifugation and were treated with 0.1% Triton X-100 and sonication to disrupt the host cells, as described previously (37). This procedure is used to reduce bacterial clumping and was previously shown to be harmless for ex vivo bacteria regarding their ability to multiply and their susceptibilities to antibiotics (37). To compare the rate of elimination of strain MRGR3 recovered from tissue cage fluids with that of the same strain grown in vitro, tissue cage bacteria were directly exposed to 0.25 μg of trovafloxacin per ml in tubes containing 1 ml of either plain MHB or MHB supplemented with 50% pooled tissue cage fluid. To make the comparison with ex vivo bacteria more relevant, bacteria grown in vitro were taken from saline-washed cultures of stationary-phase organisms (37).
Prophylaxis of tissue cage infections.
Four multiperforated polytetrafluorethylene (Teflon) tissue cages, each of which contained three polymethylmethacrylate coverslips (7 by 7 mm), were implanted subcutaneously in guinea pigs under aseptic conditions, as previously described in detail (4, 45). The purpose of inserting coverslips in the tissue cages is to detect a very low residual level of infection (detection limit, 1 CFU) after prophylaxis. At 3 weeks after implantation, tissue cage fluids were aseptically aspirated and were checked for sterility.
To study the prevention of experimental infection by the antimicrobial agents, a single dose of either levofloxacin (50 mg/kg of body weight), alatrofloxacin (50 mg/kg), or vancomycin (50 mg/kg) was administered intraperitoneally to guinea pigs 3 h before inoculation of MRSA into each tissue cage. This lag time was necessary to obtain bactericidal levels of each antimicrobial agent in each tissue cage at the time of inoculation with 0.1 ml of saline containing either 103, 104, or 105 CFU of strain MRGR3, which represented serial 10-fold dilutions of a log-phase culture in MHB. The tissue cages of an additional control group of guinea pigs received an injection of 103 CFU of strain MRGR3, which represented a 100% infectious dose.
At 24 h, 48 h, and 7 days after the local injection of strain MRGR3, quantitative cultures were performed by plating 100 μl of serially 10-fold-diluted tissue cage fluid specimens on MHA; the detection limit was 102 CFU/ml. At day 7, the cages were removed and the coverslips were cultured in MHB at 37°C for 7 days; the detection limit was 1 CFU per coverslip (37).
The numbers of tissue cage fluid specimens and coverslips protected by each antimicrobial agent from infection by an identical number of inoculated organisms were compared by Fisher's two-tailed (two-by-two) exact probability test with Bonferroni's correction for multiple comparisons. P values <0.05 were considered significant.
Treatment of chronic tissue cage infections.
Four tissue cages were implanted subcutaneously into rats as described previously (29). At 3 weeks after implantation, tissue cage fluid was aspirated and was checked for sterility. To establish a chronic local MRSA infection, the tissue cages were inoculated with 0.1 ml of saline containing 0.2 × 106 to 2 × 106 CFU of a log-phase culture of strain MRGR3. Two weeks later, all tissue cages containing more than 105 CFU/ml of fluid were included in the therapeutic protocols.
Rats infected with strain MRGR3 were randomized to receive (by the intraperitoneal route for 7 days) either twice-a-day (b.i.d.) regimens of either levofloxacin (50 mg/kg) or vancomycin (50 mg/kg) or once-a-day (q.d.) regimens of either levofloxacin (100 mg/kg) or alatrofloxacin mesylate (50 mg/kg) or were left untreated.
At 12 h after the last injection of levofloxacin and vancomycin b.i.d. or 24 h after the last injection of levofloxacin or alatrofloxacin q.d., quantitative cultures of serially 10-fold-diluted tissue cage fluid were performed on MHA. Possible bacterial clumps were disrupted by sonication (60 W, 1 min) before they were plated. Quantitative bacterial counts were determined (detection limit, 102 CFU/ml) and were expressed as log10 CFU per milliliter. The differences in CFU counts between day 1 and day 8 were determined and expressed as delta log10 CFU per milliliter. For each treatment group, the results were expressed as the means ± standard errors of the means. Comparison of the bacterial counts in the different groups was performed by one-way analysis of variance and the Newman-Keuls multiple-comparisons procedure. Data were considered significant when P was <0.05 by using two-tailed significance levels.
Resistance to antimicrobial agents.
