Abstract
A newly discovered gene, designated tcrB, which is located on a conjugative plasmid conferring acquired copper resistance in Enterococcus faecium, was identified in an isolate from a pig. The tcrB gene encodes a putative protein belonging to the CPx-type ATPase family with homology (46%) to the CopB protein from Enterococcus hirae. The tcrB gene was found in E. faecium isolated from pigs (75%), broilers (34%), calves (16%), and humans (10%) but not in isolates from sheep. Resistant isolates, containing the tcrB gene, grew on brain heart infusion agar plates containing up to 28 mM CuSO4 compared to only 4 mM for the susceptible isolates. Copper resistance, and therefore the presence of the tcrB gene, was strongly correlated to macrolide and glycopeptide resistance in isolates from pigs, and the tcrB gene was shown to be located on the same conjugative plasmid as the genes responsible for resistance to these two antimicrobial agents. The frequent occurrence of this new copper resistance gene in isolates from pigs, where copper sulfate is being used in large amounts as feed additive, suggests that the use of copper has selected for resistance.
Copper in the form of copper sulfate (CuSO4) is commonly used as a feed supplement in food animal production. In Denmark the use is most pronounced in piglets receiving concentrations of ca. 165 ppm but also in slaughter pigs (25 ppm), broilers (20 ppm), and calves (2 ppm) that receive copper sulfate in their feed (Danish Agricultural Advisory Center, unpublished data). In contrast, neither sheep nor humans receive additional supplements of copper in their diet. In fact, sheep are hypersensitive to copper and can easily suffer intoxication if the feed contains copper or if they are allowed to graze on plains fertilized with manure from pigs that have been fed copper in their diet (11). It is therefore to be expected that the intestinal flora of pigs in particular, but also of broilers, is adapted to higher concentrations of copper, whereas the intestinal flora of sheep and humans should not need this adaptation.
Copper is an essential cofactor in many of the enzymatic processes taking place within the cell. However, copper is extremely reactive and for that reason it is toxic to the cell in high concentrations. Therefore, all living organisms have developed mechanisms to maintain a suitable level of intracellular copper. Copper homeostasis in most living organisms is mediated by a group of membrane-spanning proteins called CPx-type ATPases (17), which are encoded by genes located on the chromosome. The most extensively studied copper homeostasis system in bacteria is the cop operon from the gram-positive bacterium Enterococcus hirae (reviewed in reference 20). The cop operon consists of four genes (copYZAB) controlled by the same promoter (21). The copY gene product (CopY) acts as a regulator of the promoter (18), and the copZ gene product (CopZ) is the intracellular copper chaperone responsible for intracellular copper transport (15). CopA and CopB are membrane-located proteins of the CPx-type ATPase family coded by the two most distal genes (called copA and copB, respectively) of the operon (15, 16). CopA is the protein responsible for transporting copper into the cell under copper-limiting conditions, whereas CopB excludes copper from the bacteria when intracellular copper reaches toxic levels. The cop operon is inducible upon the addition of copper to the growth medium, since this removes the negative regulator CopY from the promoter region and allows the transcription of cop genes (14).
A few cases of acquired resistance to copper have been reported. Copper resistance genes are often located on plasmids, as opposed to the chromosomal genes involved in copper homeostasis, and are in most case transferable. Acquired and transferable copper resistance has been most thoroughly studied in gram-negative bacteria such as the pco genes from Escherichia coli (7) and the cop genes from Pseudomonas syringae (13), but none of these belong to the CPx-type ATPase family. Very few cases of copper resistance in gram-positive bacteria have been reported (12), and in such cases the resistance mechanisms are unknown.
Here we describe the identification of the first transferable and plasmid-located copper resistance gene, designated tcrB (transferable copper resistance homologous to copB), encoding a putative protein belonging to the CPx-type ATPase family and the first case of transferable copper resistance in gram-positive bacteria. It confers a sevenfold increase of resistance toward copper. The gene was detected on a plasmid from an E. faecium strain isolated from a pig, but our results indicate a widespread presence of this gene in isolates from pigs, broilers, and calves in a quantity corresponding to the amounts of copper present in the diet of each group of animals. Furthermore, the tcrB gene is genetically linked to genes encoding resistance to macrolides [erm(B)] and glycopeptides (vanA) in the plasmids originating from pig isolates.
MATERIALS AND METHODS
Bacterial strains and MIC studies.
A total of 59 E. faecium isolates from pigs, 29 E. faecium isolates from broilers, 32 E. faecium isolates from calves, and 29 E. faecium isolates from humans were selected from a previously described collection (2). The isolates from pigs, broilers, and calves were collected from healthy animals at or just prior to slaughter during the first 9 months of 1998. The isolates from humans were isolated from stool samples submitted for diagnostic purposes in March 1998. All isolates had previously been tested for susceptibility to the following antimicrobial agents by using a commercial prepared dehydrated panel (SensiTitre): avilamycin, bacitracin, chloramphenicol, erythromycin, gentamicin, kanamycin, penicillin, quinupristin-dalfopristin, streptomycin, tetracycline, vancomycin, and virginiamycin (4).
