Abstract
Posaconazole (SCH 56592) was tested against 25 strains of Coccidioides immitis to determine their in vitro susceptibilities. The geometric mean 48-h MIC of posaconazole (POSA) was 0.5 μg/ml, the MIC range was 0.25 to 1 μg/ml, and the MIC at which 50% of the isolates tested are inhibited (MIC50) and the MIC90 were 0.5 and 1 μg/ml, respectively. The geometric mean 48-h MIC of itraconazole (ITRA) was 0.23 μg/ml, the MIC range was 0.125 to 0.5 μg/ml, and the MIC50 and MIC90 were both 0.25 μg/ml. Two strains of C. immitis were selected for in vivo studies on the basis of the POSA 48-h MICs for the isolates. POSA orally administered at 0.01, 0.1, 0.5, 1, 5, and 10 mg/kg of body weight/day was compared with ITRA administered at 10 and 30 mg/kg three times a day. The spleens and livers of mice that died or survived to day 50 were removed to measure the fungal burdens. Mice had ≥90% survival when they were treated with ≥0.5 mg of POSA per kg or 30 mg of ITRA per kg. Cultures of whole spleens and livers from mice treated with 10 mg of POSA per kg showed ≥70% sterilization. No sterilization of whole spleens and livers from mice treated with ITRA was seen. POSA displayed potent in vivo activity against the two strains of C. immitis tested.
Amphotericin B remains the drug of choice for the treatment of coccidioidomycosis as well as other systemic mycoses. However, amphotericin B is often toxic and is sometimes ineffective (7). Triazoles have been found to be effective in the treatment of threatening human mycoses. Itraconazole (ITRA) and fluconazole are used for the treatment of patients with mild or moderate manifestations of coccidioidomycosis and following treatment with amphotericin B in patients with severe forms of coccidioidomycosis (8). Therapy can be completed with these antifungal agents, but the response rates are only 50 to 60% (2, 9).
These unfavorable conditions have prompted a search for more effective alternatives with improved pharmacokinetic parameters and safety. Posaconazole (POSA; SCH 56592) is a broad-spectrum triazole whose activities against a variety of systemic and opportunistic fungal pathogens are under active evaluation (1, 4, 6, 10, 12, 15, 16, 17, 19). Lutz et al. (11) reported on the activity of POSA in a murine model of disseminated coccidioidomycosis. POSA exhibited greater in vivo activity than fluconazole and ITRA in both survival and tissue burden studies, with doses of 10 and 25 mg/kg of body weight curing high proportions of infected mice. Pharmacokinetic studies with POSA in mice, rats, rabbits, dogs, and monkeys showed that it has good oral bioavailability (14). The half-lives in mice, rats, and rabbits were 7 to 9 h. In contrast, the half-lives in dogs and monkeys were 15 and 23 h, respectively.
In this study, we determined the in vitro activities of POSA against isolates of Coccidioides immitis and then compared the lowest and highest MICs of POSA in vivo using a murine model.
MATERIALS AND METHODS
Antifungal agents.
Powders of POSA and ITRA for in vitro use were obtained from Schering-Plough Research Institute, Kenilworth, N.J., and Janssen Pharmaceutica, Beerse, Belgium, respectively. For in vitro susceptibility studies, stock solutions were prepared by dissolving the antifungal powder in polyethylene glycol 400 (Sigma, St. Louis, Mo.) that had been heated to 75°C in a water bath to a final concentration of 5,000 μg/ml, and the preparations were kept at room temperature until needed. We used polyethylene glycol as the solubilizing agent because it has been reported that POSA and ITRA are very stable when they are solubilized with this agent (6). For in vivo studies, POSA was suspended in 0.3% Noble agar and was given orally by gavage. ITRA was suspended in 2-hydroxypropyl-β-cyclodextrin solution and was purchased from Janssen Pharmaceutical, Titusville, N.J.; it was diluted to the appropriate concentration in sterile water and was administered orally by gavage.
In vitro activities.