The bacteria recovered from the tissue cage fluids on day 8 were screened for the emergence of resistance to levofloxacin or trovafloxacin: 100-μl samples of 10-fold-diluted tissue cage fluid or sonicated coverslips were plated onto MHA containing each fluoroquinolone at fourfold the MIC for MRSA strain MRGR3. The plates were incubated for 48 h at 37°C. The detection limit was 2 log10 CFU per ml of tissue cage fluid.
Pharmacokinetics of antimicrobial agents.
The pharmacokinetic properties of vancomycin in guinea pig (4) or rat (29) tissue cage fluids have been estimated previously.
In guinea pigs, the concentrations of levofloxacin and trovafloxacin in tissue cage fluid were measured by a bioassay at various time intervals (3, 6, 12, and 24 h) after intraperitoneal administration of a single dose of 50 mg of antimicrobial agent per kg.
In rats treated with the different regimens of levofloxacin and alatrofloxacin, the pharmacokinetics of each antimicrobial agent in tissue cage fluids were also determined by a bioassay at various time intervals (1, 3, 6, 12, and 24 h) on day 4 (to allow the equilibrium concentration of each agent to be achieved) and day 7 of therapy.
To evaluate the concentrations of levofloxacin and trovafloxacin in guinea pig or rat tissue cage fluid, we used a bioassay with Escherichia coli 1346 as the test strain (4). All standard curves for determination of the concentrations in tissue cage fluids of trovafloxacin, which is highly bound to rat serum protein components (14), were performed in phosphate-buffered saline (PBS) supplemented with 50% tissue cage fluid. In contrast, systematic supplementation of PBS with 50% tissue cage fluid for determination of levofloxacin concentrations in tissue cage fluids was omitted because the standard curves of the zone sizes versus the natural logarithm of the drug concentration were identical in the presence or absence of 50% tissue cage fluid (data not shown).
In both guinea pig and rat models, the areas under the concentration-time curve (AUCs) from 0 to 24 h (AUC0-24s) for each fluoroquinolone were estimated after administration of each antimicrobial agent by the linear trapezoidal rule.
Determination of antibiotic binding to tissue cage fluid and serum proteins.
The binding of trovafloxacin and levofloxacin to sterile pooled tissue cage fluid and serum proteins of rats was determined by ultrafiltration. One-milliliter protein-containing samples or PBS-buffered controls containing 5 μg of trovafloxacin or levofloxacin per ml were filtered through a centrifugal filter (molecular weight cutoff, 10,000) device (Ultrafree; Millipore Corp., Bedford, Mass.). Each fluoroquinolone tested for protein binding was solubilized in PBS supplemented with 50% rat tissue cage fluid or serum. The free trovafloxacin and levofloxacin concentrations in the ultrafiltrates were determined by the bioassay described above. For each fluoroquinolone, the percentage of free drug in 50% tissue cage fluid or serum was expressed as a function of that assayed in the ultrafiltrate of the PBS-buffered control.
RESULTS
MICs and MBCs from in vitro studies.
The MICs and MBCs of levofloxacin, trovafloxacin, and vancomycin for MRSA MRGR3 were 0.12 and 0.25, 0.03 and 0.06, and 1 and 2 μg/ml, respectively. Time-kill studies performed in MHB showed rapid elimination of exponential-phase cultures of strain MRGR3 grown in vitro by either 1 or 0.25 μg of either levofloxacin or trovafloxacin per ml, respectively; these concentrations represent four times the MBCs of the drugs for MRSA. The reductions in the viable counts of strain MRGR3 by levofloxacin or trovafloxacin exceeded 3 log10 CFU/ml after 3 h (data not shown). Similar reductions in the viable counts of strain MRGR3 by either 1 or 0.25 μg of either levofloxacin or trovafloxacin per ml were observed in MHB supplemented with 50% tissue cage fluid (data not shown).
Prophylaxis of tissue cage infections.
In untreated animals, 12 of 12 tissue cages challenged with 103 CFU of MRSA MRGR3 developed infection, with bacterial counts exceeding 104 CFU/ml of fluid at 24 h or later. These rates of tissue cage infections were similar to those recorded in previous studies (5, 29, 37).