A total of 22 E. faecium strains were isolated from fecal samples from sheep submitted to the Danish Veterinary Laboratory in April 2000 for diagnostic purposes. The strains were isolated and identified as previously described (3) and tested for susceptibility to antimicrobial agents as mentioned above.
A plasmid with the approximate size of 175 kb from the copper-resistant E. faecium A17sv1 was chosen for sequencing. E. faecium A17sv1 was isolated from a pig (deposited in the strain collection at the Pasteur Institute [http://www.pasteur.fr], accession number CIP 106701) in 1995. Of the previously mentioned antimicrobial agents, the A17sv1 isolate was resistant to erythromycin, penicillin, tetracycline, and vancomycin. Only the erythromycin and vancomycin resistance determinants are located on the plasmid (1). The strain has a characteristic pulsed-field gel electrophoretic type (9), and the genes conferring resistance to macrolides [erm(B) on Tn917] and glycopeptides (vanHAX on Tn1546) are located on this plasmid which is transferable by conjugation (1).
As recipient in all conjugation studies, the E. faecium reference strain BM4105RF was used. This strain is resistant to rifampin (25 μg/ml) and fusidic acid (25 μg/ml) but not to any of the other antibiotics tested in the SensiTitre panel. For cloning in E. coli, the electrocompetent E. coli strain XL10 Gold (Stratagene) was used.
Copper gradient assay.
The isolates were streaked on blood agar plates (Colombia agar supplemented with 5% bovine blood) and incubated overnight at 37°C. A single colony from each strain was restreaked on brain heart infusion (BHI) agar plates containing 1 mM CuSO4 · 5H2O (Sigma-Aldrich) to ensure the induction of copper resistance. This concentration is well below the inhibitory concentration for susceptible bacteria. Agar plates containing a copper gradient were prepared by overlaying 25-ml slants of solidified BHI agar containing 28 mM copper sulfate (CuSO4 · 5H2O, pH = 7) with 25 ml of BHI agar on square petri dishes (9 by 9 cm). The second layer was made the same day as the plates were used in order to avoid diffusion of the copper sulfate. From the agar plates containing 1 mM copper sulfate, colonies were picked and resuspended in 0.9% NaCl to a cell density of ca. 108 cells/ml (McFarland = 0.5). Then, 30 μl of this suspension was then streaked across the plates containing the copper gradient and incubated at 37°C for 24 h. The length of growth from the bottom of the plate was measured and compared to the known susceptible strain BM4105RF.
MIC determination of copper sulfate by the agar dilution method.
The MIC of copper sulfate was determined for 22 selected isolates as recommended by the NCCLS guidelines. BHI agar plates containing 0, 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, and 40 mM copper sulfate (CuSO4 · 5H2O) adjusted to pH = 7 was inoculated with a single drop of cells (i.e., ca. 5 × 105 cells) picked from an agar plate containing 1 mM copper sulfate and suspended in a 0.9% NaCl solution adjusted to McFarland = 0.5 as described above. The plates were then incubated at 37°C for 16 to 20 h, and the growth was assessed.
Construction of the Sau3AI library from pA17sv1.
The E. faecium strain A17sv1 was inoculated in an Erlenmeyer flask containing 300 ml of BHI medium supplemented with 32 μg of vancomycin and 16 μg of erythromycin/ml and grown at 37°C with rotary shaking (120 to 150 rpm). Plasmid DNA was then purified by using a modified protocol for the Qiagen Plasmid Midi kit (Qiagen). Cells were harvested in a Beckman centrifuge at 6,000 × g for 10 min and resuspended in 10 ml of P1 buffer (50 mM Tris-Cl, pH 8.0; 10 mM EDTA) containing 100 μg of RNase (Qiagen) and 20 mg of lysozyme (Sigma-Aldrich)/ml. The suspension was placed on a rotary shaker (250 rpm) at 37°C for 15 min, and then 10 ml of P2 buffer (200 mM NaOH, 1% sodium dodecyl sulfate) and 10 ml of P3 buffer (3.0 M potassium acetate; pH 5.5) was added as described in the Qiagen protocol. The DNA was then purified on a Qiagen Midi column as described by the Qiagen Plasmid Midi protocol.
Plasmid DNA was partially digested by using 0.00125 U of Sau3AI restriction endonuclease (Amersham Pharmacia Biotech)/μl for 1 h and run on a 1% Tris-borate EDTA agarose gel. A gel piece containing fragments of ca. 2 to 6 kb was cut from the gel and purified by using the GFX PCR DNA and a gel band purification kit (Amersham Pharmacia Biotech). This digested DNA was ligated to the pUC18 vector, which was cut with BamHI (Amersham Pharmacia Biotech) and dephosphorylated (USB). After ligation, the DNA was transformed into electrocompetent E. coli XL10 Gold. Transformants were selected on BHI agar containing 100 μg of ampicillin/ml, and DNA was purified by using a QIAprep Spin Miniprep kit (Qiagen). Clones containing inserts were subjected to nucleotide sequencing as described below.
