Abstract
Thiolactomycin (TLM) is an antibiotic that inhibits bacterial type II fatty acid synthesis at the condensing enzyme step, and β-ketoacyl-acyl carrier protein synthase I (FabB) is the relevant target in Escherichia coli. TLM resistance is associated with the upregulation of efflux pumps. Therefore, a tolC knockout mutant (strain ANS1) was constructed to eliminate the contribution of type I secretion systems to TLM resistance. Six independent TLM-resistant clones of strain ANS1 were isolated, and all possessed the same missense mutation in the fabB gene (T1168G) that directed the expression of a mutant protein, FabB(F390V). FabB(F390V) was resistant to TLM in vitro. Leucine is the only other amino acid found at position 390 in nature, and the Staphylococcus aureus FabF protein, which contains this substitution, was sensitive to TLM. Structural modeling predicted that the CG2 methyl group of the valine side chain interfered with the positioning of the C11 methyl on the isoprenoid side chain of TLM in the binary complex, whereas the absence of a bulky methyl group on the leucine side chain permitted TLM binding. These data illustrate that missense mutations that introduce valine at position 390 confer TLM resistance while maintaining the vital catalytic properties of FabB.
Bacterial fatty acid synthesis is critical to bacterial growth and survival and is carried out by a universal set of enzymes that are encoded by a distinct group of highly related genes termed the type II system (6, 34). There are two essential classes of condensing enzymes that function as key regulators of the pathway. FabH, β-ketoacyl-acyl carrier protein (ACP) synthase III, catalyzes the first step in the pathway and governs the rate of initiation of new acyl chains. The initiation condensing enzyme uses acyl coenzyme A (acyl-CoA) as a substrate and has a His-Asn-Cys active-site triad. FabB and FabF, synthases I and II, respectively, are chain elongation condensing enzymes that control fatty acid composition and influence the rate of fatty acid production, and these enzymes possess a His-His-Cys catalytic triad.
Two natural products inhibit type II fatty acid synthesis by blocking the activity of one or more of the β-ketoacyl-ACP synthases. Cerulenin is an irreversible inhibitor of the elongation class of condensing enzymes, β-ketoacyl-ACP synthases I and II (FabB and FabF) (7, 19, 41), and forms a covalent adduct with the active-site cysteine (18). Cerulenin is not selective for type II systems because it is also a potent inhibitor of the condensation reaction catalyzed by the mammalian multifunctional (type I) fatty acid synthase (30, 31). However, cerulenin and related compounds have antineoplastic activity (22) and reduce food intake and body weight in mice (23), suggesting that this type of inhibitor may have utility in the treatment of human disorders of lipid metabolism. The structures of the FabF-cerulenin (27) and FabB-cerulenin (32) complexes show that the hydrophobic tail of the antibiotic slides into the substrate-binding tunnel and that the functional groups bind to the active-site histidines in a fashion that mimics the condensation transition state (32). Thiolactomycin (TLM) is a unique thiolactone that reversibly inhibits type II but not metazoan type I fatty acid synthase (11, 12). In contrast to cerulenin, TLM binds to the condensing enzyme active site in a mode that mimics the interaction of the enzyme with malonyl-ACP (32). TLM has a broad spectrum of activity against many pathogens (29). Also, the antibiotic is not toxic to mice and affords significant protection against urinary tract and intraperitoneal bacterial infections (26). TLM is active against gram-negative anaerobes associated with periodontal disease (10) and exhibits antimycobacterial action by virtue of its inhibition of mycolic acid synthesis (21, 39). TLM also has activity against malaria (42) and trypanosomes (28), thereby extending the potential for using this template as a platform for drug development.
Understanding the mechanisms of acquired resistance is important to the future development of TLM as an effective therapeutic agent. Spontaneous TLM resistance occurs at a high frequency in wild-type populations of Escherichia coli (16). A series of genetic and biochemical experiments demonstrated that these resistant isolates arose from mutations that inactivated the EmrR transcriptional repressor, thus increasing the expression of the EmrAB efflux pump in E. coli (8, 24). The intrinsic resistance of Pseudomonas aeruginosa to TLM is also attributed to active efflux of the antibiotic (37). The overexpression of FabB, accomplished through the introduction of the fabB gene on a multicopy plasmid, also confers TLM resistance (40); this finding directly illustrates that FabB is a relevant target for TLM in vivo. However, alterations in FabB expression or mutations in the fabB gene that lead to TLM resistance have not been discovered. In this report, we examine the efficacy of TLM for strains devoid of TolC-dependent type I secretory activity and use these strains to specifically select for missense mutations in the fabB gene that confer TLM resistance.
MATERIALS AND METHODS
Materials and bacterial strains.