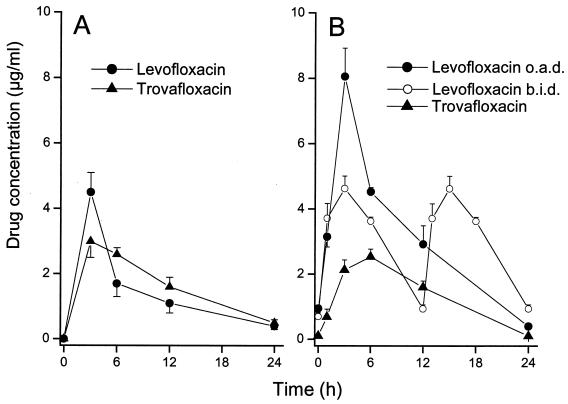
The mean peak and residual levels of levofloxacin and trovafloxacin in tissue cage fluids (n = 4) were 4.5 and 3.0 μg/ml, respectively, at 3 h (time of bacterial inoculation) and 0.4 and 0.5 μg/ml, respectively, at 24 h. Thus, a single prophylactic dose of each fluoroquinolone produced bactericidal levels in tissue cage fluid for the next 24-h period. In comparison, the mean concentrations of vancomycin in tissue cage fluid determined in a previous study (4) were 7.1, 12.2, and 2.0 μg/ml at 3, 6, and 24 h, respectively. The tissue cage fluid AUC0-24 for trovafloxacin was 37.3 mg · h/liter, being slightly higher than that for levofloxacin, which was 33.9 mg · h/liter.
Table 1 shows that both levofloxacin and alatrofloxacin reduced the colony counts below the detection limit of 102 CFU/ml of tissue cage fluid within 48 h in all tissue cages challenged with either 103 (n = 12) or 104 (n = 12) CFU of MRSA MRGR3. Both fluoroquinolones were significantly (P < 0.05) more efficient than vancomycin in reducing colony counts below the detection limit at 48 h in tissue cages (n = 12) challenged with 105 CFU of strain MRGR3. At 7 days, however, a significant proportion of tissue cage fluids that were scored as culture negative at 48 h showed evidence of bacterial regrowth. For each prophylactic regimen, the proportion of tissue cage fluid samples yielding bacterial regrowth from 48 h to 7 days increased as a function of increased levels of bacterial challenge (Table 1). However, 50% of tissue cage fluids challenged with 105 CFU (n = 12) of strain MRGR3 were still culture negative at 7 days for levofloxacin-treated animals, while <8% (0 of 12) of tissue cage fluids were protected from MRSA infection for vancomycin-treated animals (P < 0.05). The differences between levofloxacin- and vancomycin-treated animals were even more impressive when the percentages of culture-negative coverslips (limit of detection, 1 CFU) were compared for both treatment groups. While 67 and 50% of the coverslips from levofloxacin-treated animals challenged with either 104 or 105 CFU of MRGR3, respectively, were scored as culture negative, none of the coverslips from vancomycin-treated animals challenged with either inoculum was protected from infection (Table 1).
TABLE 1.
Comparison of levofloxacin, alatrofloxacin, and vancomycin in the prophylactic treatment of tissue cage infection caused by S. aureus MRGR3
| Antibiotic (dose [mg/kg]) and no. of S. aureus CFU inoculated | No. of culture-negative samplesa/no. of samples analyzed (%) after:
|
||
|---|---|---|---|
| 48 h, fluid | 7 days
|
||
| Fluid | Coverslip | ||
| None (control), 103 | 0/12 (0) | 0/12 (0) | 0/12 (0) |
| Levofloxacin (50) | |||
| 103 | 12/12 (100) | 8/12 (67) | 8/12 (67) |
| 104 | 12/12 (100) | 8/12 (67) | 8/12 (67) |
| 105 | 11/12 (92) | 6/12 (50) | 6/12 (50) |
| Alatrofloxacin (50) | |||
| 103 | 12/12 (100) | 12/12 (100) | 9/12 (75) |
| 104 | 12/12 (100) | 8/12 (67) | 3/12 (25) |
| 105 | 9/12 (75) | 2/12 (17) | 1/12 (8) |
| Vancomycin (50) | |||
| 103 | 12/12 (100) | 12/12 (100) | 8/12 (67) |
| 104 | 9/12 (75) | 3/12 (25) | 0/12b (0) |
| 105 | 0/12c (25) | 0/12b (0) | 0/12b (0) |
The limits of detection were 102 CFU/ml and 1 CFU for tissue cage fluids and coverslips, respectively.
P < 0.05 for levofloxacin versus vancomycin.