Twenty-five isolates of C. immitis were grown for 10 days at 35°C on potato flakes agar slants prepared in-house (18). Table 1 shows the origins and numbers of these strains. The isolates were evaluated by broth macrodilution method M38-P of the National Committee for Clinical Laboratory Standards (13). The mycelium was overlaid with sterile distilled water, and suspensions were made by softly scraping the colonies with wooden applicators. The conidia and small mycelial fragment suspensions were vortexed and adjusted (1 × 104 to 5 × 104 CFU/ml) at a 95% transmittance setting at 530 nm with a spectrophotometer (Spectronic 21; Milton Roy Company). POSA and ITRA were tested in RPMI 1640 with l-glutamine and morpholinepropanesulfonic acid buffer at a concentration of 165 mM (Angus, Niagara Falls, N.Y.). The final concentrations of both drugs were 0.015 to 8 μg/ml.
TABLE 1.
Accession numbers and sources of C. immitis strains
| UTHSC strain no.a | Source |
|---|---|
| 98-1884 | Back fluid |
| 97-1895 | Clinical isolate (source unknown) |
| 98-571 | Cerebrospinal fluid |
| 98-573 | Blood |
| 98-1002 | Cerebrospinal fluid |
| 98-1889 | Sputum |
| 97-2532 | Wrist |
| 98-1193 | Bronchial washing |
| 98-1037 | Clinical isolate (source unknown) |
| 98-1696 | Sputum |
| 98-7 | Cerebrospinal fluid |
| 98-293 | Cerebrospinal fluid |
| 98-706 | Blood |
| 98-449 | Lung tissue |
| 98-1194 | Lung tissue |
| 97-960 | Bronchial washing |
| 98-1412 | Blood |
| 98-1142 | Tissue |
| 98-703 | Lung tissue |
| 98-572 | Knee fluid |
| 97-2036 | Sputum |
| 98-1578 | Lung abscess |
| 98-1208 | Knee bone |
| 97-817 | Cerebrospinal fluid |
| 97-1089 | Axilla abscess |
UTHSC, The University of Texas Health Science Center.
Previously prepared frozen tubes containing 0.1 ml of drug were allowed to thaw and were inoculated with 0.9 ml of the suspensions. A drug-free growth control tube was included for each isolate. The tubes were incubated at 35°C. The MICs were read at 24 and 48 h. The MIC was defined as the concentration in the first tube with an 80% or greater reduction in turbidity (visual reading) in contrast to that for the drug-free control tube (13).
A Paecilomyces variotii strain, strain UTHSC 90-459, was included in all in vitro tests for quality control purposes.
Animals.
Outbred male ICR mice (age, 4 to 6 weeks; weight, 25 to 30 g at the start of the study) were purchased from Harlan Sprague-Dawley Laboratories. Ten mice were randomly assigned to each treatment or control group for each survival and tissue burden study. They were housed in cages with five mice each. The mice were provided food and water ad libitum.
Microorganisms.
Two strains of C. immitis for which POSA MICs were different were selected for this study. They were strain 97-960 (for which the lowest POSA MIC was 0.25 μg/ml and the lowest ITRA MIC was 0.125 μg/ml) and strain 97-2036 (for which the highest POSA MIC was 1 μg/ml and the highest ITRA MIC was 0.5 μg/ml). Four-week-old cultures in the mycelial phase were maintained on potato dextrose agar plates. Arthroconidia were obtained by a previously described procedure (3). They were then filtered through glass wool, washed three times, suspended in sterile saline, and counted in a hemocytometer.
In vivo activities.
A previously described model of systemic coccidioidomycosis was used to determine in vitro activities (5). Mice received an intravenous injection of 200 arthroconidia of C. immitis. Quantitative cultures by serial dilution were used to confirm the inoculum size. In the first in vivo study, POSA was given once daily at 0.01, 0.1, 0.5, 1, 5, and 10 mg/kg of body weight orally by gavage in a 0.2-ml volume and ITRA was given at 10 and 30 mg/kg three times a day (for total daily doses of 30 and 90 mg/kg) orally by gavage on days 2 through 22 postinfection. The control group of infected mice received 0.3% Noble agar orally by gavage. Deaths were recorded through 50 days postinfection. Moribund mice were killed, and their deaths were recorded as occurring on the next day. Survivors were killed at day 50 by methoxyflurane (metofane) inhalation followed by cervical dislocation. The spleens and livers of the dead mice and the survivors that had been killed were removed aseptically. The organs were homogenized in 2 ml of sterile saline, and the entire volume of homogenate was plated onto potato dextrose agar and incubated at 35°C for a week to determine the viabilities of the fungi in the organs.