PCR and cloning.
Based on the high sequence homology to the HRA-1 gene--a gene coding for a putative CPx-type ATPase (19) obtained from the NCBI GenBank database (GenBank accession number ECU16658) of one of the cloned Sau3AI fragments from the pA17sv1 library described above--two primers were designed to cover the complete open reading frame: primer 432 (5′-GGGGAATTCTATAATAAGGAGGAATTAAAAATGAGG-3′) carrying an EcoRI site (underlined) and primer 433 (5′-CGCGGATCCAAATAACTGTTTATTTTGGA-3′) carrying a BamHI site (underlined) in a PCR by using the Expand High Fidelity PCR system (Boehringer Mannheim). The 2,180-bp PCR product was digested with EcoRI and BamHI (BioLabs) and ligated into the E. coli vector pUC19, which was likewise digested with the two enzymes. The ligated DNA was transformed into electroporation-competent E. coli XL10 Gold cells, and plasmid DNA was purified by using the QIAprep Spin Miniprep kit (Qiagen). Inserts were detected by restriction enzyme analysis. A plasmid containing the PCR fragment was then subjected to sequencing as described below.
DNA sequencing.
Cycle sequencing of plasmid DNA and PCR products was carried out according to the manufacturer's instructions by using an AmpliTaq dye terminator kit and a 373A automatic sequencer (Applied Biosystems/Perkin-Elmer, Foster City, Calif.) with DNASIS software (Hitachi Software Engineering Co., Ltd) and the WinseqEZ for windows 95/98 (courtesy of F. G. Hansen, Biocentrum DTU, Lyngby, Denmark). The following primers were used for sequencing of the tcrB gene: primer 415 (5′-TGACAATAAGGCAACGATTTC-3′), primer 416 (5′-CCAGGCATGATGTCCTTG-3′), primer 456 (5′-AACTGGTGAGTTTAAAGTATTAGATG-3′), primer 462 (5′-ATCTTGAGGAGATTGATTAGCTAG-3′), and primer 511 (5′-GGAAAGGCAACTGAATATCC-3′).
Detection of the tcrB gene.
Boiling lysates were made as previously described (10). For detection of the tcrB gene within all of the tested strains, primers 415 and 416 (see above) were used in a standard PCR on the boiling lysates.
Transferability of resistance.
Conjugation of macrolide and copper resistance was performed with eight macrolide-resistant isolates from pigs by using the filter-mating procedure as described by Clewell et al. (8). E. faecium BM4105RF, resistant to rifampin and fusidic acid, was used as a recipient. Transconjugants were selected on BHI agar plates containing rifampin (25 μg/ml), fusidic acid (25 μg/ml), and erythromycin (16 μg/ml). In the case of selection on 16 and 28 mM copper sulfate (CuSO4 · 5H2O), the melted agar was adjusted to pH = 7 after the addition of the copper sulfate.
Statistical methods.
The association between resistance to copper and erythromycin and vancomycin was calculated by the chi-square test by using EpiInfo version 6.02. The significant level was 5% (two-tailed test).
Nucleotide sequence accession number.
The complete sequence of the tcrB gene has been submitted to the GenBank database (http://www.ncbi.nlm.nih.gov; accession number AY048044).
RESULTS
Copper resistance among E. faecium isolates from animals and humans.
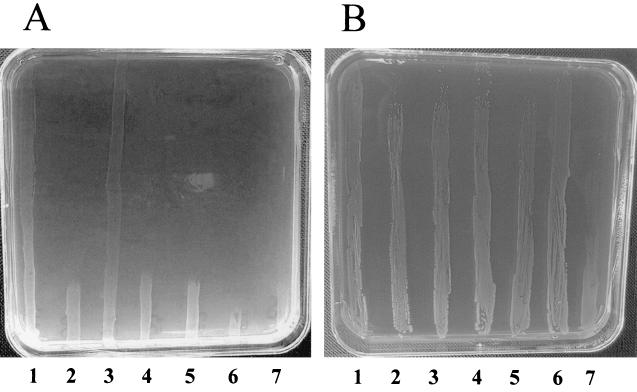
In order to examine the copper resistance status in different reservoirs, 59 E. faecium strains isolated from pigs, 29 strains isolated from broilers, 32 strains isolated from calves, 22 strains isolated from sheep, and 29 strains isolated from humans were studied. The E. faecium isolates could be differentiated into two separate groups: one group which did not grow on high concentrations of copper and one group which tolerated even the highest amounts of copper within the plates (28 mM copper sulfate). An example is shown in Fig. 1A. Between the five groups, large differences in the number of copper resistant isolates were found (Table 1). Most resistance was found in isolates of porcine (76%) and poultry (34%) origin, and less resistance was found in isolates from calves (16%) and from humans (10%); we did not find any copper resistance among the sheep isolates.