The sources of supplies were as follows: [2-14C]malonyl-CoA (specific activity, 55 mCi/mmol), Amersham Biosciences, Inc.; and ACP, Sigma Chemical Co. Myristoyl-ACP was prepared by the acyl-ACP synthetase method (14, 35). Purified FadD and FabB proteins were obtained as described previously (13, 15). The fabF gene from Staphylococcus aureus was amplified from chromosomal DNA by using primer pair 5′-GGATCCGAGTCAAAATATAAGAGTAGTTATTACA and 5′-GGATCCTGTGCTGTCGCTCATCTTAG to engineer a BamHI site (underlined) at the initiator methionine, which was changed to a proline residue, and a BamHI site downstream of the stop codon. The PCR products were cloned in plasmid pCR2.1 and sequenced. The sequence-verified clone was transferred to pET-15b by using BamHI, and S. aureus FabF was expressed and purified by affinity chromatography as described previously (13, 15). Purified Bacillus subtilis FabF was a gift from Keum-Hwa Choi and was prepared as described previously (36). TLM isolated from a fermentation broth was a gift from John Lonsdale, Glaxo/SmithKline. All other chemicals were reagent grade or better.
The bacterial strains used in this study were derivatives of E. coli K-12: strain UB1005 (metB1 relA1 spoT1 gyrA216 λ− λr F−) (2); strain CDM5 (emrR [Tlmr] metB1 relA1 spoT1 gyrA216 λ− λr F−) (16, 24); strain JT10 (metB1 relA1 spoT1 gyrA216 λ− λr F−) containing pFabB (40), which overexpresses FabB; strain ANS1 (metB1 relA1 spoT1 gyrA216 tolC::Tn10 λ− λr F−); and strain ANS6 (fabB390 metB1 relA1 spoT1 gyrA216 tolC::Tn10 λ− λr F−). Strain ANS1 was constructed by P1-mediated transduction of tolC::Tn10 in strain EP1581 into strain UB1005, followed by selection for tetracycline resistance. Strain ANS6 was obtained by selection for the growth of strain ANS1 on 24 μM TLM plates as described below. Plasmids were constructed by moving the XbaI-BamHI fragment of the His-tagged version of either the fabH or the fabB gene (14, 15) in pET-15b into pBluescript II KS(+), respectively.
Condensing enzyme assay.
The FabB-FabF condensation assay was essentially the same as that described previously (9). Briefly, condensing enzymes assay mixtures contained 45 μM myristoyl-ACP, 50 μM [2-14C]malonyl-CoA, 100 μM ACP, 25 ng of FabD (malonyl transacylase), and condensing enzyme FabB, FabB(F390V), or FabF (0 to 50 ng) in a final volume of 40 μl. The mixtures were incubated at 37°C for 15 min, reduced with borohydride, extracted into toluene, and quantitated by scintillation counting. Assays were linear with time and protein. Protein content was determined with the Bradford assay (3).
Isolation of TLM-resistant fabB mutants.
The sensitivity of strains to TLM was determined by spotting colonies onto rich agar plates containing geometrically increasing amounts of TLM. The MIC was reported as the concentration of TLM at which no bacterial growth was observed. Strain ANS1 was grown in rich broth in the absence of TLM, and approximately 107 cells were plated onto rich media containing 24, 48, and 96 μM TLM. A total of six independent colonies were obtained at a frequency of one colony per plate (four on plates containing 24 μM TLM and two on plates containing 48 μM TLM). The fabB gene (including the promoter region) was amplified from each of the six isolates by using primers EcFabB109F (5′-GATCTTAGCGATGTGTGTAAGG) and EcFabB1498R (5′-TCGGATGCGACGCTGGC). The fragments were cloned in plasmid pCR2.1 and sequenced by using the two primers listed above plus an internal primer, EcFabB566F (5′-GCGTTTCCAGGTGTTCGG). All isolates contained the same missense mutation. The mutated fabB gene in pCR2.1 was digested with AgeI and BamHI, and this fragment was transferred to a similarly digested FabB-pET-15b expression vector. The FabB(F390V) protein was expressed and purified by affinity chromatography as described previously (15).
Fatty acid analysis.
Cultures (5 ml) of E. coli strains were grown to mid-log phase in M9 minimal medium (25) supplemented with 1% Casamino Acids (ICN), 0.4% glucose, 0.01% methionine, 0.0005% thiamine, and 10 μM β-alanine and were harvested by centrifugation. The cell pellet was suspended in 1 ml of water, the lipids were extracted as described by Bligh and Dyer (1), and fatty acid methyl esters were prepared by the addition of 2 ml of HCl-methanol to the dry extract. The fatty acid methyl esters were fractionated by using a Hewlett-Packard model 5890 gas chromatograph equipped with a flame ionization detector and a glass column (internal diameter, 2 m by 4 mm) containing 3% SP2100-coated Supelcoport (100/120 mesh) and operated at 190°C. Fatty acid methyl esters were identified by comparing their retention times with those of standards (Matreya).
Structural modeling.