In the second in vivo study, mice were treated with POSA at 0.1, 0.5, and 1 mg/kg/day orally by gavage and ITRA at 10 and 30 mg/kg three times a day from days 2 through 22 postinfection. The control group of infected mice received 0.3% Noble agar orally by gavage. Deaths were recorded through day 24. The livers, spleens, and lungs of the dead mice and the survivors that had been killed were removed, weighed, and homogenized in 1 to 5 ml of sterile saline; and serial 10-fold dilutions were placed onto potato dextrose agar plates and incubated at 35°C for a week to determine the number of viable CFU in each organ.
All studies were conducted by use of laboratory safety level 3 criteria for infectious agents (20).
Analysis of data.
For survival studies, the log rank and Wilcoxon tests were used. The P values for determination of significance varied because of correction for multiple comparisons. For tissue burden studies, Dunnett's one-tailed t test was used and a P value of ≤0.05 was determined to be significant when the values were compared only with those for the controls.
RESULTS
Antifungal susceptibilities.
The POSA MICs ranged from 0.25 to 1 μg/ml, and the geometric mean POSA MIC was 0.5 μg/ml. The POSA MICs at which 50 of the isolates tested were inhibited (MIC50) and the MIC90 were 0.5 and 1 μg/ml, respectively. In comparison, the ITRA MICs ranged from 0.125 to 0.5 μg/ml, and the geometric mean ITRA MIC was 0.23 μg/ml. The ITRA MIC50 and MIC90 were both 0.25 μg/ml.
In vivo activities.
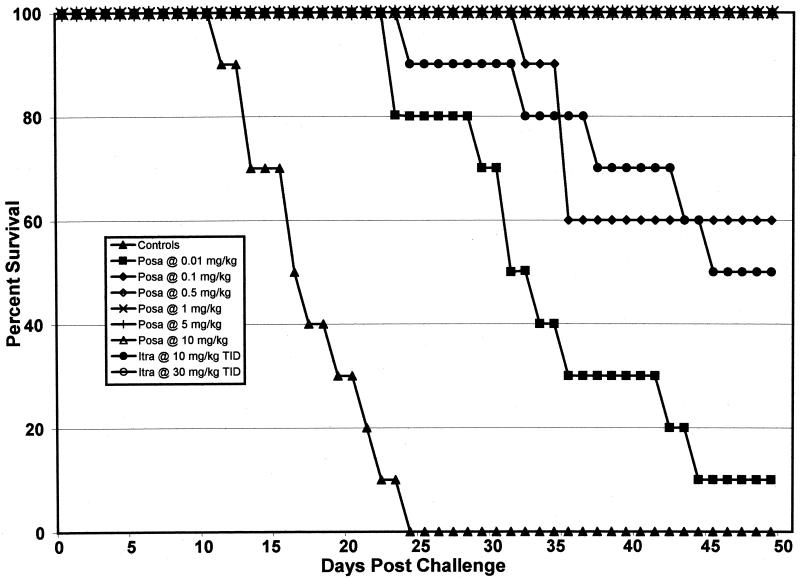
The results of the survival study with strain 97-960 are displayed in Fig. 1. All control mice died between days 12 and 25. Mice showed 100% survival to day 50 when treated with ≥0.5 mg of POSA per kg once daily and 30 mg of ITRA per kg three times a day. POSA doses of ≥0.01 mg/kg and ITRA doses of 10 mg/kg significantly prolonged survival (P ≤ 0.05). Fungal burdens for the entire livers and spleens of mice that died or for those of mice that survived to day 50 postchallenge were determined. Cultures of homogenized organs showed 50 and 70% sterilization of both organs when mice were treated with 5 and 10 mg of POSA per kg, respectively. ITRA resulted in no sterilization, even when it was used at 30 mg/kg (Table 2). The results of the survival study with mice infected with isolate 97-2036 are shown in Fig. 2. All of the control mice died between days 14 and 27. Ninety to 100% of the mice survived when the POSA treatment dose was ≥0.5 mg/kg. Significant prolongation of survival was noted at POSA doses of ≥0.01 mg/kg and ITRA doses of 10 mg/kg (P ≤ 0.05). Cultures of homogenized entire organs showed 30 and 80% sterilization for both organs when the mice were treated with 5 and 10 mg of POSA per kg, respectively. No sterilization of either organ was observed with ITRA. The rates of recovery of C. immitis from whole spleens and livers of mice treated with POSA and ITRA are shown in Table 2.