P < 0.05 for levofloxacin or alatrofloxacin versus vancomycin
Treatment of chronic tissue cage infections.
At day 4 of therapy, the mean peak and residual levels of levofloxacin and trovafloxacin in tissue cage fluids (n = 6) of animals treated with q.d. regimens were 8.1 and 2.5 μg/ml and 0.4 and 0.1 (limit of detection) μg/ml, respectively (Fig. 1B). In comparison, average peak and residual levels in the tissue cage fluids of animals treated with levofloxacin b.i.d. were 4.6 and 0.9 μg/ml, respectively. Similar concentrations of either fluoroquinolone were recorded at day 7 of therapy (data not shown). Since residual levels of levofloxacin and trovafloxacin were significantly higher than their respective MBCs, these fluoroquinolones were present in tissue cage fluids at concentrations constantly exceeding their MBCs for the test strain. At day 4 of therapy, the tissue cage fluid AUC0-24 was 74.6 mg · h/liter for levofloxacin administered q.d., 72.5 mg·h/liter for levofloxacin administered b.i.d., and 31.9 mg·h/liter for trovafloxacin.
FIG. 1.
Levels of levofloxacin and trovafloxacin in tissue cage fluid of guinea pigs that received a single 50-mg/kg dose of antimicrobial agents (A) or rats on day 4 of b.i.d. therapy with 50 mg of levofloxacin per kg or q.d. (o.a.d.) therapy with either 100 mg of levofloxacin per kg or 50 mg of alatrofloxacin per kg (B).
The average peak and trough levels of vancomycin in tissue cage fluid determined in a previous study (29) were 12 and 2 μg/ml at 4 and 12 h, respectively.
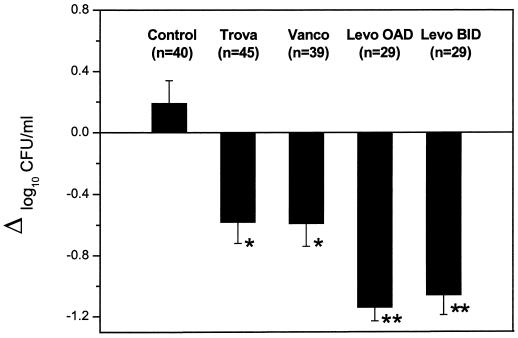
At the onset of therapy, average bacterial counts for 129 tissue cages infected with MRSA strain MRGR3 were 7.30 ± 0.13 log10 CFU/ml for controls (n = 40), 7.28 ± 0.12 log10 CFU/ml for animals receiving levofloxacin q.d. (n = 29), 7.22 ± 0.12 log10 CFU/ml for animals receiving levofloxacin b.i.d. (n = 29), 7.19 ± 0.11 log10 CFU/ml for animals receiving alatrofloxacin (n = 45), and 7.50 ± 0.08 log10 CFU/ml for animals receiving vancomycin (n = 39). At the end of the 7-day treatment period, bacterial counts in the tissue cages of control animals showed a slight and nonsignificant increase of 0.19 ± 0.15 log10 CFU/ml (n = 40). In contrast, both the q.d. and b.i.d. levofloxacin regimens (Fig. 2) led to significant reductions in bacterial counts in tissue cage fluids of 1.14 ± 0.09 (n = 29) and 1.06 ± 0.13 log10 (n = 29) CFU/ml, respectively. In comparison, the alatrofloxacin and vancomycin regimens led to significantly (P < 0.05) lower reductions in bacterial counts in tissue cage fluids of 0.58 ± 0.14 (n = 45) and 0.59 ± 0.15 log10 (n = 39) CFU/ml, respectively.
FIG. 2.
Decrease in viable counts of S. aureus MRGR3 in tissue cage fluids of rats treated with the different regimens for 7 days. Abbreviations: Trova, trovafloxacin; Vanco, vancomycin; Levo OAD, levofloxacin q.d.; Levo BID, levofloxacin b.i.d.
Screening of resistant organisms during therapy.
The potential emergence of quinolone-resistant mutants during therapy of chronic tissue cage infections by MRSA strain MRGR3 was studied. No MRGR3 isolates resistant to either levofloxacin or trovafloxacin were recovered from the tissue cage fluids of animals treated with any of the fluoroquinolone regimens for 7 days.
Activity of trovafloxacin against bacteria grown in vitro and in vivo.