FIG. 1.
Copper gradient BHI agar plate containing between 0 mM (bottom of the plate) and 28 mM (top of the plate) copper sulfate. (A) Lanes 1 and 3 contains copper-resistant strains, whereas lanes 2, 4, 5, 6, and 7 all contain copper susceptible strains. (B) Lanes 1, 3, and 5 contain copper-resistant donor strains, whereas lanes 2, 4, and 6 contain their respective transconjugants, and lane 7 contains the recipient strain (BM4105RF).
TABLE 1.
The number of E. faecium isolates examined from different origins, the occurrence of copper resistance, and the approximate use of copper sulfate in each group
| Origin | No. of isolates | No. of copper-resistant isolates (%) | CuSO4 (ppm) in feed |
|---|---|---|---|
| Pig | 59 | 45 (76) | 165/25a |
| Broiler | 29 | 10 (34) | 20 |
| Calve | 32 | 5 (16) | 2 |
| Sheep | 22 | 0 (0) | 0 |
| Human | 29 | 3 (10) | 0 |
That is, 165 ppm for weaners and 25 ppm for slaughter pigs.
The copper resistance phenotype is biphasic.
The growth of a total of 22 selected isolates from the five populations was tested on plates containing increasing amounts of copper sulfate in order to determine the MIC of copper for the two groups. Isolates, which were susceptible to copper on gradient agar, were able to grow on plates containing up to 4 mM copper sulfate. The copper-resistant strains, however, showed a biphasic growth phenotype, being able to grow well on plates containing 0, 2, 4, 16, and 28 mM copper sulfate, but they did not grow, or grew very poorly, on 8, 12, 20, and 24 mM, as well as at concentrations of copper sulfate that were >28 mM. Only after prolonged incubation (3 to 4 days) was very weak growth visible on the plates containing 8, 12, 20, and 24 mM CuSO4.
Correlation between copper resistance and resistance to macrolide and glycopeptides.
As the tested isolates are part of our DANMAP surveillance program (3), it was possible to correlate the copper resistance with resistance to macrolides and glycopeptides of each of the isolates (Table 2). Among the isolates from pigs, a strong correlation between copper resistance and both macrolide resistance (P < 0.0001) and glycopeptide resistance (P = 0.026) was found. Of the 45 copper-resistant pig isolates, 41 were resistant to macrolides and 14 were resistant to both macrolides and glycopeptides. The strains, which were resistant to glycopeptides, were all simultaneously resistant to macrolides and copper, and all strains resistant to macrolide were resistant to copper. Four strains were resistant to copper without being resistant to either macrolides or glycopeptides.
TABLE 2.
Number of copper-resistant (Copr) and copper-susceptible (Cops) E. faecium isolates from each group of animals and humans correlated with resistance to macrolide (Eryr) and glycopeptide (Vanr) resistance
| Source and copper resistance phenotype | No. of E. faecium isolates
|
|||
|---|---|---|---|---|
| Eryr | Erys | Vanr | Vans | |
| Piga | ||||
| Copr | 41 | 4 | 14 | 31 |
| Cops | 0 | 14 | 0 | 14 |
| Broilerb | ||||
| Copr | 5 | 5 | 3 | 7 |
| Cops | 11 | 8 | 0 | 19 |
| Calfc | ||||
| Copr | 2 | 3 | 0 | 5 |
| Cops | 2 | 25 | 0 | 27 |
| Sheepc | ||||
| Copr | 2 | 3 | 0 | 5 |
| Cops | 2 | 25 | 0 | 27 |
| Humanc | ||||
| Copr | 1 | 2 | 0 | 3 |
| Cops | 12 | 14 | 0 | 26 |
The level of Copr is significantly higher for Eryr versus Erys (P < 0.0001) and for Vanr versus Vans (P = 0.026)
The level of Copr is not significantly higher for Eryr versus Erys but it is significantly higher for Vanr versus Vans (P = 0.033).
The level of Copr is neither significantly higher (P > 0.05) for Eryr versus Erys nor for Vanr versus Vans.
To investigate whether this correlation was a result of the same macrolide- and copper-resistant clone present in the pig population in Denmark, 19 of the 59 isolates were randomly selected for pulsed-field gel electrophoresis. Only four of the macrolide- and copper-resistant isolates were the same clone, and they were all glycopeptide resistant, thus confirming the previously published clonality of this subset of E. faecium (1). This finding indicates that clonality cannot explain the correlation between copper and macrolide resistance.