The models of FabB(F390V) and FabB(F390L) were created from the coordinates of the binary complex of FabB-TLM (protein data bank ID 1FJ4). The binary complex was chosen because it is known that TLM induces conformational changes in the active site of the wild-type protein (32) that we think would likely be present in any complex of TLM with the mutant proteins. The amino acid subsitutions of valine and leucine for phenylalanine were made by using the program O (17), and the models, with TLM and water removed, were energy minimized by using the Kollman all-atom force field (38). Energy minimization was carried out by using the program Maximin2 within the Sybyl molecular modeling environment (SYBYL 6.7; Tripos Inc., St. Louis, Mo.). The binary complex of FabB(F390V)-TLM was made by using the same energy minimization software and the Tripos force field (5). Flexible docking with the program FlexX (33) was used to model the binding of TLM to FabB, FabB(F390V), and FabB(F390L). The initial position and conformation of TLM were chosen to place TLM outside the active site, near the entrance to the active-site tunnel. In all docking calculations, 30 solutions were requested, although for FabB(F390L), only 23 were found by FlexX.
RESULTS
Isolation of TLM-resistant FabB mutants.
Previous work implicated efflux pumps as the major determinants of TLM sensitivity in E. coli. The only TLM-resistant strains isolated to date have arisen from mutations in the emrR gene that have resulted in the upregulation of the emrAB efflux pump (8, 24). Therefore, we constructed strain ANS1 (tolC::Tn10) to abrogate the activity of all TolC-dependent type I secretion and multidrug efflux systems (4, 20). Accordingly, we found that the TLM MIC of 150 μM for parent strain UB1005 dropped to 3 μM for strain ANS1 (Table 1). This significant increase in the sensitivity of strain ANS1 to TLM illustrated that pumps operating via the TolC-dependent efflux system were a major determinant of the sensitivity of strains to this antibiotic.
TABLE 1.
TLM, cerulenin, triclosan, and chloramphenicol resistance profiles for bacterial strainsa
| Strainb | MIC (μM) of:
|
|||
|---|---|---|---|---|
| TLM | Cerulenin | Triclosan | Chloramphenicol | |
| UB1005 (wild type) | 150 | 200 | 0.9 | 12 |
| CDM5 (emrR) | >800 | 200 | 0.9 | 12 |
| ANS1 (tolC) | 3 | 20 | 0.02 | 6 |
| ANS1/pFabB | 6 | 200 | 0.02 | 6 |
| ANS1/pFabB(F390V) | >400 | 100 | 0.02 | 6 |
| ANS6 (fabB390) | 50 | 20 | 0.02 | 6 |
| ANS6/pFabH | 50 | 5 | 0.01 | 6 |
| ANS6/pFabB | 50 | 50 | 0.02 | 6 |
The MICs of the four antibiotics were determined by spotting the indicated strains on rich plates containing progressively higher concentrations of antibiotics. The MIC was the concentration of antibiotic at which there was no growth.
The relevant genotypes of the strains are shown in parenthesis; all strains are derivatives of strain UB1005. The presence of a plasmid is indicated, followed by a description of the protein that is expressed by the particular plasmid construct.
We used strain ANS1 to select for TLM resistance by spreading sensitive cells grown on regular medium on plates containing 24 or 48 μM TLM and selecting a total of six independent colonies for analysis. Colonies arose with a frequency of about 1 in 107 cells plated, and all strains retained their TLM resistance phenotype when cultured in the absence of the drug. The fabB gene from each isolate was amplified by PCR as described in Materials and Methods and sequenced. All six isolates contained the same single-base-pair missense mutation (T1168G), and one of these, strain ANS6, was selected for further experiments in this study. The allele was termed fabB390 because it was predicted to encode a FabB(F390V) mutant protein. We were unable to discern an altered growth phenotype for strain ANS6. The strain grew with the same doubling time as the parent strain on both rich and minimal media.
We performed several additional experiments with the same protocol as that used to obtain resistant mutants of strain ANS1 by using TLM-resistant strain ANS6 as the starting point in an attempt to select for strains with higher levels of TLM resistance. We were unable to isolate colonies that were able to grow on higher concentrations of TLM than strain ANS6 (three attempts). This result was investigated further by introducing plasmids that overexpressed either FabH or FabB into strain ANS6 and examining the effect on TLM resistance (Table 1). The introduction of the FabB-expressing plasmid into strain ANS1 increased TLM resistance twofold to 6 μM. In contrast, the introduction of a plasmid expressing the FabB(F390) mutant protein increased the resistance of strain ANS1 to greater than 400 μM TLM. These data show that the expression of FabB(F390V) alone is sufficient to confer TLM resistance. Neither FabH nor FabB expression was able to alter TLM resistance in strain ANS6, indicating that neither of these proteins alone was responsible for the TLM sensitivity of strain ANS6. The resistance of strain ANS6 was selective for TLM. Strain ANS6 did not exhibit increased resistance to cerulenin, a condensing enzyme inhibitor, triclosan, a fatty acid biosynthesis inhibitor, or chloramphenicol (Table 1).
Phenotype of strain ANS6.
