Abstract
E2A proteins regulate multiple stages of thymocyte development and suppress T-cell lymphoma. The activity of E2A proteins throughout thymocyte development is modulated by signals emanating from the pre-TCR and TCR. Here we demonstrate that E2A is required for the complete arrest in both differentiation and proliferation observed in thymocytes with defects in proteins that mediate pre-TCR signaling, including LAT, Lck and Fyn. We show that E2A proteins are required to prevent the accumulation of TCRβ negative cells beyond the pre-TCR checkpoint. E2A-deficient thymocytes also exhibit abnormal cell-cycle progression prior to pre-TCR expression. Furthermore, we demonstrate that E47 can act in concert with Bcl-2 to induce cell-cycle arrest in vitro. These observations indicate that E2A proteins function during early thymocyte development to block cell-cycle progression prior to the expression of TCRβ. In addition, these data provide further insight into how deficiencies in E2A lead to T lymphoma.
Keywords: β-selection, cell cycle, E proteins, Id, T-cell development
Introduction
The development of αβ T cells in the thymus is a process ordered by the sequential rearrangement and expression of T-cell antigen receptor (TCR) genes (Zuniga-Pflucker and Lenardo, 1996). Shortly after lineage commitment, T-cell progenitors initiate TCRβ chain rearrangements. Murine thymocytes at this stage are characterized by the absence of surface expression of the CD4 and CD8 coreceptors, together with high CD25 and low CD44 levels. Such cells also express high levels of Bcl-2, and are quiescent (Veis et al, 1993; Hoffman et al, 1996; Voll et al, 2000). Upon rearrangement and expression of a functional TCRβ chain and its assembly into a pre-TCR complex, thymocytes initiate a developmental transition characterized by rapid proliferation and expansion. During this phase, murine thymocytes first downregulate Bcl-2 and CD25, and then begin to express CD8, followed by CD4. CD4 and CD8 double positive (DP) cells, which comprise about 80% of normal adult thymocytes, then exit the cell cycle, initiate TCRα gene rearrangement, and undergo positive and negative selection to allow maturation of only cells with moderate affinity for antigen/MHC complexes expressed by thymic antigen presenting cells. Positively selected thymocytes downregulate expression of either CD4 or CD8 to become mature CD8 or CD4 single positive (SP) T cells.
Studies employing mice with targeted mutations have demonstrated the importance of the pre-TCR complex for transition from the CD4, CD8 double negative (DN) to the DP stage (Kruisbeek et al, 2000). Deficiencies in proteins required for pre-TCR assembly result in arrest at the CD25+, CD44low (DN3) stage. Such proteins include the components of the pre-TCR complex, as well as factors required for TCR gene rearrangement. A number of cytoplasmic proteins have also been identified as critical for pre-TCR signaling. For example, thymocytes with mutations in the adaptor proteins LAT and SLP-76, or in both the Lck and Fyn proto-oncogenes are also arrested at the DN3 stage. Furthermore, both overexpression of the activity of signal transduction pathway components such as Lck, or null mutations in proteins such as Csk that act to inhibit TCR-mediated signaling have been shown to promote the aberrant maturation of thymocytes with pre-TCR expression defects. Such studies have, in particular, suggested an important role for signaling through the Ras-MAP kinase pathway in transducing signals from the TCR and pre-TCR. Furthermore, genetic analyses have also demonstrated that transcription factors such as NF-κB and β-catenin play important roles in the regulation of the DN to DP transition (Voll et al, 2000; Gounari et al, 2001).
Class I helix–loop–helix (HLH), or E proteins, comprise a class of transcription factors that have important roles in lymphocyte development (Massari and Murre, 2000). These proteins were originally identified by their ability to bind E box elements (CANNTG) in the immunoglobulin (Ig) enhancer. The E2A gene encodes two E proteins, designated as E12 and E47, which are generated through alternate RNA splicing. Mice with null mutations in the E2A locus exhibit a complete block in B-cell development, before the point of lineage commitment (Bain et al, 1994; Zhuang et al, 1994). T-cell development is also partially inhibited in E2A-deficient mice prior to lineage commitment (Bain et al, 1997a). These data demonstrate the importance of E2A proteins at or before the initial phases of lymphocyte differentiation.