Among the isolates from broilers, only 5 of the 10 copper-resistant strains were also resistant to macrolides (Table 2) but the three isolates, which were resistant to glycopeptides, were all copper resistant (P = 0.033). Only five isolates from calves were resistant to copper and, of these, only two isolates were resistant to macrolides and no isolates were resistant to glycopeptides. Within the bacterial population from sheep, we did not observe resistance to macrolides, glycopeptides, or copper. Among the human isolates, three were found to be copper resistant, but only one of these was also resistant to macrolides. None of the isolates of human origin were resistant to glycopeptides.
The copper resistance phenotype is transferable by conjugation.
Of the 41 copper- and macrolide-resistant isolates obtained from pigs, 8 were randomly selected and conjugated with the macrolide- and copper-susceptible E. faecium strain BM4105RF as described in Materials and Methods. The transconjugants were scored on agar plates containing erythromycin, rifampin, and fusidic acid. These transconjugants were tested on gradient agar (Fig. 1B) containing copper sulfate as described above. In all eight cases, the copper resistance cotransferred with the macrolide resistance, indicating that the copper resistance determinant is located on the same transferable element as the macrolide resistance gene, which in all cases was shown to be the erm(B) gene by PCR (data not shown). Additionally, three of the transconjugants had also become resistant to tetracycline, three were kanamycin resistant, and four were resistant to streptomycin (of these, two transconjugants were resistant to all four antibiotics, as well as copper resistant). None of the transconjugants were resistant to quinupristin-dalfopristin. Therefore, the transferable plasmids do in some cases contain several other resistances apart from the macrolide and copper resistances.
Identification of a plasmid-located CopB-like CPx type ATPase.
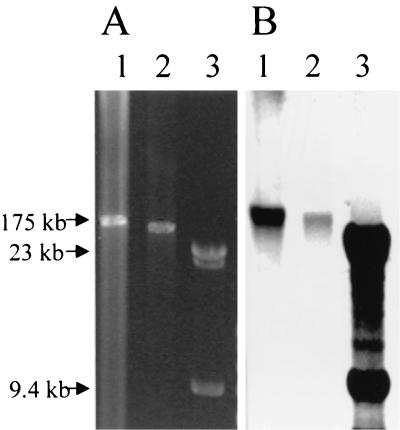
In order to locate the gene responsible for the copper resistance phenotype, a large plasmid (ca. 175 kb) from the copper-resistant E. faecium pig isolate A17sv1 was purified, and a DNA library was constructed. This plasmid is transferable and carries genes for glycopeptide and macrolide resistance. Sequencing fragments of the library revealed part of an open reading frame with strong homology to a previously sequenced gene from E. coli coding for a protein (HRA-1) of the CPx type ATPase family (19). From this, it was possible to sequence the complete sequence of the open reading frame (orf710), which codes for a putative protein of 710 amino acids showing 92% identity to the HRA-1 protein and 46% identity to the CopB protein from E. hirae, suggesting it to be involved in copper efflux. Furthermore, this protein had all of the key features indicative for a CPx type ATPase (17), including the CPH motif thus far only identified in CopB from E. hirae. The purified plasmid from the wild-type strain A17sv1, and the corresponding transconjugant in BM4105RF was cut with SmaI, which only cuts once in the plasmid and subsequently run on an agarose gel (Fig. 2A). To confirm the presence of the orf710 gene on the plasmid, this gel was blotted to a nitrocellulose membrane and a Southern blot hybridization was performed with a tcrB-specific probe (Fig. 2B).
FIG. 2.
(A) Ethidium bromide-stained 0.5% agarose gel containing the purified plasmid from the wild-type A17sv1 strain (lane 1) and its corresponding transconjugant in BM4105RF digested with SmaI (lane 2). A Lambda HindIII molecular marker has been added (lane 3). (B) Southern blot of the same gel with a tcrB probe and a Lambda probe to detect the location of the tcrB gene and the Lambda fragments, respectively.
Only copper-resistant isolates carry the orf710 gene.
From the sequence of the orf710 gene, a specific set of primers was constructed to examine boiling lysates of all of the tested strains for the presence of the orf710 gene as described in Materials and Methods. Only the isolates with the copper-resistant phenotype tested positive for the orf710 gene, whereas all of the isolates susceptible to copper tested negative in the PCR. Likewise, the eight copper-resistant transconjugants described above from the macrolide-resistant pig isolates, but not BM4105RF itself, tested positive. Therefore, we propose the orf710 gene to be assigned the name tcrB (transferable copper resistance gene homologous to copB).
Transconjugants can be selected on 16 mM CuSO4.