In the absence of an altered growth phenotype on standard laboratory media, we compared the fatty acid composition of strain ANS6 to that of parent strain ANS1, since FabB is known to play a critical role in unsaturated fatty acid biosynthesis. The strains were grown to a density of 5 × 108 cells/ml in M9 minimal medium containing glucose as the carbon source; the lipids were extracted, and their fatty acid composition was determined by gas chromatography as described in Materials and Methods. We found no significant differences in the unsaturated fatty acid content or the chain-length distribution in strain ANS6 compared to strain ANS1. The unsaturated/saturated fatty acid ratios in membrane phospholipids were 1.01 in strain ANS1 and 0.96 in strain ANS6. Thus, the expression of the fabB390 allele does not impair the ability of FabB to perform its function in unsaturated fatty acid synthesis in vivo.
TLM resistance of the FabB(F390V) protein.
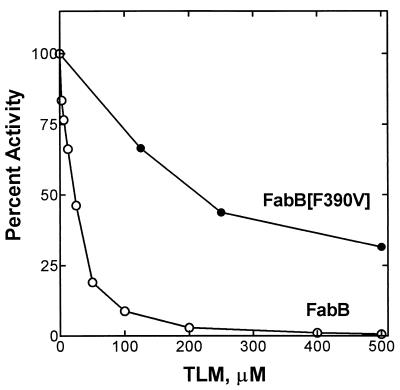
FabB(F390V) was expressed and purified to compare its biochemical properties with those of wild-type FabB. The specific activity of FabB(F390V) (0.26 ± 0.092 nmol/min/μg) was similar to that of the wild-type protein (0.34 ± 0.013 nmol/min/μg) when myristoyl-ACP was used as the substrate. However, unlike wild-type FabB, which is sensitive to TLM (50% inhibitory concentration [IC50], ≈20 μM), FabB(F390V) was clearly resistant to TLM in the in vitro assay (Fig. 1); the IC50 for FabB(F390V) under these assay conditions was 200 μM TLM. These data show that the substitution of valine for phenylalanine at position 390 in FabB impairs the interaction of TLM with FabB without seriously compromising the catalytic competence of the protein.
FIG. 1.
Activity and inhibition of FabB and FabB(F390V) by TLM. Wild-type FabB was sensitive to TLM, with an apparent IC50 of ≈20 μM under these assay conditions, whereas for the FabB(F390V) mutant, the apparent IC50 for TLM inhibition was ≈200 μM. The FabB and FabB(F390V) proteins were purified and assayed in the presence of the indicated concentrations of TLM as described in Materials and Methods.
Sensitivity of condensing enzymes containing leucine at position 390 to TLM.
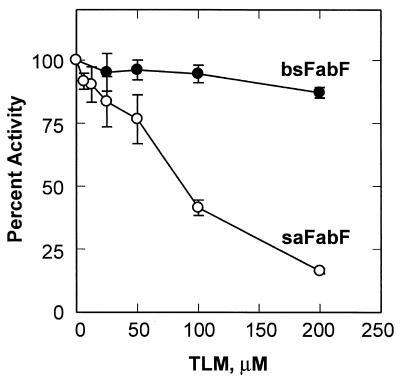
A survey of the amino acid sequences of the elongation condensing enzymes (FabB and FabF) from different organisms revealed that 34 out of the 39 genera contained phenylalanine at the position corresponding to amino acid 390 in E. coli FabB. However, five genera (all gram-positive bacteria) contained a leucine at position 390 instead of phenylalanine. The effect of the leucine substitution with respect to TLM sensitivity is equivocal. One of the variants, B. subtilis FabF, is known from previous work (36) to be resistant to TLM, suggesting the possibility that, like F390V variants, F390L variants are also TLM resistant. However, S. aureus also has the F390L replacement, and this organism is sensitive to TLM (29). To investigate this finding further, we overexpressed and purified S. aureus FabF and compared its properties to those of B. subtilis FabF to determine directly if a leucine at position 390 confers TLM resistance. Although both FabF proteins have the same replacement, they responded quite differently to TLM in the in vitro assay. B. subtilis FabF was resistant to TLM, retaining almost 100% activity at 200 μM TLM. On the other hand, S. aureus FabF was sensitive to this antibiotic, with an IC50 of ≈80 μM (Fig. 2). The different responses of B. subtilis FabF and S. aureus FabF to TLM treatment suggested that the replacement of Phe with Leu does not significantly impair TLM binding and that there is another residue(s) in B. subtilis FabF that renders it TLM resistant. These data also explain why we did not isolate fabB missense mutants that generated a FabB(F390L) protein following TLM selection. A single-base missense mutation in fabB would have resulted in an altered gene that would have produced FabB(F390L), yet we did not obtain such a mutant in our screens.
FIG. 2.
Inhibition of S. aureus and B. subtilis FabF proteins by TLM. The B. subtilis FabF (bsFabF) and S. aureus FabF (saFabF) proteins were purified and assayed in the presence of the indicated concentrations of TLM as described in Materials and Methods. Error bars show standard deviations.
Structural basis for TLM resistance in FabB(F390V).
