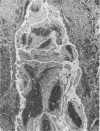
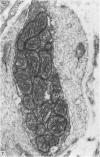
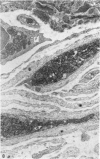
Full text
PDF

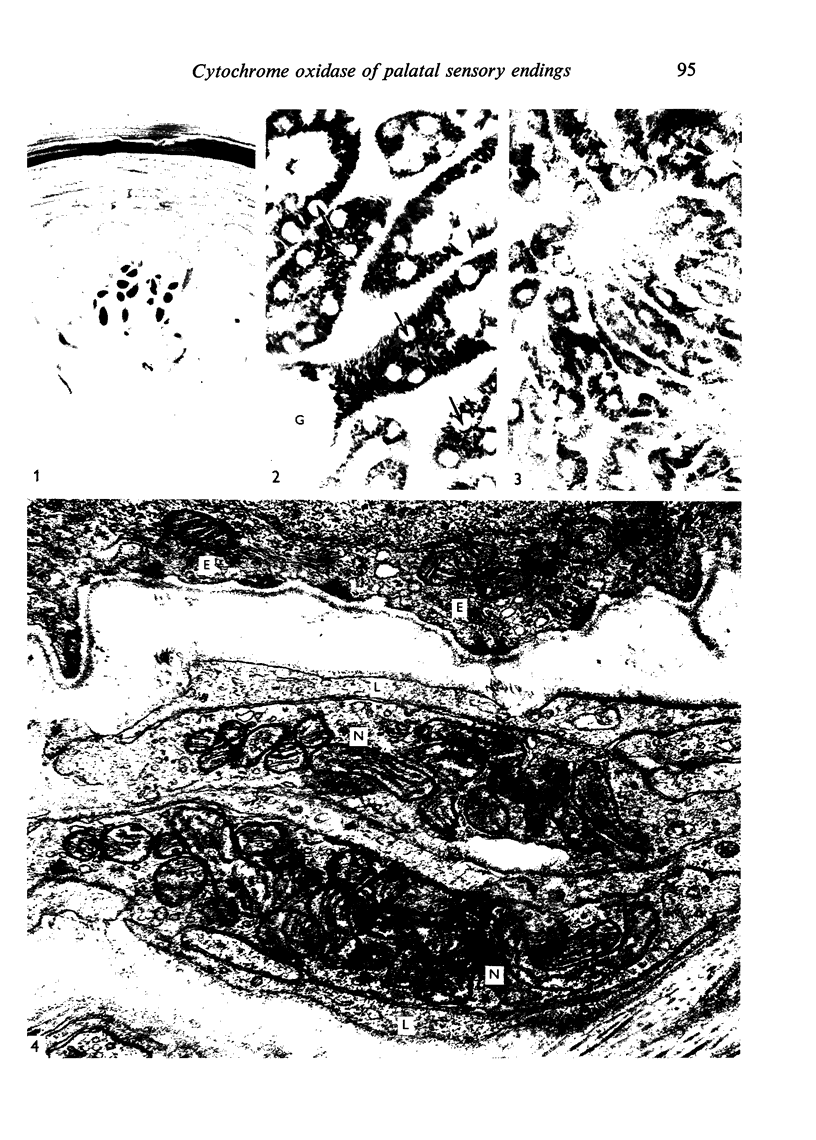

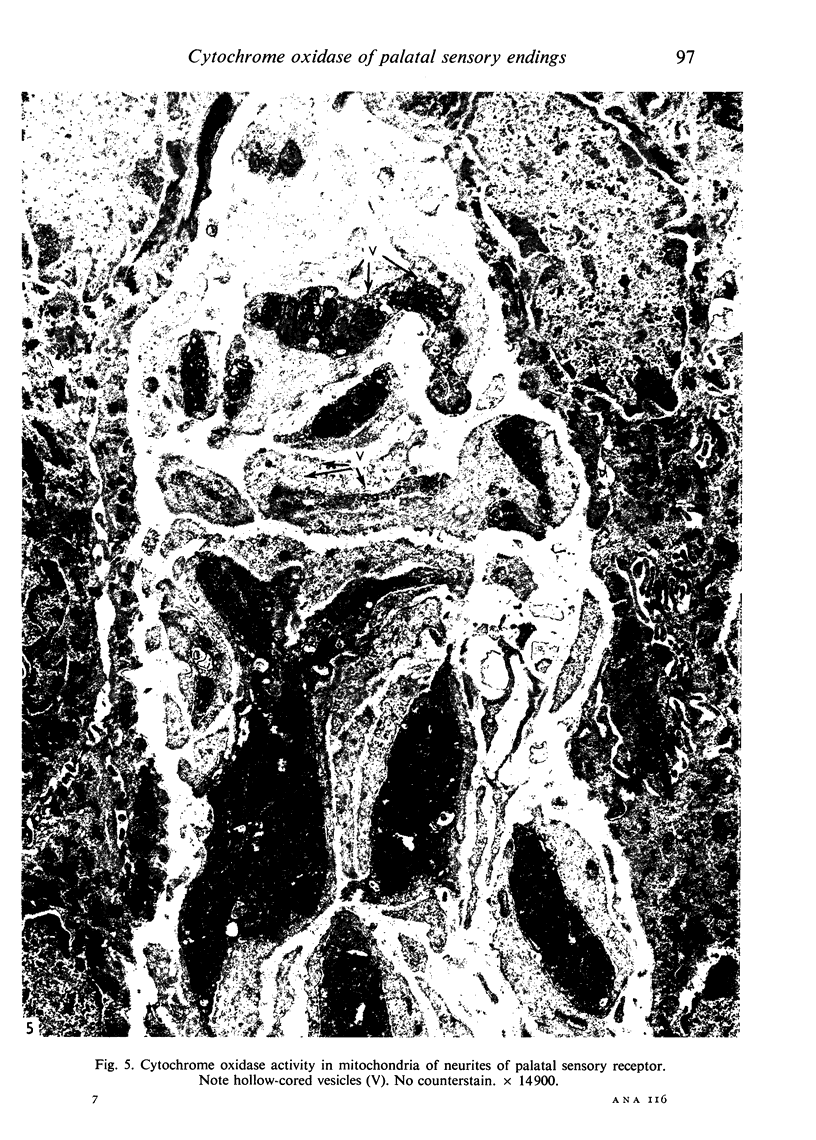

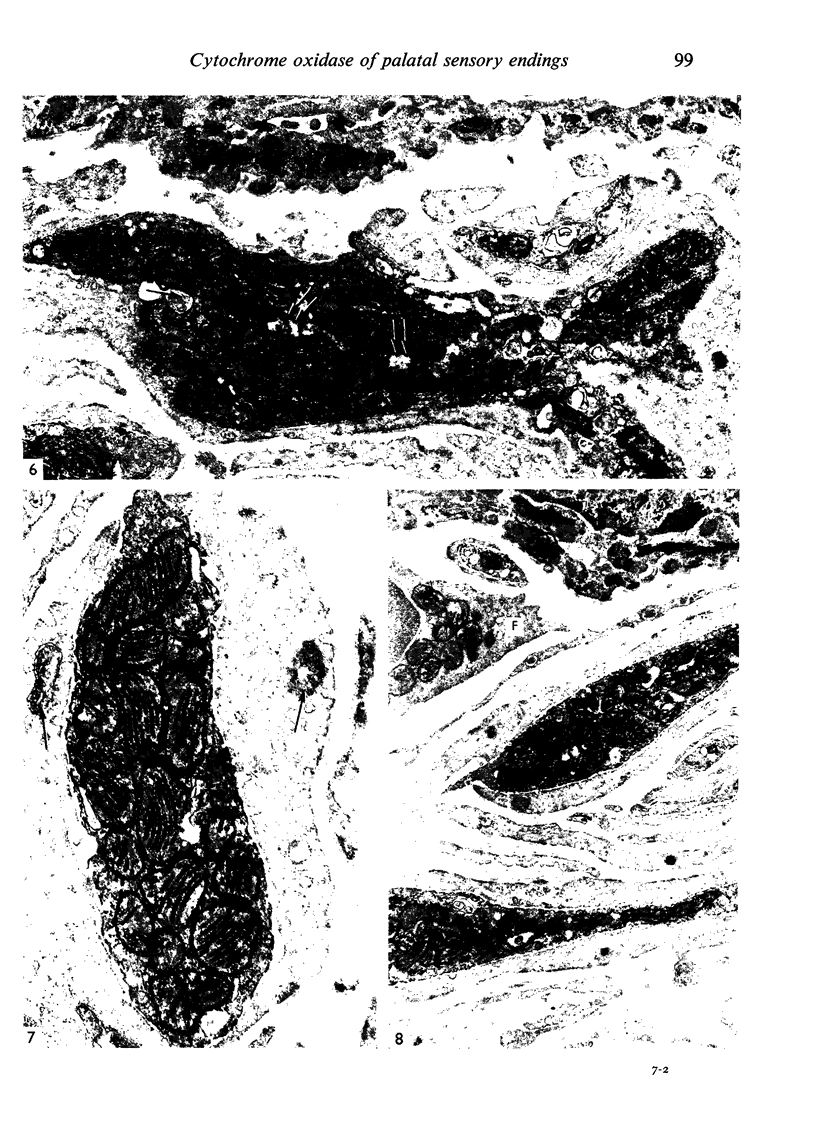



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEICHMAR H., DE ROBERTIS E. Submicroscopic morphology of the infrared receptor of pit vipers. Z Zellforsch Mikrosk Anat. 1962;56:748–761. doi: 10.1007/BF00336332. [DOI] [PubMed] [Google Scholar]
- CAUNA N., ROSS L. L. The fine structure of Meissner's touch corpuscles of human fingers. J Biophys Biochem Cytol. 1960 Oct;8:467–482. doi: 10.1083/jcb.8.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós F., Kerpel-Fronius S. Electron histochemical observation of succinic dehydrogenase activity in various parts of neurons. Exp Brain Res. 1969;8(1):66–78. doi: 10.1007/BF00234926. [DOI] [PubMed] [Google Scholar]
- Hajós F., Kerpel-Fronius S. Electron microscope histochemical evidence for a partial or total block of the tricarboxylic acid cycle in the mitochondria of presynaptic axon terminals. J Cell Biol. 1971 Oct;51(1):216–222. doi: 10.1083/jcb.51.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger A., Blomstrand C., Lehninger A. L. Comparative studies on mitochondria isolated from neuron-enriched and glia-enriched fractions of rabbit and beef brain. J Cell Biol. 1970 May;45(2):221–234. doi: 10.1083/jcb.45.