The lower level of in vivo activity of trovafloxacin against chronic tissue cage infection compared to those of both the q.d. and the b.i.d. regimens of levofloxacin might be explained by several factors, such as the differential protein binding of each fluoroquinolone, the selectively altered susceptibility of tissue cage bacteria to killing by either antibiotic, and/or the differential extra- and intraleukocytic bactericidal activities of each antimicrobial agent.
Determination of drug-protein binding by ultrafiltration indicated that trovafloxacin was more highly bound to sterile tissue cage fluid (ca. 90%) and serum (>90%) proteins than levofloxacin, whose level of binding to these rat protein components (ca. 30%) was quite low.
The activity of 0.25 μg of trovafloxacin per ml against bacteria recovered from chronically infected rat tissue cage fluids was also evaluated in unsupplemented MHB or MHB supplemented with 50% sterile tissue cage fluid. The reduction in viable counts of tissue cage bacteria reached 2 and 3 log10 CFU/ml at 6 and 24 h, respectively, in both tissue cage fluid-supplemented and unsupplemented MHB, whereas the decrease for stationary-phase organisms grown in vitro was 3 log10 CFU/ml at 3 h in both cage fluid-supplemented and unsupplemented MHB (data not shown). Overall, these data demonstrate that tissue cage fluid components do not severely interfere with the activity of trovafloxacin, even against organisms recovered from the in vivo milieu, which could explain the inferior activity of this fluoroquinolone compared to that of levofloxacin in the model of chronic tissue cage infections.
DISCUSSION
In the last decade, the in vivo activities of fluoroquinolones against a wide spectrum of bacterial infections have been extensively studied (22). The agents most frequently studied for the treatment of acute or chronic fluoroquinolone-susceptible S. aureus infections were ciprofloxacin, ofloxacin, and pefloxacin. These agents proved effective for the treatment of skin and soft tissue infections (20) and osteomyelitis, septic arthritis, and implant-related infections (8, 21, 27, 28, 44, 46). In contrast to the aforementioned fluoroquinolones, the most recently developed fluoroquinolones that have activities against gram-positive organisms and that are endowed with optimized pharmacokinetic properties and tissue penetration following oral or intravenous administration have mostly been targeted toward microbial pathogens of major respiratory tract infections other than S. aureus (22). In addition, the ongoing increase in hospital strains of MRSA exhibiting resistance to several antibiotics including fluoroquinolones has strongly restricted comparative clinical studies testing the efficacies of newer quinolones against deep-seated or foreign-body-associated S. aureus infections (22).
Experimental models of S. aureus aortic-valve endocarditis in rats (13, 14) or rabbits (3, 6, 23, 26) assessed the efficacies of levofloxacin (6, 13) and trovafloxacin (3, 14, 23, 26) for the treatment of such life-threatening infections. The in vivo activities of the newer fluoroquinolones were generally compared with those of ciprofloxacin (13, 14, 26), vancomycin (3, 6, 13, 14, 23, 26), or beta-lactams (3, 6, 13, 14, 26). Two comparative studies demonstrated that levofloxacin had improved in vivo efficacy over that of ciprofloxacin (13) in a rat model of S. aureus endocarditis or a murine model of acute hematogenous pyelonephritis (19). In the first study, the improved in vivo activity of levofloxacin compared to that of ciprofloxacin was explained by a significantly lower rate of emergence of staphylococcal variants with decreased susceptibilities to fluoroquinolones (13). Additional studies confirmed that ciprofloxacin was more prone than levofloxacin (6, 15) or trovafloxacin (14, 16, 23) to select for quinolone-resistant derivatives of S. aureus in vitro or in vivo.