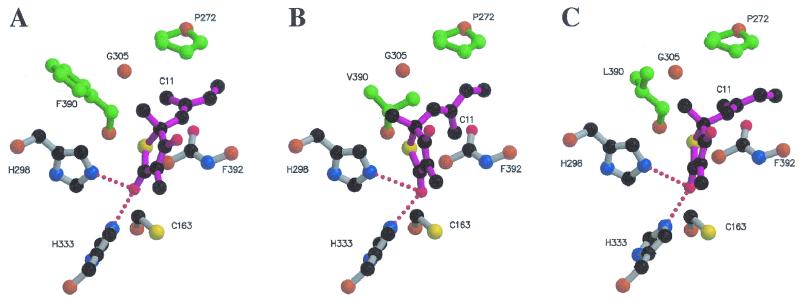
By directly placing TLM into the active site of modeled FabB(F390V) by using the wild-type binding mode, it was immediately apparent that a steric clash would result between the CG2 methyl group of Val390 and the C11 methyl of TLM (Fig. 3). Attempts to energy minimize this complex failed to resolve the steric clash and resulted in grossly distorted methyl groups in both valine and TLM that could not be improved by further energy minimization. This result strongly implied that steric incompatibility is the structural basis for the decreased affinity of TLM for FabB(F390V). To further support this conclusion, a docking calculation was performed by using the program FlexX (33). This procedure was validated by using the algorithm to dock TLM into the crystal structure of the wild-type protein and to determine if the analysis would reproduce the observed binding mode. The results are shown in Table 2, and the highest-scoring docking solution accurately reproduced the known structure to within 0.74 Å (root mean square [RMS]). When the calculation was repeated with FabB(F390V), the highest-scoring docking solution showed the isoprenoid group of TLM (C11) rotated by 90° relative to the wild-type binding mode to avoid a clash with the CG2 methyl group of Val390 (compare Fig. 3A and B). In addition, the highest-scoring solutions were highly distorted from the wild-type binding mode, as indicated by the RMS differences between these solutions and the crystal structure (Table 2). The calculated free energies for these modeled complexes [compare FabB and FabB(F390V)] (Table 2) confirm that the binding modes of the mutant are much less energetically favorable than that of the wild-type protein.
FIG. 3.
Models of binary complexes of FabB, FabB(F390V), and FabB(F390L) with TLM. Only the key TLM-interacting residues in the FabB active site are shown. TLM is represented with purple bonds, and red dots indicate hydrogen bonds formed between His298 and His333 and the carbonyl oxygen of TLM. (A) Crystal structure of the FabB-TLM binary complex (32). The side chain of Phe390 points away from the C11 methyl group of TLM. (B) Highest-scoring FabB(F390V)-TLM complex derived from docking calculations, showing that TLM cannot bind in the same orientation in the mutant protein. The CG2 methyl group of Val390 interferes with the proper positioning of the C11 methyl group of TLM, causing the drug to bind at the active site with the C11 of TLM rotated into a position that prevents favorable stacking interactions of the isoprenoid side chain of TLM with Pro272 and the peptide bond of Phe392. The side chain of Val390 cannot rotate away from the C11 of TLM. (C) Highest-scoring FabB(F390L)-TLM complex derived from docking calculations. The side chain of Leu390 is rotated away from the C11 methyl group of TLM in the same orientation as the Phe390 aromatic side chain, retaining the stacking interactions with Pro272 and the peptide bond of Phe392.
TABLE 2.
Results of docking calculationsa
| FabB | FabB (F390V)
|
|
FabB (F390L)
|
||||
|---|---|---|---|---|---|---|---|
| Free energy (kJ/mol) | RMS | Free energy (kJ/mol) | RMS | Free energy (kJ/mol) | RMS | ||
| −14.15 | 0.74 | −9.86 | 1.26 | −13.79 | 0.77 | ||
| −13.38 | 0.67 | −7.84 | 4.26 | −10.50 | 1.04 | ||
| −13.17 | 0.45 | −7.22 | 1.30 | −10.34 | 0.63 | ||
| −13.11 | 0.73 | −4.27 | 13.72 | −10.09 | 1.16 | ||
| −12.96 | 0.89 | −4.11 | 14.07 | −9.79 | 1.04 | ||
| −12.77 | 0.54 | −4.09 | 13.89 | −9.78 | 1.16 | ||
| −12.66 | 0.55 | −4.07 | 13.90 | −9.06 | 0.74 | ||
| −11.72 | 0.91 | −4.01 | 9.39 | −8.97 | 0.67 | ||
| −11.35 | 0.51 | −4.01 | 9.39 | −8.31 | 0.65 | ||
| −10.98 | 1.30 | −3.99 | 13.69 | −7.69 | 0.41 | ||
The top 10 scoring solutions are shown. RMS values are derived from differences between the coordinates of modeled TLM and the coordinates of TLM from the crystal structure of the FabB-TLM binary complex (32).
The FabB(F390L) mutant was modeled and then used in the FlexX TLM docking calculation to understand how the presence of leucine at position 390 affects TLM sensitivity. The calculated free energy of the best docking solution for the FabB(F390L)-TLM complex was nearly the same as that for the FabB-TLM complex (Table 2). The highest-scoring solution showed that TLM binds to the FabB(F390L) model to within an RMS deviation of 0.77 Å of the value for the observed FabB-TLM structure (Fig. 3C).