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., HIMMELHOCH S. R. Seasonal and starvation-induced changes in enzymatic pattern of frog nephron. Am J Physiol. 1961 Nov;201:781–785. doi: 10.1152/ajplegacy.1961.201.5.781. [DOI] [PubMed] [Google Scholar]
- Kerpel-Fronius S., Hajós F. A method for the electron microscopic demonstration of cytochrome oxidase in fresh and formalin-prefixed tissues. Histochemie. 1967;10(3):216–223. doi: 10.1007/BF00304868. [DOI] [PubMed] [Google Scholar]
- Liisberg M. F. A new histophysiological concept of the striated tubules based on a very simple and new method for staining of mitochondria. Acta Anat (Basel) 1969;73(4):496–509. doi: 10.1159/000143314. [DOI] [PubMed] [Google Scholar]
- MERRILLEES N. C. The fine structure of muscle spindles in the lumbrical muscles of the rat. J Biophys Biochem Cytol. 1960 Jul;7:725–742. doi: 10.1083/jcb.7.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K., Oura C., Pallie W. Fine structure of Pacinian corpuscles in the mesentery of the cat. J Cell Biol. 1969 Dec;43(3):539–552. doi: 10.1083/jcb.43.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle W. K., Jr Sensory nerve endings shown by nitro blue tetrazolium in isolated muscle spindles of the cat. Stain Technol. 1971 Nov;46(6):311–314. doi: 10.3109/10520297109067881. [DOI] [PubMed] [Google Scholar]