Because some thymocytes do mature in an E2A-deficient background, it has been possible to identify roles for E2A proteins at later stages of T-cell development. A number of lines of evidence suggest that E proteins, and E2A proteins in particular, are involved in regulating the transitions both from DN to DP and DP to mature SP. Signaling from both the pre-TCR and TCR acts to inhibit E-protein DNA-binding activity and to stimulate expression of the E-protein inhibitor Id3, a member of a family of proteins that interfere with E-protein DNA binding (Bain et al, 2001). Furthermore, deficiencies in E2A have been found to accentuate thymocyte positive selection (Bain et al, 1999). In contrast, Id3-null mice exhibit inefficient positive selection (Rivera et al, 2000). These data suggest that E proteins act as an attenuator of signaling that leads to positive selection, although it should be noted that the effects of E2A deficiencies on positive selection may be due partially to extrinsic factors (Pan et al, 2002). E2A proteins also play a similar role at the DN to DP transition. Deficiencies in E2A, or overexpression of Id proteins, abrogate the developmental block normally observed in thymocytes with defects in pre-TCR expression (Engel et al, 2001; Kim et al, 2002). Thus, E2A proteins act as a gatekeeper at two stages of thymocyte development, and passage through these checkpoints requires the inhibition of E-protein activity through signaling from the TCR or pre-TCR complexes.
A growing body of evidence indicates that both E and Id proteins can act to regulate cell-cycle progression. Overexpression of myogenic bHLH proteins or E proteins impairs proliferation in NIH 3T3 cells (Peverali et al, 1994). Id2 has been shown to reverse cell-cycle arrest by the retinoblastoma (Rb) protein through direct interaction with Rb (Lasorella et al, 2000). Id1 has been demonstrated to block E-protein- and Ets-protein-mediated activation of the cdk inhibitor p16/INK4a (Alani et al, 2001; Ohtani et al, 2001). E and Id proteins also regulate tumorigenesis. A deficiency in E2A proteins leads to the spontaneous development of thymic lymphoma (Bain et al, 1997a; Yan et al, 1997). In contrast, transgene-directed overexpression of both Id1 and Id2 in mouse thymocytes results in the rapid development of T-cell lymphoma similar to that described for E2A-deficient mice (Kim et al, 1999; Morrow et al, 1999). Furthermore, Id1, Id2 and Id3 expression is activated in a large variety of tumors (Asp et al, 1998; Rockman et al, 2001; Nishimori et al, 2002; Wang et al, 2002). These data suggest that E and Id proteins play opposing roles in regulating cell proliferation and oncogenic transformation.
We have extended our examination of the contribution of pre-TCR signaling and E2A proteins to the regulation of both cell-cycle status and β selection. We have found that E2A proteins are required to enforce the developmental arrest of thymocytes with mutations that prevent pre-TCR signaling, and that these aberrantly developing thymocytes are cycling. We also demonstrate roles for E47 in cell-cycle repression prior to β selection, and in preventing the accumulation of TCRβ− cells at the DN4 stage. Furthermore, we show that the ectopic expression of E47 together with Bcl-2 induces cell-cycle arrest in E2A-deficient lymphoma lines. These data suggest a direct role for E2A in the inhibition of aberrant proliferation in the absence of pre-TCR signaling, and are consistent with a model in which high levels of E2A enforce both the developmental and proliferative block at the pre-TCR checkpoint. This model also predicts that release from this checkpoint requires downregulation of E-protein activity mediated by signaling through the pre-TCR signaling pathway. The role defined for E2A as an inhibitor of aberrant proliferation is also consistent with its function as a suppressor of lymphoma.
Results
E2A proteins are necessary to prevent thymocyte differentiation in the absence of pre-TCR signaling
Our previous data demonstrated that E2A proteins were required to prevent the aberrant development of thymocytes with defects that prevent TCRβ expression. However, low levels of pre-TCR components are expressed on DN thymocytes in the absence of TCRβ, and these incomplete pre-TCR complexes have the potential to initiate developmental progression (Levelt et al, 1993; Jacobs et al, 1994; Shinkai and Alt, 1994). Thus, it remained uncertain as to whether a deficiency of E2A proteins could allow for developmental progression in the complete absence of pre-TCR signaling. In order to address this question, E47 mutant mice were bred into a LAT-deficient background. LAT is an adaptor molecule required for signaling from the TCR complex and for developmental progression past the DN3 stage (Zhang et al, 1998; Zhang et al, 1999). Unlike thymocytes with defects in TCRβ expression, LAT-deficient thymocytes cannot be driven to develop by pre-TCR crosslinking (Zhang et al, 1999). Thymocytes were isolated from E47−/−, LAT −/− deficient mice and analyzed by flow cytometry. Strikingly, an E47-deficient background completely abrogated the developmental arrest normally observed in LAT-null mice, as the vast majority of E47−/−, LAT−/− thymocytes were DP (Figure 1A).