DISCUSSION
Our experiments validate the hypothesis that efflux pump activity is the most important determinant of TLM sensitivity in E. coli and, by extension, other gram-negative bacteria. Although genetic evidence points to FabB as the important TLM target in E. coli (40), earlier selection schemes for TLM-resistant strains did not yield any fabB mutants. Instead, increased TLM resistance was associated with mutations that upregulate the EmrAB efflux pump (8). Sequence analysis demonstrated that a defect in the emrR gene, a transcriptional repressor that controls the expression of the emrAB operon, is responsible for acquired TLM resistance (24). Any mutation that inactivates the DNA-binding activity of EmrR will confer TLM resistance to E. coli, thus explaining the high frequency of TLM-resistant bacteria. For our experiments, a tolC knockout mutant was constructed to address the contribution of all TolC-dependent efflux pumps to TLM sensitivity. Strikingly, the TLM MIC of 150 μM for wild-type strain UB1005 was reduced to 3 μM for strain ANS1 (tolC::Tn10), illustrating that TolC-coupled pumps are a major determinant of TLM sensitivity. Also, the TLM MIC of 25 μM for strain SJ261 (emrB::Tn10) (8; this work) and the MIC of 3 μM for strain ANS1 (tolC::Tn10) argue that pumps other than the EmrAB system also contribute to TLM resistance. These observations indicate that the effectiveness of TLM in gram-negative bacteria could be significantly increased by changes in the molecule that abrogate its affinity for efflux pumps, even if these modifications lower its potency toward the elongation condensing enzymes.
Our data show that a missense mutation in the fabB gene is sufficient to increase the resistance of cells to TLM. Previous work clearly pointed to FabB as the relevant cellular target for TLM, based on overexpression experiments (40). However, TLM-resistant fabB genes never arose from selections for strains for which the TLM MIC was elevated. Ablation of efflux pump activation as a mechanism for resistance by introduction of the tolC::Tn10 knockout mutation eliminated the high background of upregulated pump mutants and revealed a second mechanism for acquired TLM resistance. Six independent TLM-resistant clones were analyzed, and all possessed the same missense mutation that gave rise to the expression of FabB(F390V). Although FabH is also inhibited by TLM, the IC50 for FabH is 110 μM (32), and mutations in its gene were therefore not anticipated and were not found. The IC50 of TLM for FabB(F390V), estimated from Fig. 1, was apparently higher than the MIC for strain ANS6 (Table 1). One reason for this observation may be that there is a second, more sensitive TLM target than FabB(F390V) in strain ANS6. This idea, however, is not consistent with the observation that the expression of multiple copies of FabB(F390V) increased the TLM MIC for the sensitive strain to a higher level than for ANS6 (Table 1). TLM is a competitive inhibitor of the condensing enzymes with respect to malonyl-ACP (11, 32), and the most likely explanation for the discrepancy between the in vitro and in vivo results is that the concentration of malonyl-ACP in the in vitro assay is high, whereas the concentration of malonyl-ACP in vivo is much lower and may be a limiting factor in fatty acid synthesis (6). One explanation for the inability to obtain more mutants more resistant to TLM than strain ANS6 with the same approach may be that there are multiple targets. Since it is known that both FabH and FabB are targets and the biochemical data indicate that FabH and FabB(F390V) are about equally sensitive to TLM, a further increase in TLM resistance in strain ANS6 may require mutations in both of their genes. Accordingly, the overexpression of either FabB or FabH in strain ANS6 did not increase resistance to TLM, indicating that neither protein is the sole target for the antibiotic in this strain (Table 1). These results are consistent with the concept that there are two targets, postulated to be FabH and FabB, in strain ANS6, although we cannot rule out the existence of an as-yet-unidentified TLM target that becomes important in resistant strain ANS6.
The structural modeling showed that the source of the resistance conferred by the substitution of valine for phenylalanine at position 390 arises from the steric clash between the C11 methyl of TLM and the CG2 methyl of Val390 (Fig. 3). In agreement with these experiments, the energy minimization docking calculation predicted that the sensitivity of the mutant strain to TLM would be significantly reduced. The calculation also showed that the steric clash distorts TLM within the active site and forces the rotation of the isoprenoid tail within the binding pocket (Fig. 3B). Price et al. pointed out the importance of the stacking of the planar double-bonded isoprenoid moiety between the delocalized electron systems of the peptide bond between residues 391 and 392 and Pro272 in the binding of TLM to wild-type FabB (32). The rotated isoprenoid group in the FabB(F390V) complex no longer optimally packs into this hydrophobic binding cleft, and the disruption of this crucial interaction significantly contributes to the lower binding affinity (Table 2). It is also important to note that in the modeling of the mutants (both F390V and F390L), the presence of the amino acid substitution does not significantly alter the conformation of the active-site residues, Cys163, His298, and His333 (Fig. 3). Thus, it would be predicted from the models that these mutants would retain enzyme activity. The substitution of leucine for phenylalanine does not pose the same problem for TLM binding (Fig. 3C), since there is no CG2 methyl in leucine. The docked structure shows TLM binding to FabB(F390L) in the same mode as in the wild type, with the isoprenoid stacked neatly between the proline and the peptide bond. The important interactions between the delocalized electron systems are preserved, and the calculated binding energy (Table 2) predicts that this variant will be as sensitive to TLM as the wild type. This result explains why leucine, the only other residue found at this position in nature, still permits TLM inhibition (Fig. 2). If this notion is correct, then the resistance that B. subtilis FabF shows against TLM would be predicted to arise from another source, since the leucine at position 390 should not diminish TLM binding to the active site of this enzyme. A determination of the underlying cause for TLM resistance in B. subtilis will require the analysis of site-directed mutants.