A useful property of subcutaneous tissue cage models of implant-associated infections due to S. aureus (5, 7, 29, 36, 37) or S. epidermidis (38, 43) is the possibility of the direct assessment of the levels of each antimicrobial agent in tissue cage fluids. This allows one to make direct estimates of the tissue cage concentration-time profile of each antimicrobial agent used for prophylaxis or therapy in an extravascular compartment. In recent years, pharmacodynamic modeling of the therapeutic efficacies of antimicrobial agents has been developed as powerful tool that combines the pharmacokinetic properties of each agent with the antimicrobial susceptibilities of its microbial targets (25, 41). The pharmacodynamic aspects of fluoroquinolone therapy, in particular, ciprofloxacin and levofloxacin therapy, have been extensively studied and have indicated that the plasma AUC/MIC ratios, maximum concentration of drug in serum (Cmax)/MIC ratios, and site of infection are the most important predictors of successful clinical and microbiological outcomes (18, 34, 35). To some extent, the AUC/MIC and Cmax/MIC ratios extrapolated from the local concentration of either levofloxacin or trovafloxacin in tissue cage fluids of rats with respect to the low MIC of either quinolone for strain MRGR3 seemed to be significantly higher than the breakpoints predicting successful outcomes of therapy with fluoroquinolones in pharmacodynamic studies with human patients (34, 35). However, another important aspect of the bactericidal activities of levofloxacin or trovafloxacin recorded in infected tissue cage fluids is their relatively poor efficacies, as scored by the marginal elimination of tissue cage bacteria even after 7 days of intensive therapy, in contrast to the rapid rates of elimination of the same organisms grown in vitro by equivalent antibiotic concentrations.
A number of local factors such as the differential protein binding of each fluoroquinolone, the selectively altered susceptibility of tissue cage bacteria to killing by either antibiotic, and/or the differential extra- and intraleukocytic bactericidal activities of each antimicrobial agent might explain the disappointing activities of these antibiotics. While the presence of sterile tissue cage fluid proteins did not significantly influence the bactericidal activity of levofloxacin, tissue cage fluid components moderately antagonized the killing effect of trovafloxacin against strain MRGR3 either collected from a stationary-phase culture or freshly removed from infected tissue cage fluids. Indeed, the bactericidal activity of trovafloxacin at a low concentration (0.25 μg/ml) in the presence of 50% sterile tissue cage fluid, equivalent to four times its MBC for log-phase organisms in unsupplemented MHB, was adequate for the elimination of tissue cage bacteria. Another factor potentially explaining the in vivo activities of levofloxacin compared to those of trovafloxacin might be a selectively increased binding of the latter fluoroquinolone compared to that of the former fluoroquinolone by some unknown local tissue cage fluid components specifically triggered by the chronic infection process. However, this hypothesis is difficult to address experimentally, in particular because infected tissue cage fluids have variable but frequently abundant contents of inflammatory cells such as polymorphonuclear neutrophils. Thus, both fluid-phase and cellular components of infected tissue cages might contribute to the binding and inactivation of different fluoroquinolones in a selective manner, as was also recorded in a previous study (5).
Further studies are required to evaluate the roles of additional parameters that might explain the significant differences in activities between levofloxacin and trovafloxacin against tissue cage S. aureus infections. We are lacking comparative studies with which to evaluate the intracellular antibacterial activity of trovafloxacin versus that of levofloxacin against S. aureus organisms ingested by granulocytes. Finally, the susceptibility to either fluoroquinolone of surface-attached organisms compared to that of fluid-phase organisms in chronically infected tissue cages might also be considered a potential contributor to the superior in vivo efficacy of levofloxacin compared to that of trovafloxacin.
In conclusion, the data presented here further emphasize the value of performing experiments with animals for the primary evaluation of new therapeutic agents. In particular, comparative studies of different fluoroquinolones with similar in vitro activities but different in vivo efficacies in pertinent experimental models may help investigators discover important parameters influencing their in vivo activities.
Acknowledgments
This work was supported in part by research grants from Aventis Pharma (Zürich, Switzerland) and Pfizer and grants 3200-63710.00 (to P.V.) and 632-57950.99 (to J.S.) from the Swiss National Foundation.
We thank Manuela Bento for technical assistance and José Entenza for useful advice.