Figure 1.
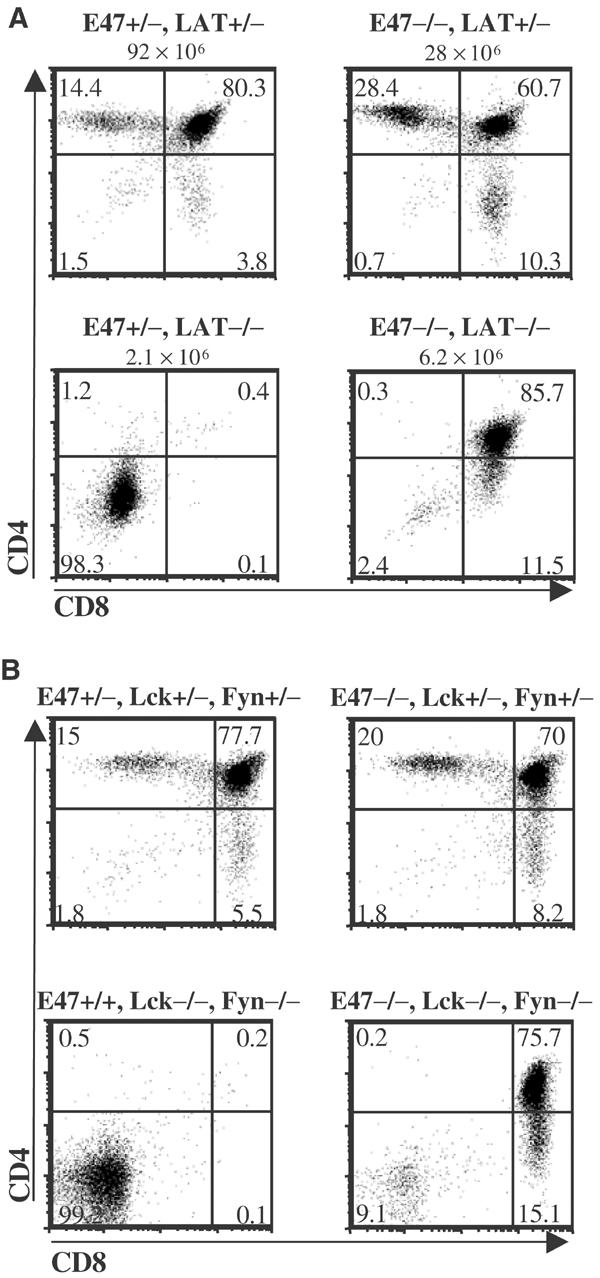
E2A proteins are required for developmental arrest in the absence of TCR signaling. (A) Thymocytes from littermates that were heterozygous (+/−) or homozygous (−/−) for null mutations in the LAT and E47 genes were analyzed for surface expression of the CD4 and CD8 coreceptor molecules. Numbers above each plot indicate thymus cellularity. Results were representative of five separate experiments. (B) Thymocytes from mice that were wild type (+/+), heterozygous (+/−) or homozygous null (−/−) for the Fyn, Lck and E47 genes were analyzed for surface expression of the CD4 and CD8 coreceptor molecules. The E47+/+, Fyn−/−, Lck−/− mouse analyzed was from a separate litter from the other three mice. Numbers in each quadrant represent percentages of the total.
The initiation of pre-TCR and TCR signaling normally requires phosphorylation of the CD3 and ζ chains by Lck, although Fyn activity can partially substitute in the absence of Lck (Cantrell, 1996; Groves et al, 1996; van Oers et al, 1996). Our previous data demonstrated that Lck-mediated signaling rapidly induced the expression of Id3 to inhibit E-protein DNA-binding activity (Bain et al, 2001). To determine whether E47 enforces the pre-TCR checkpoint in the absence of Lck/Fyn activity, triple null mutant mice were generated that lacked the expression of Lck, Fyn and E47. Thymocytes were isolated from the various compound mice and analyzed for the expression of CD4 and CD8. The absence of E47 allowed the development of Lck−/−, Fyn−/− thymocytes to the DP stage (Figure 1B). These data demonstrate that E47 is essential for developmental arrest in thymocytes with defects that prevent pre-TCR signaling.