FIG. 1.
Survival study after intravenous infection with 200 arthroconidia of C. immitis strain 97-960 (MIC, 0.25 μg/ml). Therapy was given on days 2 to 22 after infection. The control group received sterile distilled water. There were 10 animals per group. The survivors were killed on day 50. TID, three times a day.
TABLE 2.
Recovery of C. immitis isolates 97-960 and 97-2036 from organs of mice treated with POSA and ITRA
| Treatment (dose [mg/kg])a | % Sterilization
|
|||
|---|---|---|---|---|
| Isolate 97-960b
|
Isolate 97-2036c
|
|||
| Spleen | Liver | Spleen | Liver | |
| Controls | 0 | 0 | 0 | 0 |
| POSA (0.01) | 0 | 0 | 0 | 0 |
| POSA (0.1) | 0 | 0 | 0 | 0 |
| POSA (0.5) | 0 | 0 | 0 | 0 |
| POSA (1) | 0 | 0 | 0 | 0 |
| POSA (5) | 60 | 50 | 50 | 30 |
| POSA (10) | 80 | 70 | 80 | 80 |
| ITRA (10) | 0 | 0 | 0 | 0 |
| ITRA (30) | 0 | 0 | 0 | 0 |
Each group had 10 mice. The mice were monitored through day 50 postchallenge. The organs were homogenized, and whole organs were plated onto potato dextrose agar and incubated at 30°C for a week.
MIC, 0.25 μg/ml.
MIC, 1 μg/ml.
FIG. 2.
Survival study after intravenous infection with 200 arthroconidia of C. immitis strain 97-2036 (MIC, 1 μg/ml). Therapy was given on days 2 to 22 after infection. The control group received sterile distilled water. There were 10 animals per group. The survivors were killed on day 50. TID, three times a day.
The results of the second in vivo study are shown in Table 3. Tissue burdens were determined as the mice died or on day 24 postchallenge for those that survived. Treatment with POSA at ≥0.1 mg/kg significantly reduced the counts in the spleens, livers, and lungs relative to the counts in the organs of the controls in a dose-dependent manner for both isolates tested. Treatment with ITRA at 10 and 30 mg/kg also significantly reduced the counts in the organs for both isolates of C. immitis. For the spleen, lung, and liver, POSA given at 0.1, 0.5, and 1 mg/kg was more potent than ITRA given at 10 mg/kg three times a day in reducing the numbers of CFU per organ (P = 0.01 to <0.0001). There were no statistically significant differences between POSA administered at 0.1 and 0.5 mg/kg and ITRA administered at 30 mg/kg three times a day (P > 0.05). However, a significant improvement in the reduction of the fungal burden in each organ was observed with POSA administered at 1 mg/kg in comparison with that observed with ITRA administered at 30 mg/kg three times a day (P < 0.0001).
TABLE 3.