REFERENCES
- 1.Acar, J. F., F. W. Goldstein, and J. Duval. 1983. Use of rifampin for the treatment of serious staphylococcal and gram-negative bacillary infections. Rev. Infect. Dis. 5(Suppl. 3):S502-SS506. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, A. S., and K. Lam. 1985. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J. Infect. Dis. 151:157-165. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, A. S., C. Li, and M. Ing. 1998. Efficacy of trovafloxacin, a new quinolone antibiotic, in experimental staphylococcal endocarditis due to oxacillin-resistant strains. Antimicrob. Agents Chemother. 42:1837-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchenaki, N., P. Vaudaux, E. Huggler, F. A. Waldvogel, and D. P. Lew. 1990. Successful single-dose prophylaxis of Staphylococcus aureus foreign body infection in guinea pigs by fleroxacin. Antimicrob. Agents Chemother. 34:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagni, A., C. Chuard, P. Vaudaux, J. Schrenzel, and D. P. Lew. 1995. Comparison of sparfloxacin, temafloxacin, and ciprofloxacin for prophylaxis and treatment of experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 39:1655-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, H. F., Q. Xiang, Q. Liu, L. L. Chow, and C. Hackbarth. 1999. Efficacy of levofloxacin for experimental aortic-valve endocarditis in rabbits infected with viridans group streptococcus or Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2742-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuard, C., M. Herrmann, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1991. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob. Agents Chemother. 35:2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drancourt, M., A. Stein, J. N. Argenson, A. Zannier, G. Curvale, and D. Raoult. 1993. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob. Agents Chemother. 37:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin, R., G. Modin, S. Kunz, R. Rich, O. Zak, and M. Sande. 1990. Comparative efficacies of ciprofloxacin, pefloxacin, and vancomycin in combination with rifampin in a rat model of methicillin-resistant Staphylococcus aureus chronic osteomyelitis. Antimicrob. Agents Chemother. 34:1014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dworkin, R. J., B. L. Lee, M.A. Sande, and H. F. Chambers. 1989. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet ii:1071-1073. [DOI] [PubMed] [Google Scholar]
- 11.Eng, R. H., S. M. Smith, F. J. Buccini, and C. E. Cherubin. 1985. Differences in ability of cell-wall antibiotics to suppress emergence of rifampicin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 15:201-207. [DOI] [PubMed] [Google Scholar]
- 12.Eng, R. H., S. M. Smith, M. Tillem, and C. Cherubin. 1985. Rifampin resistance. Development during the therapy of methicillin-resistant Staphylococcus aureus infection. Arch. Intern. Med. 145:146-148. [DOI] [PubMed] [Google Scholar]
- 13.Entenza, J. M., J. Vouillamoz, M. P. Glauser, and P. Moreillon. 1997. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41:1662-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entenza, J. M., J. Vouillamoz, M. P. Glauser, and P. Moreillon. 1999. Efficacy of trovafloxacin in treatment of experimental staphylococcal or streptococcal endocarditis. Antimicrob. Agents Chemother. 43:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, M. E., and W. B. Titlow. 1998. Levofloxacin selects fluoroquinolone-resistant methicillin-resistant Staphylococcus aureus less frequently than ciprofloxacin. J. Antimicrob. Chemother. 41:285-288. [DOI] [PubMed] [Google Scholar]
- 16.Evans, M. E., and W. B. Titlow. 1998. Selection of fluoroquinolone-resistant methicillin-resistant Staphylococcus aureus with ciprofloxacin and trovafloxacin. Antimicrob. Agents Chemother. 42:727.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faville, R. J., Jr., D. E. Zaske, E. L. Kaplan, K. Crossley, L. D. Sabath, and P. G. Quie. 1978. Staphylococcus aureus endocarditis. Combined therapy with vancomycin and rifampin. JAMA 240:1963-1965. [DOI] [PubMed] [Google Scholar]
- 18.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frosco, M. B., J. L. Melton, F. P. Stewart, B. A. Kulwich, L. Licata, and J. F. Barrett. 1996. In vivo efficacies of levofloxacin and ciprofloxacin in acute murine hematogenous pyelonephritis induced by methicillin-susceptible and -resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 40:2529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentry, L. O. 1993. Treatment of skin and soft tissue infections with quinolone antimicrobial agents, p. 413-422. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents. American Society for Microbiology, Washington, D.C.
- 21.Greenberg, R. N., M. T. Newman, S. Shariaty, and R. W. Pectol. 2000. Ciprofloxacin, lomefloxacin, or levofloxacin as treatment for chronic osteomyelitis. Antimicrob. Agents Chemother. 44:164-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper, D. C. 2000. New uses for new and old quinolones and the challenge of resistance. Clin. Infect. Dis. 30:243-254. [DOI] [PubMed] [Google Scholar]
- 23.Kaatz, G. W., S. M. Seo, J. R. Aeschlimann, H. H. Houlihan, R. C. Mercier, and M. J. Rybak. 1998. Efficacy of trovafloxacin against experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 42:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaatz, G. W., S. M. Seo, S. L. Barriere, L. M. Albrecht, and M. J. Rybak. 1989. Ciprofloxacin and rifampin, alone and in combination, for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 33:1184-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karabalut, N., and G. L. Drusano. 1993. Pharmacokinetics of the quinolone antimicrobial agents, p. 195-223. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents. American Society for Microbiology, Washington, D.C.