E47 regulates the proliferation of LAT-deficient thymocytes
Thymuses from mice deficient in both E2A and genes required for TCRβ expression are hypercellular relative to littermates deficient for TCRβ expression but not E2A (Engel et al, 2001). These data suggested that E2A proteins were required for the proliferative arrest of thymocytes with defects in pre-TCR expression. However, an increase in thymic cellularity would also be predicted if a deficiency in E2A enhanced the survival of thymocytes with defects in TCRβ expression. We have examined this question further through the analysis of thymocytes deficient for both E47 and LAT. We first determined the thymic cellularity of mice deficient in both E47 and LAT as compared to LAT-deficient littermates that were heterozygous or wild type for E47. We found that a deficiency in E2A proteins resulted in small but consistent increases in the cellularity of thymuses from LAT-deficient mice (P=0.04) (Figure 2A). To test whether a deficiency in E2A proteins allowed for the aberrant proliferation of LAT-null thymocytes, we subjected E47−/−, LAT−/− mice and littermate controls to 2-bromodeoxyuridine (BrdU) pulse label analysis. Mice were injected with BrdU just prior to being killed and thymus extraction. The thymocytes were stained with antibodies against surface markers to define developmental subsets as well as with an anti-BrdU antibody. We then determined BrdU incorporation rates in various thymocyte subsets using flow cytometry, focusing on the immature CD8 single positive (ISP) subset. ISP cells comprise a transitional phase between the DN and DP stages, and are normally rapidly proliferating (Zuniga-Pflucker and Lenardo, 1996). We found that a large fraction of the ISP cells from mice doubly deficient for E47 and LAT were also proliferating (Figure 2B). These data demonstrated unambiguously that a deficit of E2A proteins allowed for both the aberrant differentiation and proliferation of LAT-deficient thymocytes. However, it should be noted that the percentage of BrdU+ cells among E47−/−, LAT−/− ISPs was consistently lower than in ISPs from littermates that were heterozygous for LAT or E47. Thus, some of the mechanisms by which pre-TCR signaling promoted proliferation were independent of an inhibition of E2A activity.
Figure 2.
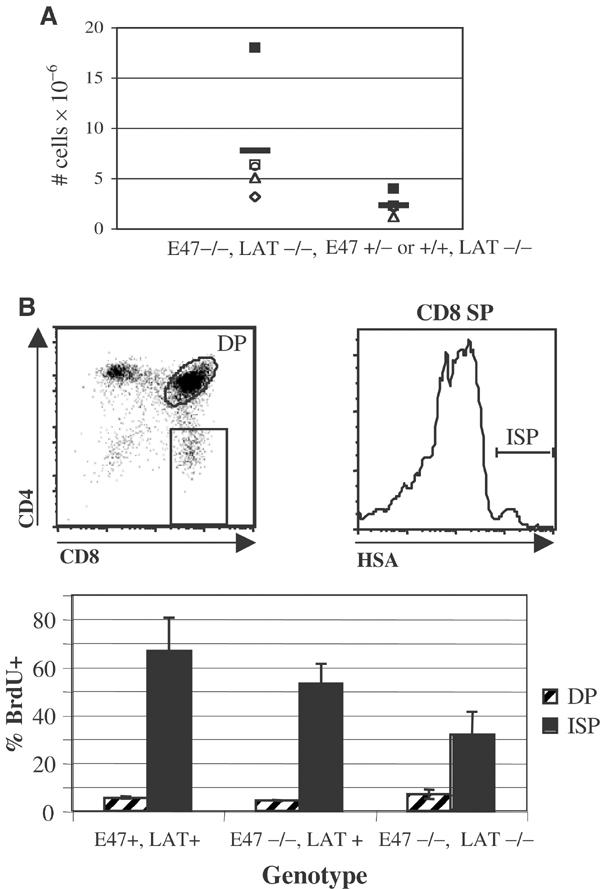
Deficiencies in E2A proteins allow for the aberrant proliferation of thymocytes with mutations that prevent pre-TCR signaling. (A) Scatter plot depicting thymus cellularity in five E47−/−, LAT−/− mice and LAT−/− littermates that were E47+/− or +/+. Littermates are represented by unique symbols. Horizontal lines indicate mean values for each genotype. (B) BrdU incorporation in ISP and DP subsets identified in E47−/−, LAT−/− thymocytes as compared to the same subsets isolated from littermates that were homozygous null for E47 only, as well as from littermates with both E47 and LAT activity. Mice were administered BrdU shortly before being killed. Bar graph depicts the percentages of BrdU+ cells within the ISP (CD8SP, HSAhigh, black bars) and DP (hatched bars) subsets, which were defined according to the dot plot and histogram. Similar data were obtained when the ISP gate was defined as CD8SP, TCRlow (data not shown). The mean and s.d. from five mice representing four littermate sets are reported.