Fungal burdens in spleens, livers, and lungs of mice infected with 200 arthroconidia of C. immitis strains 97-960 and 97-2036
| Treatment (dose [mg/kg]) | Mean log10 CFU/organ
|
|||||
|---|---|---|---|---|---|---|
| Strain 97-960a
|
Strain 97-2036b
|
|||||
| Spleen | Liver | Lungs | Spleen | Liver | Lungs | |
| Controls | 6.147 | 6.317 | 6.165 | 6.139 | 6.296 | 6.143 |
| POSA (0.1) | 4.366 | 4.505 | 4.450 | 4.316 | 4.448 | 4.408 |
| POSA (0.5) | 3.382 | 3.489 | 3.445 | 3.403 | 3.515 | 3.394 |
| POSA (1) | 2.068 | 2.305 | 2.212 | 2.100 | 2.068 | 2.082 |
| POSA (10) | 5.134 | 5.277 | 5.459 | 5.089 | 5.272 | 5.244 |
| ITRA (30) | 3.380 | 3.432 | 3.437 | 3.305 | 3.506 | 3.597 |
MIC, 0.25 μg/ml.
MIC, 1 μg/ml.
DISCUSSION
POSA is a new antifungal agent with a spectrum of activity which includes Candida spp., Cryptococcus neoformans, and a large group of invasive filamentous fungi. In this study, POSA displayed excellent in vitro activity against all clinical isolates of C. immitis tested. Under standardized laboratory conditions, POSA was similar in activity to ITRA. The MIC ranges, the geometric mean MICs, the MIC50s, and the MIC90s of the two drugs were nearly identical. Nevertheless, POSA was considerably more effective than ITRA in a murine model of systemic coccidioidomycosis.
The two clinical isolates of C. immitis used in the study exhibited slightly different in vitro profiles of susceptibility (POSA MICs, 0.25 and 1 μg/ml, and ITRA MICs, 0.125 and 0.5 μg/ml). When a survival study was performed with isolate 97-960, POSA at ≥0.5 mg/kg/day as well as ITRA at 30 mg/kg three times a day was capable of preventing death, with 100% survival at day 50. Studies with a second, more resistant isolate, isolate 97-2036, showed that POSA at ≥1 mg/kg/day and ITRA at 30 mg/kg three times a day were effective at preventing mortality, with 100% survival at day 50.
Studies of the fungal burdens for entire livers and spleens of mice treated with POSA at 5 and 10 mg/kg/day for both C. immitis isolates showed increased percentages of sterilization. ITRA showed no sterilization even when it was used at 30 mg/kg three times a day. In quantitative tissue burden studies with the two isolates, all doses of POSA (0.1, 0.5, and 1 mg/kg) and ITRA (10 and 30 mg/kg) significantly reduced the counts in the spleens, livers, and lungs to levels lower than those in the controls in a dose-dependent manner. There were no statistically significant differences in the results of the tissue burden studies between the two strains for either POSA or ITRA. The in vitro activity of POSA was reflected in its potent in vivo activity in the murine model of coccidioidomycosis used in the present study. The dramatic activity of POSA was evidenced in the tissue burden studies; no other triazole has sterilized all these organs.
Our study yielded results similar to those obtained in a previous study by Lutz et al. (11) evaluating POSA for the treatment of coccidioidomycosis in a murine model. However, their in vitro results with five strains of C. immitis revealed POSA MICs that ranged from 0.39 to 3.13 μg/ml. These small differences could be the result of the application of dissimilar in vitro methodologies. They used Synthetic Amino Acid Medium-Fungi as the medium for in vitro testing and an inoculum of 103 CFU, and MICs were determined after 6 to 7 days of incubation at room temperature. The Silveira strain was used in the in vivo study, and the POSA MIC was 0.39 μg/ml for that strain (11).
At present, POSA is in phase III clinical trials for the treatment of severe fungal infections. In a study with 20 patients suffering from chronic pulmonary or nonmeningeal disseminated coccidioidomycosis, patients received 400 mg of POSA per day for up to 6 months. Fifteen patients completed the treatment. At the end of 16 weeks of treatment, the median score was reduced to 40% of the baseline score, and at 24 weeks, the median score was reduced to 22% of the baseline score (A. Catanzaro, G. Cloud, D. Stevens, B. Levine, P. Williams, R. Johnson, R. Sanchez, L. Perez, A. Rendon, L. Mirels, J. Lutz, M. Holloway, D. Blum, M. Murphy, and J. Galgiani, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J1417, p. 382, 2000). Thus, POSA could expand the armamentarium of antifungal agents used for the treatment of human coccidioidomycosis.