In order to investigate whether copper sulfate could be used to select for transconjugants instead of macrolide, E. faecium A17sv1 was selected as the donor for conjugation with BM4105RF and plated out onto rifampin-fusidic acid agar plates also containing one of the following: (i) 16 mM erythromycin (16 μg/ml), (ii) 16 mM vancomycin (32 μg/ml), (iii) 16 mM copper sulfate, or (iv) 28 mM copper sulfate. Conjugation frequencies (i.e., the number of transconjugants per donor) from the different plates were as follows: for the plates containing vancomycin, rifampin, and fusidic acid the frequency was 1.7 × 103; for the plates containing erythromycin, rifampin, and fusidic acid the frequency was 1.7 × 103; for the plates containing 16 mM CuSO4, rifampin, and fusidic acid the frequency was 0.94 × 103; and for the plates containing 28 mM CuSO4, rifampin, and fusidic acid the frequency was zero. The frequencies for selection on erythromycin, vancomycin, and 16 mM copper sulfate are of the same magnitude, although the amount of transconjugants on the copper plates is lower than on the two other sets of plates, whereas selection on 28 mM copper sulfate did not yield any transconjugants. Not surprisingly, all transconjugants regardless of the selection method were simultaneously resistant to macrolides, glycopeptides, and copper sulfate.
DISCUSSION
Copper sulfate is a common feed supplement to pigs, broilers, and calves in Denmark. This mineral remains mostly unabsorbed in the gut and thus creates an intestinal environment with a high concentration of copper. Bacteria within this environment are therefore likely to have acquired copper resistance genes, or otherwise be adapted to these conditions, in order to survive. Here we set out to test five different populations of E. faecium of animal and human origin for their ability to grow in high concentrations of copper. We found a good agreement between the amount of copper used in the different populations and the level of copper resistance found in each group. The highest level of copper resistance (76%) was found in isolates from slaughter pigs. This is in good agreement with the fact that pigs in Denmark receive 165 ppm of copper sulfate as weaners (<35 kg) and ca. 25 ppm as slaughter pigs (>35 kg). Chickens, for which 34% of the isolates were resistant to copper, receive roughly the same amount of copper sulfate (ca. 20 ppm) as slaughter pigs. The difference in the level of copper resistance between these two populations could be explained by the high concentrations given to weaners, which could establish a large copper-resistant population in these animals. Calves also receive copper sulfate in their feed but in much smaller concentrations than pigs and broilers (ca. 2 ppm). Here only a few (16%) copper-resistant isolates were found, which is in good agreement with the amount of copper given to them. None of the bacteria isolated from sheep showed any resistance toward copper, which may not be surprising, since these animals are hypersensitive to this metal and therefore should have very low concentrations of copper in the gut. Among the human isolates, we were able to identify three isolates (10%) that were resistant to copper. The explanation for the presence in humans of these three copper-resistant isolates is most likely that they are of animal origin or from contaminated food, since the diet of humans does not contain excess copper.
In all of the copper-resistant isolates we identified a new transferable copper resistance gene, tcrB, from E. faecium. The putative protein of 710 amino acids, encoded by the tcrB gene, belongs to the CPx-type ATPase family of heavy metal transporters. A gene (HRA-1) that is almost identical to the tcrB gene (93% identity at the DNA level) found here in E. faecium has previously been suggested to originate from E. coli (19), but we find this unlikely. In the study by Trenor et al., a human small intestine cDNA library was screened with degenerated primers for human homologues of the human CPx-type ATPases related to Menkes and Wilson disease. These authors found two open reading frames of bacterial origin from this library and suspected them to originate from the E. coli host used to generate the library. However, we tested 20 different E. coli strains, including several of our K-12 strains, by PCR with the specific primers for both the HRA-1 gene and the tcrB gene (data not shown). None of these yielded a product, showing that the gene is not present in any of our tested E. coli strains. Furthermore, since 1994, several E. coli genomes have been sequenced without revealing any sequences with close homology to HRA-1. Also, since the GC content of the tcrB gene is 42%, it is more likely to originate from a bacterium with a low GC content, such as enterococci, than from E. coli, which normally has a GC ratio of ca. 50%. Finally, a search in the NCBI BLAST database of the TcrB protein reveals an overall amino acid identity of 46% to the CopB protein from E. hirae as the most homologous protein, and only 30% identity to anything of E. coli origin, which further confirms its membership of the Enterococcus family. We therefore propose the true origin of the HRA-1 gene as well as the tcrB gene to be from enterococci and not from E. coli.
The presence of the tcrB gene in all of the isolates was tested by PCR, and it was shown to be harbored by all copper-resistant E. faecium isolates regardless of the origin of the bacteria. In contrast, we did not find any isolates susceptible to copper, which had the gene. It therefore seems plausible to conclude that this gene is responsible for most of the copper resistance found in E. faecium of animal and human origin, at least in Denmark.