E2A proteins contribute to the cell-cycle arrest of DN3 thymocytes
We also used BrdU pulse label analysis to address whether the cell-cycle status of various thymocyte developmental subsets is affected by a deficit in E2A proteins. Thymocytes from BrdU-pulsed mice were stained for the expression of the appropriate markers and analyzed by flow cytometry. Interestingly, a significantly higher fraction of E47-null DN3 thymocytes incorporated BrdU than the same subset in wild-type mice of similar age and genetic background (Figure 3A). A smaller, somewhat variable increase in BrdU+ cells was also observed in E47 +/− DN3 thymocytes relative to wild-type cells from the same thymic subset (Figure 3A). The DP and SP populations did not show increased BrdU incorporation in E47-deficient mice (Figure 3A and data not shown). DN3 thymocytes normally consist primarily of G1-arrested cells undergoing TCRβ gene rearrangement. However, a significant fraction of DN3 cells have productively rearranged TCRβ and re-entered the cell cycle (Hoffman et al, 1996). Thus, the higher percentage of BrdU-incorporating cells within the DN3 subset of E2A-deficient thymocytes could have been due to an increased fraction of TCRβ-expressing cells. We tested for this possibility using two strategies, both of which involved the staining of thymocytes for intracellular TCRβ protein (icβ). Our first approach was to separately analyze BrdU incorporation and icβ levels within DN3 thymocytes from E2A-deficient and heterozygous and wild-type littermates or age- and strain-matched controls. A deficiency for E2A did not significantly affect the percentages of icβ+ cells or levels of icβ expression within the DN3 population, and correcting for differences in TCRβ expression did not alter the observed differences in BrdU incorporation (Supplementary Figure S1 and data not shown). We also simultaneously analyzed DN3 thymocytes from BrdU-pulsed mice for both BrdU and icβ. Using this approach we demonstrated that the icβ− fraction of E2A-deficient DN3 thymocytes had a significantly higher percentage of BrdU+ cells (Figure 3B, Supplementary Figure S2). These data indicate that E2A proteins act to maintain DN3 thymocytes in G1 arrest prior to the expression of TCRβ.
Figure 3.
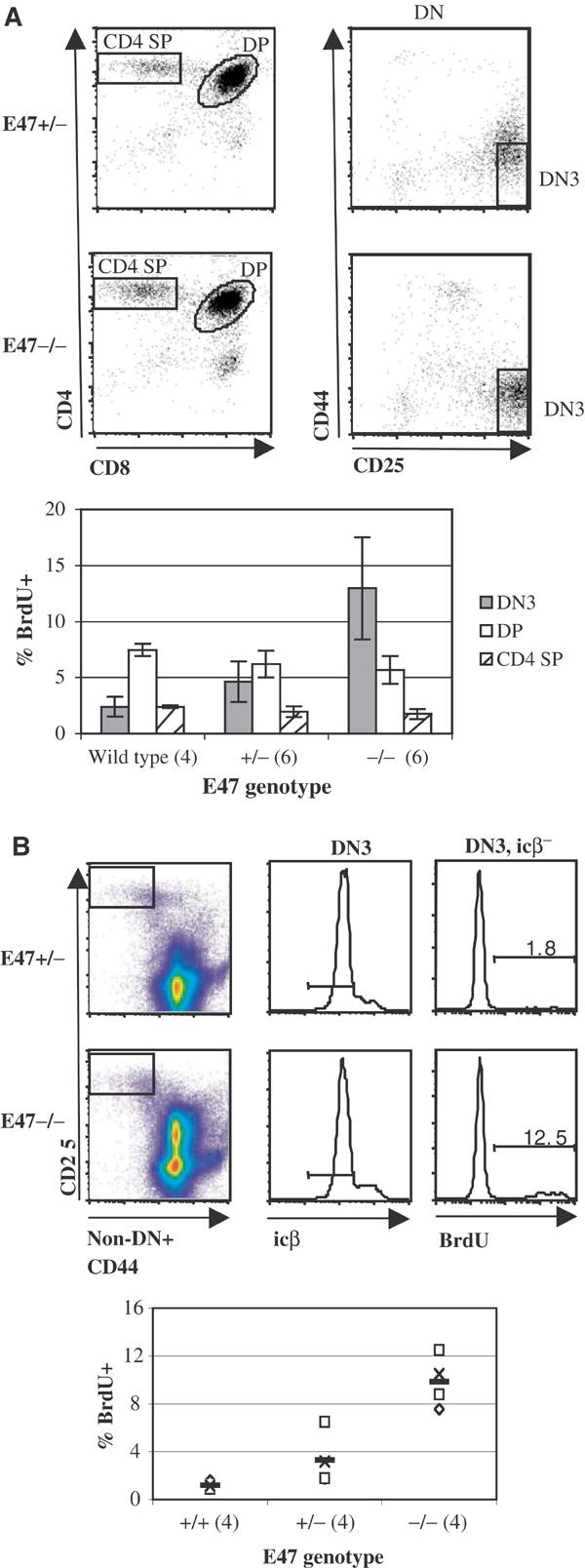
Aberrant cell-cycle activity within the DN3 subset of E47−/− thymocytes. (A) Dot plots: defined electronic gates in E47+/− (top plots) and E47−/− (bottom plots) BrdU-pulsed littermates. Left plots: total thymocytes stained with antibodies against CD8 and CD4. Right plots: electronically gated DN thymocytes stained for CD25 and CD44. Bar graph: mean BrdU incorporation within the DN3 (solid gray bars), DP (open bars) and CD4 SP (hatched bars) thymoycte subsets from E47 wild type, heterozygous (+/−) and homozygous null (−/−) mice. The number of mice analyzed from each genotype is indicated in parentheses. The −/− and +/− mice were obtained from three litters, while the wild-type mice were from separate litters, but