Acknowledgments
This work was supported by National Institutes of Health grants AI49320, GM34496, GM45737, and GM44973; Cancer Center (CORE) support grant CA 21765; and the American Lebanese Syrian Associated Charities.
We thank Pam Jackson and Amy Sullivan for expert technical assistance and Keum-Hwa Choi for providing the B. subtilis FabF protein.
REFERENCES
- 1.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 2.Booth, B. R. 1980. Cell surface proteins of E. coli. Biochem. Biophys. Res. Commun. 94:1029-1036. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, S. K. 2001. Type I secretion and multidrug efflux: transport through the TolC channel-tunnel. Trends Biochem. Sci. 26:3-6. [DOI] [PubMed] [Google Scholar]
- 5.Clark, M., R. D. Cramer, I. I. I., and N. Van Opdenbosch. 1989. Validation of the general purpose Tripos 5.2 force field. J. Comput. Chem. 10:982-1012. [Google Scholar]
- 6.Cronan, J. E., Jr., and C. O. Rock. 1996. Biosynthesis of membrane lipids, p. 612-636. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 7.D'Agnolo, G., I. S. Rosenfeld, J. Awaya, S. Omura, and P. R. Vagelos. 1973. Inhibition of fatty acid biosynthesis by the antibiotic cerulenin. Specific inactivation of β-ketoacyl-acyl carrier protein synthetase. Biochim. Biophys. Acta 326:155-166. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa, H., J.-T. Tsay, S. Jackowski, Y. Takamura, and C. O. Rock. 1993. Thiolactomycin resistance in Escherichia coli is associated with the multidrug resistance efflux pump encoded by emrAB. J. Bacteriol. 175:3723-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garwin, J. L., A. L. Klages, and J. E. Cronan, Jr. 1980. Structural, enzymatic, and genetic studies of β-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli. J. Biol. Chem. 255:11949-11956. [PubMed] [Google Scholar]
- 10.Hamada, S., T. Fujiwara, H. Shimauchi, Ogawa, T., T. Nishihara, T. Koga, T. Neheshi, and T. Matsuno. 1990. Antimicrobial activities of thiolactomycin against Gram-negative anaerobes associated with periodontal disease. Oral Microbiol. Immunol. 5:340-345. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi, T., O. Yamamoto, H. Sasaki, A. Kawaguchi, and H. Okazaki. 1983. Mechanism of action of the antibiotic thiolactomycin inhibition of fatty acid synthesis of Escherichia coli. Biochem. Biophys. Res. Commun. 115:1108-1113. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, T., O. Yamamoto, H. Sasaki, and H. Okazaki. 1984. Inhibition of fatty acid synthesis by the antibiotic thiolactomycin. J. Antibiot. (Tokyo) 37:1456-1461. [DOI] [PubMed] [Google Scholar]
- 13.Heath, R. J., and C. O. Rock. 1995. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J. Biol. Chem. 270:26538-26542. [DOI] [PubMed] [Google Scholar]
- 14.Heath, R. J., and C. O. Rock. 1996. Inhibition of β-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J. Biol. Chem. 271:10996-11000. [DOI] [PubMed] [Google Scholar]
- 15.Heath, R. J., and C. O. Rock. 1996. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 271:27795-27801. [DOI] [PubMed] [Google Scholar]
- 16.Jackowski, S., C. M. Murphy, J. E. Cronan, Jr., and C. O. Rock. 1989. Acetoacetyl-acyl carrier protein synthase: a target for the antibiotic thiolactomycin. J. Biol. Chem. 264:7624-7629. [PubMed] [Google Scholar]
- 17.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 47:110-119. [DOI] [PubMed] [Google Scholar]
- 18.Kauppinen, S., M. Siggaard-Anderson, and P. van Wettstein-Knowles. 1988. β-Ketoacyl-ACP synthase I of Escherichia coli: nucleotide sequence of the fabB gene and identification of the cerulenin binding residue. Carlsberg Res. Commun. 53:357-370. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi, A., H. Tomoda, S. Nozoe, S. Omura, and S. Okuda. 1982. Mechanism of action of cerulenin on fatty acid synthetase. Effect of cerulenin on iodoacetamide-induced malonyl-CoA decarboxylase activity. J. Biochem. (Tokyo) 92:7-12. [DOI] [PubMed] [Google Scholar]
- 20.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature (London) 405:914-919. [DOI] [PubMed] [Google Scholar]