The growth of resistant strains, containing the tcrB gene, in increasing concentrations of copper sulfate, which were toxic for the susceptible strains, was biphasic in the sense that cells containing the gene grew well on plates containing 16 and 28 mM copper sulfate but very poorly on 8 to 12 mM or 20 to 24 mM copper sulfate. This could indicate that the expression of the tcrB gene is inducible upon increasing amounts of copper and that the induction occurs in two stages: one at a copper concentration of ∼16 mM and one at ∼28 mM. Since nothing is yet known about the regulation of tcrB, it is difficult to explain this genetically. One explanation could be that the tcrB gene is part of an operon very similar to the cop operon of E. hirae. E. faecium itself is most likely to contain a chromosomally located cop operon to maintain copper homeostasis in addition to the plasmid-acquired tcrB operon, and therefore the CopY and possibly CopZ proteins from the chromosome could be interfering with the regulation of the tcrB expression. The sequence of the E. faecium chromosome has recently been made available online (http://www.jgi.doe.gov/JGI_microbial/html/enterococcus/enterococcus_mainpage.html). A BLAST search here, with the E. hirae copYZAB gene cluster as a template, did reveal a similar copYZAB gene cluster (72% overall identity), further confirming the presence of a chromosomally located copper homeostasis gene cluster. Another possibility is that a plasmid-located influx gene similar to copA is coregulated with the tcrB gene and therefore is counteracting the effect of the TcrB protein at certain concentrations of copper.
Among the isolates from pigs we found a strong correlation between copper resistance and both macrolide resistance and glycopeptide resistance. We have previously shown the glycopeptide and macrolide resistance genes to be linked within this population of E. faecium isolates (1, 6), but now it is clear that the tcrB gene, and thus the copper resistance phenotype, is likewise linked to these two resistance genes. This is further fueled by the fact that we were able to purify a 175-kb plasmid from a pig isolate (E. faecium A17sv1) carrying the genes for glycopeptide resistance (the vanA gene cluster) and macrolide resistance [the erm(B) gene], along with the tcrB gene.
In order to examine the transferability of the tcrB gene, we tested eight randomly selected macrolide-resistant strains isolated from pigs for their ability to transfer the tcrB gene to a recipient susceptible to copper along with the macrolide resistance [coded by the erm(B) gene]. In all eight cases we were able to cotransfer the tcrB gene and thus the copper resistance phenotype with the macrolide resistance. From this, we cannot be completely certain that the two genes are always located on the same transferable element in E. faecium isolates from pigs. However, considering the strong correlation between macrolide and copper resistance and the detection of the tcrB gene on the same plasmid as the erm(B) gene from E. faecium A17sv1, it seems likely that these two genes are physically linked in our pig isolates. In a similar manner, glycopeptide resistance seems to be linked to the copper resistance in the pig isolates. In isolates from broilers, there were no significant correlations between copper resistance and macrolide resistance, but the three isolates, which were resistant to glycopeptides, were all resistant to copper. Even though this is based on three isolates only, this was statistically significant (P = 0.033). Copper and glycopeptide resistance could therefore be linked in isolates from broilers as well, but this is currently under investigation.
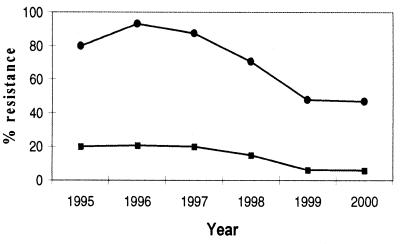
In Denmark, the two animal growth promoters tylosin and avoparcin, which belong to the macrolides and glycopeptides, respectively, have been banned (macrolides in 1998 and glycopeptides in 1995) in order to reduce the resistance of bacteria, which can possess a risk to the public health. From the in vitro results presented here it is clear that copper sulfate alone is a selective factor for conjugation and transfer of the resistances to macrolides and glycopeptides in E. faecium, when the resistance genes are located on the same transferable element. A major question is whether the continued use of copper sulfate as an additive, particularly on pig farms, can maintain resistance within the E. faecium population. The genetic linkage found here could ultimately lead to a situation in which the use of copper sulfate maintains resistance to macrolides and glycopeptides among E. faecium in pigs. This will ultimately have to await feeding experiments of pigs with or without copper sulfate. Since the use of copper sulfate in pig production is still very common, this could explain why the occurrence of macrolide-resistant E. faecium in Denmark seems to have reached a plateau of ca. 47% while that of glycopeptide-resistant E. faecium has reached a plateau of ca. 6% (5), with virtually no change from 1999 to 2000 (Fig. 3). Tylosin is, however, still used for the treatment of infections in pigs, and this could also be the explanation for macrolide resistance in Denmark. Copper could likewise select for resistance among other bacterial populations. However, these aspects will have to await further data.
FIG. 3.
Percent resistance of E. faecium isolates from pigs to macrolides (•) and glycopeptides (▪) from 1995 to 2000 (5).
Besides copper and antibiotic agents used for therapy, a large number of other chemical substances with antibacterial activity, such as detergents, are used in human and animal environments. It must be expected that bacteria in increasing frequency have adapted to these substances.