REFERENCES
- 1.Al-Abdely, H. M., L. Najvar, R. Bocanegra, A. Fothergill, D. Loebenberg, M. G. Rinaldi, and J. R. Graybill. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob. Agents Chemother. 44:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catanzaro, A., J. N. Galgiani, B. E. Levine, P. K. Sharkey-Mathis, J. Fierer, D. A. Stevens, S. W. Chapman, G. Cloud, and the NIAID Mycoses Study Group. 1995. Fluconazole in the treatment of chronic pulmonary and non meningeal disseminated coccidioidomycosis. Am. J. Med. 98:249-256. [DOI] [PubMed] [Google Scholar]
- 3.Clemons, K. V., L. H. Hanson, A. M. Perlman, and D. A. Stevens. 1990. Efficacy of SCH39304 and fluconazole in a murine model of disseminated coccidioidomycosis. Antimicrob. Agents Chemother. 34:928-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connoly, P., J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 1999. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 43:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galgiani, J. N., S. H. Sun, K. V. Clemons, and D. A. Stevens. 1990. Activity of cilofungin against Coccidioides immitis: differential in vitro effects on mycelia and spherules correlated with in vivo studies. J. Infect. Dis. 162:944-948. [DOI] [PubMed] [Google Scholar]
- 6.Galgiani, J. N., and M. L. Lewis. 1997. In vitro studies of activities of the antifungal triazoles SCH 56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob. Agents Chemother. 41:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 8.Gallis, H. A. 1996. Amphotericin B: a commentary on its role as an antifungal agent and as a comparative agent in clinical trials. Clin. Infect. Dis. 22(Suppl. 2):S145-S147. [DOI] [PubMed] [Google Scholar]
- 9.Graybill, J. R., D. A. Stevens, J. N. Galgiani, W. E. Dismukes, G. A. Cloud, and the NIAID Mycoses Study Group. 1990. Itraconazole treatment of coccidioidomycosis. Am. J. Med. 89:282-290. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick, W. R., R. K. McAtee, A. Fothergill, D. Loebenberg, M. G. Rinaldi, and T. F. Patterson. 2000. Efficiency of SCH 56592 in a rabbit model of invasive aspergillosis. Antimicrob. Agents Chemother. 44:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz, J. E., K. V. Clemons, B. H. Aristizabal, and D. A. Stevens. 1997. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob. Agents Chemother. 41:1558-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. In vitro activity of a new triazole antifungal agent, Sch 56592, against clinical isolates of filamentous fungi. Mycopathologia 141:73-77. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard. NCCLS document M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Nomeir, A. A., P. Kumari, M. J. Hilbert, S. Gupta, D. Loebenberg, A. Cacciapuoti, R. Hare, G. H. Miller, C.-C. Lin, and M. N. Cayen. 2000. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 44:727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oakley, K. L., C. B. Moore, and D. W. Denning. 1997. In vitro activity of SCH-56592 and comparison with activities of amphotericin B and itraconazole against Aspergillus spp. Antimicrob. Agents Chemother. 41:1124-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perfect, J. R., G. M. Cox, R. K. Dodge, and W. A. Schell. 1996. In vitro and in vivo efficacies of the azole SCH 56592 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 40:1910-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., S. Messer, and R. N. Jones. 1997. Activity of a new triazole, Sch 56592, compared with those of four other antifungal agents tested against clinical isolates of Candida spp. and Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 41:233-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldi, M. G. 1982. Use of potato flakes agar in clinical mycology. J. Clin. Microbiol. 15:1159-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugar, A. M., and X-P. Liu. 1996. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob. Agents Chemother. 40:1314-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services. 1999. Biosafety in microbiological and biomedical laboratories, 4th ed. U.S. Government Printing Office, Washington, D.C.