- 26.Kim, Y. S., Q. Liu, L. L. Chow, H. F. Chambers, and M. G. Tauber. 1998. Comparative efficacy of trovafloxacin in experimental endocarditis caused by ciprofloxacin-sensitive, methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 42:3325-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew, D. P., and F. A. Waldvogel. 1993. Use of quinolones for treatment of osteomyelitis and septic arthritis, p. 371-379. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents. American Society for Microbiology, Washington, D.C.
- 28.Lew, D. P., and F.A. Waldvogel. 1999. Use of quinolones in osteomyelitis and infected orthopaedic prosthesis. Drugs 58:85-91. [DOI] [PubMed] [Google Scholar]
- 29.Lucet, J. C., M. Herrmann, P. Rohner, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1990. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:2312-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.Norden, C. W., and M. Shaffer. 1983. Treatment of experimental chronic osteomyelitis due to Staphylococcus aureus with vancomycin and rifampin. J. Infect. Dis. 147:352-357. [DOI] [PubMed] [Google Scholar]
- 32.Peterson, L. R. 1993. Quinolone resistance in clinical practice: occurrence and importance, p. 119-137. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents. American Society for Microbiology, Washington, D.C.
- 33.Peterson, L. R., J. N. Quick, B. Jensen, S. Homann, S. Johnson, J. Tenquist, C. Shanholtzer, R. A. Petzel, L. Sinn, and D. N. Gerding. 1990. Emergence of ciprofloxacin resistance in nosocomial methicillin-resistant Staphylococcus aureus isolates. Resistance during ciprofloxacin plus rifampin therapy for methicillin-resistant S. aureus colonization. Arch. Intern. Med. 150:2151-2155. [PubMed] [Google Scholar]
- 34.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 35.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, F. A. Wong, and M. Corrado. 1998. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob. Agents Chemother. 42:1098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaad, H. J., C. Chuard, P. Vaudaux, P. Rohner, F. A. Waldvogel, and D. P. Lew. 1994. Comparative efficacies of imipenem, oxacillin and vancomycin for therapy of chronic foreign body infection due to methicillin-susceptible and -resistant Staphylococcus aureus. J. Antimicrob. Chemother. 33:1191-1200. [DOI] [PubMed] [Google Scholar]
- 37.Schaad, H. J., C. Chuard, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1994. Teicoplanin alone or combined with rifampin compared with vancomycin for prophylaxis and treatment of experimental foreign body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 38:1703-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwank, S., Z. Rajacic, W. Zimmerli, and J. Blaser. 1998. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob. Agents Chemother. 42:895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tebas, P., R. Martinez Ruiz, F. Roman, P. Mendaza, J. C. Rodriguez Diaz, R. Daza, and J. M. de Letona. 1991. Early resistance to rifampin and ciprofloxacin in the treatment of right-sided Staphylococcus aureus endocarditis. J. Infect. Dis. 163:204-205. [DOI] [PubMed] [Google Scholar]
- 40.Tshefu, K., W. Zimmerli, and F. A. Waldvogel. 1983. Short-term administration of rifampin in the prevention or eradication of infection due to foreign bodies. Rev. Infect. Dis. 5(Suppl.):S474-S480. [DOI] [PubMed] [Google Scholar]
- 41.Turnidge, J. D. 1991. Prediction of antibiotic dosing intervals from in vitro susceptibility, pharmacokinetics and post-antibiotic effect: theoretical considerations. Scand. J. Infect. Dis. Suppl. 74:137-141. [PubMed] [Google Scholar]
- 42.Wenzel, R. P., M. D. Nettleman, R. N. Jones, and M. A. Pfaller. 1991. Methicillin-resistant Staphylococcus aureus: implications for the 1990s and effective control measures. Am. J. Med. 91:221S-227S. [DOI] [PubMed] [Google Scholar]
- 43.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96-102. [DOI] [PubMed] [Google Scholar]
- 44.Widmer, A. F., A. Gaechter, P. E. Ochsner, and W. Zimmerli. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 14:1251-1253. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerli, W., F. A. Waldvogel, P. Vaudaux, and U. E. Nydegger. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146:487-497. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, and P. E. Ochsner. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections--a randomized controlled trial. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]