In conclusion, a new transferable copper resistance gene was identified. Copper resistance in E. faecium isolated from both animal and human reservoirs showed a good correlation between the use of copper sulfate in the feed and the number of copper-resistant bacteria isolated from each reservoir. Furthermore, copper resistance was genetically linked to macrolide and glycopeptide resistance in E. faecium isolates from pigs.
Acknowledgments
This study was supported by a grant from the Danish Directorate for Development (98-3324).
We thank Dorte S. Nielsen, Helene Christensen, and Rene Hendriksen for their excellent help and technical assistance and Anette Villadsen for help with some of the transferability studies.
REFERENCES
- 1.Aarestrup, F. M. 2000. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 38:2774-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 3.Aarestrup, F. M., F. Bager, N. E. Jensen, M. Madsen, A. Meyling, and H. C. Wegener. 1998. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS 106:606-622. [DOI] [PubMed] [Google Scholar]
- 4.Aarestrup, F. M., H. Kruse, E. Tast, A. M. Hammerum, and L. B. Jensen. 2000. Associations between the use of antimicrobial agents for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers and pigs in Denmark, Finland, and Norway. Microb. Drug Resist. 6:63-70. [DOI] [PubMed] [Google Scholar]
- 5.Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bager, F., F. M. Aarestrup, M. Madsen, and H. C. Wegener. 1999. Glycopeptide resistance in Enterococcus faecium from broilers and pigs following discontinued use of avoparcin. Microb. Drug Resist. 5:53-56. [DOI] [PubMed] [Google Scholar]
- 7.Brown, N. L., S. R. Barrett, J. Camakaris, B. T. Lee, and D. A. Rouch. 1995. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 17:1153-1166. [DOI] [PubMed] [Google Scholar]
- 8.Clewell, D. B., F. Y. An, B. A. White, and C. Gawron-Burke. 1985. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J. Bacteriol. 162:1212-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammerum, A. M., V. Fussing, F. M. Aarestrup, and H. C. Wegener. 2000. Characterization of vancomycin-resistant and vancomycin-susceptible Enterococcus faecium isolates from humans, chickens and pigs by RiboPrinting and pulsed-field gel electrophoresis. J. Antimicrob. Chemother. 45:677-680. [DOI] [PubMed] [Google Scholar]
- 10.Jensen, L. B., P. Ahrens, L. Dons, R. N. Jones, A. M. Hammerum, and F. M. Aarestrup. 1998. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J. Clin. Microbiol. 36:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr, L. A., and H. D. McGavin. 1991. Chronic copper poisoning in sheep grazing pastures fertilized with swine manure. J. Am. Vet. Med. Assoc. 198:99-101. [PubMed] [Google Scholar]
- 12.Leelawatcharamas, V., L. G. Chia, P. Charoenchai, N. Kunajakr, C. Q. Liu, and N. W. Dunn. 1997. Plasmid-encoded copper resistance in Lactococcus lactis. Biotechnol. Lett. 19:639-643. [Google Scholar]
- 13.Lim, C. K., and D. A. Cooksey. 1993. Characterization of chromosomal homologs of the plasmid-borne copper resistance operon of Pseudomonas syringae. J. Bacteriol. 175:4492-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odermatt, A., R. Krapf, and M. Solioz. 1994. Induction of the putative copper ATPases, CopA and CopB, of Enterococcus hirae by Ag+ and Cu2+, and Ag+ extrusion by CopB. Biochem. Biophys. Res. Commun. 202:44-48. [DOI] [PubMed] [Google Scholar]
- 15.Odermatt, A., and M. Solioz. 1995. Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J. Biol. Chem. 270:4349-4354. [DOI] [PubMed] [Google Scholar]
- 16.Odermatt, A., H. Suter, R. Krapf, and M. Solioz. 1993. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 268:12775-12779. [PubMed] [Google Scholar]
- 17.Solioz, M., and C. Vulpe. 1996. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem. Sci. 21:237-241. [PubMed] [Google Scholar]
- 18.Strausak, D., and M. Solioz. 1997. CopY is a copper-inducible repressor of the Enterococcus hirae copper ATPases. J. Biol. Chem. 272:8932-8936. [DOI] [PubMed] [Google Scholar]
- 19.Trenor, C., III, W. Lin, and N. C. Andrews. 1994. Novel bacterial P-type ATPases with histidine-rich heavy-metal-associated sequences. Biochem. Biophys. Res. Commun. 205:1644-1650. [DOI] [PubMed] [Google Scholar]
- 20.Wunderli-Ye, H., and M. Solioz. 1999. Copper homeostasis in Enterococcus hirae. Adv. Exp. Med. Biol. 448:255-264. [DOI] [PubMed] [Google Scholar]
- 21.Wunderli-Ye, H., and M. Solioz. 1999. Effects of promoter mutations on the in vivo regulation of the cop operon of Enterococcus hirae by copper(I) and copper(II). Biochem. Biophys. Res. Commun. 259:443-449. [DOI] [PubMed] [Google Scholar]