- 21.Kremer, L., J. D. Douglas, A. R. Baulard, C. Morehouse, M. R. Guy, D. Alland, L. G. Dover, J. H. Lakey, W. R. Jacobs, Jr., P. J. Brennan, D. E. Minnikin, and G. S. Besra. 2000. Thiolactomycin and related analogues as novel anti-mycobacterial agents targeting KasA and KasB condensing enzymes in Mycobacterium tuberculosis. J. Biol. Chem. 275:16857-16864. [DOI] [PubMed] [Google Scholar]
- 22.Kuhajda, F. P., E. S. Pizer, J. N. Li, N. S. Mani, G. L. Frehywot, and C. A. Townsend. 2000. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. USA 97:3450-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loftus, T. M., D. E. Jaworsky, G. L. Frehywot, C. A. Townsend, G. V. Ronnett, M. D. Lane, and F. P. Kuhajda. 2000. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288:2379-2381. [DOI] [PubMed] [Google Scholar]
- 24.Lomovskaya, O., K. Lewis, and A. Matin. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 177:2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Miyakawa, S., K. Suzuki, T. Noto, Y. Harada, and H. Okazaki. 1982. Thiolactomycin a new antibiotic. IV. Biological properties and chemotherapeutic activity in mice. J. Antibiot. (Tokyo) 35:411-419. [DOI] [PubMed] [Google Scholar]
- 27.Moche, M., G. Schneider, P. Edwards, K. Dehesh, and Y. Lindqvist. 1999. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. J. Biol. Chem. 274:6031-6034. [DOI] [PubMed] [Google Scholar]
- 28.Morita, Y. S., K. S. Paul, and P. T. Englund. 2000. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science 288:140-143. [DOI] [PubMed] [Google Scholar]
- 29.Noto, T., S. Miyakawa, H. Oishi, H. Endo, and H. Okazaki. 1982. Thiolactomycin, a new antibiotic. III. In vitro antibacterial activity. J. Antibiot. (Tokyo) 35:401-410. [DOI] [PubMed] [Google Scholar]
- 30.Omura, S. 1976. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Microbiol. Rev. 40:681-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omura, S. 1981. Cerulenin. Methods Enzymol. 72:520-532. [PubMed] [Google Scholar]
- 32.Price, A. C., K. H. Choi, R. J. Heath, Z. Li, C. O. Rock, and S. W. White. 2001. Inhibition of β-ketoacyl-[acyl carrier protein] synthases by thiolactomycin and cerulenin: structure and mechanism. J. Biol. Chem. 276:6551-6559. [DOI] [PubMed] [Google Scholar]
- 33.Rarey, M., B. Kramer, T. Lengauer, and G. Kelbe. 1996. Predicting receptor-ligand interactions by an incremental construction algorithm. J. Mol. Biol. 261:470-489. [DOI] [PubMed] [Google Scholar]
- 34.Rock, C. O., and J. E. Cronan, Jr. 1996. Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim. Biophys. Acta 1302:1-16. [DOI] [PubMed] [Google Scholar]
- 35.Rock, C. O., and J. L. Garwin. 1979. Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J. Biol. Chem. 254:7123-7128. [PubMed] [Google Scholar]
- 36.Schujman, G. E., K.-H. Choi, S. Altabe, C. O. Rock, and D. de Mendoza. 2001. Response of Bacillus subtilis to cerulenin and the acquisition of resistance. J. Bacteriol. 183:3032-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweizer, H. P. 1998. Intrinsic resistance to inhibitors of fatty acid biosynthesis in Pseudomonas aeruginosa is due to efflux: application of a novel technique for generation of unmarked chromosomal mutations for the study of efflux systems. Antimicrob. Agents Chemother. 42:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh, U. C., and P. A. Kollman. 1984. An approach to computing electrostatic charges for molecules. J. Comput. Chem. 5:129-145. [Google Scholar]
- 39.Slayden, R. A., R. E. Lee, J. W. Armour, A. M. Cooper, I. M. Orme, P. J. Brennan, and G. S. Besra. 1996. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis. Antimicrob. Agents Chemother. 40:2813-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsay, J.-T., C. O. Rock, and S. Jackowski. 1992. Overproduction of β-ketoacyl-acyl carrier protein synthase I imparts thiolactomycin resistance to Escherichia coli K-12. J. Bacteriol. 174:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vance, D. E., I. Goldberg, O. Mitsuhashi, K. Bloch, S. Omura, and S. Nomura. 1972. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem. Biophys. Res. Commun. 48:649-656. [DOI] [PubMed] [Google Scholar]
- 42.Waller, R. F., P. J. Keeling, R. G. K. Donald, B. Striepen, E. Handman, N. Kang-Unnasch, A. F. Cowman, G. S. Besra, D. Roos, and G. I. McFadden. 1998. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 95:12352-12357. [DOI] [PMC free article] [PubMed] [Google Scholar]