- PEASE D. C. Infolded basal plasma membranes found in epithelia noted for their water transport. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):203–208. doi: 10.1083/jcb.2.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEASE D. C., QUILLIAM T. A. Electron microscopy of the pacinian corpuscle. J Biophys Biochem Cytol. 1957 May 25;3(3):331–342. doi: 10.1083/jcb.3.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUILLIAM T. A. New problems in the functional activity of the Pacinian corpuscle. J Biophys Biochem Cytol. 1958 May 25;4(3):341–342. doi: 10.1083/jcb.4.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUILLER C. Physiological and pathological changes in mitochondrial morphology. Int Rev Cytol. 1960;9:227–292. doi: 10.1016/s0074-7696(08)62748-5. [DOI] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman A. M., Ueno H., Morizono Y., Wasserkrug H. L., Katzoff L., Hanker J. S. Electron microscopic demonstration of dehydrogenase activity with a new osmiophilic ditetrazolium salt (TC-NBT). J Histochem Cytochem. 1967 Jan;15(1):1–13. doi: 10.1177/15.1.1. [DOI] [PubMed] [Google Scholar]
- Weiss P. A., Mayr R. Neuronal organelles in neuroplasmic ("axonal") flow. I. Mitochondria. Acta Neuropathol. 1971;5(Suppl):187–197. [PubMed] [Google Scholar]
- Weiss P., Pillai A. Convection and fate of mitochondria in nerve fibers: axonal flow as vehicle. Proc Natl Acad Sci U S A. 1965 Jul;54(1):48–56. doi: 10.1073/pnas.54.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]