matched to the +/− and −/− littermates with respect to age- and background strain. (B) Simultaneous analysis of BrdU and icβ expression in DN3 thymocytes from E47−/− and E47+/− mice. Dot plots and histograms: electronic gating procedure used to identify and measure BrdU incorporation within the DN3, icβ− subsets from one littermate pair of E47+/− (top graphs) and E47−/− (bottom graphs) mice. Gated populations are indicated by boxes in the left pseudo-color plots, and horizontal lines in the middle histograms. See Supplementary Figure S2 and Supplementary Materials and Methods for additional details. Bar graph: %BrdU+ in DN3, icβ− cells from four E47−/− and +/− 6-week-old littermates, as well as four age and background-matched wild-type mice. E47−/− and +/− littermates are indicated by unique symbols, while horizontal lines denote mean values for each genotype.
E2A proteins are required for the elimination of icβ− DN4 thymocytes
The DN4 subset normally consists primarily of thymoyctes with productive TCRβ rearrangements that are rapidly proliferating and differentiating towards the DP stage (Hoffman et al, 1996). To determine the effect of a deficiency in E47 on proliferation within the DN4 compartment, mice were pulse-labeled with BrdU and then thymocytes were stained and analyzed by flow cytometry to assess BrdU incorporation by DN4 cells. In contrast to the effects of E2A protein deficiency on cell-cycle status within the DN3 subset, we observed that E47-null DN4 thymocytes proliferated at a much lower rate than E47 heterozygote littermates (Figure 4A). Since an E47 deficiency allows developmental progression from the DN to the DP compartment in the context of mutations that prevent TCRβ expression, we considered the possibility that the lower fraction of BrdU-incorporating cells within the DN4 compartment was caused by the presence of a TCRβ− population of cells that had differentiated beyond the pre-TCR checkpoint. To address this possibility, DN4 cells were analyzed for the presence of BrdU incorporation and icβ expression. Indeed, such analysis revealed that these differences in cell-cycle status could be accounted for by a dramatically reduced percentage of icβ+ cells in the E2A-deficient DN4 population (Figures 4B–C). In both E47-null and control mice, the majority of DN4 icβ+ cells were BrdU+, while most icβ− DN4 cells did not incorporate BrdU (Figure 4C). However, whereas most DN4 thymocytes from E47 heterozygous or wild-type backgrounds were icβ+, the majority of E47–/– DN4 cells were icβ−. The icβ− cells within the E47–/– DN4 subset as defined in Figure 4 expressed uniformly high levels of Thy 1, and thus were not non-T-lineage contaminants (data not shown). Thus, E2A proteins act to prevent the accumulation of DN4 thymocytes that failed to express TCRβ. These data imply that whereas E2A proteins are required for full enforcement of the β selection checkpoint, they are not required to inhibit cell-cycle progression within the DN4, icβ− subset.
Figure 4.
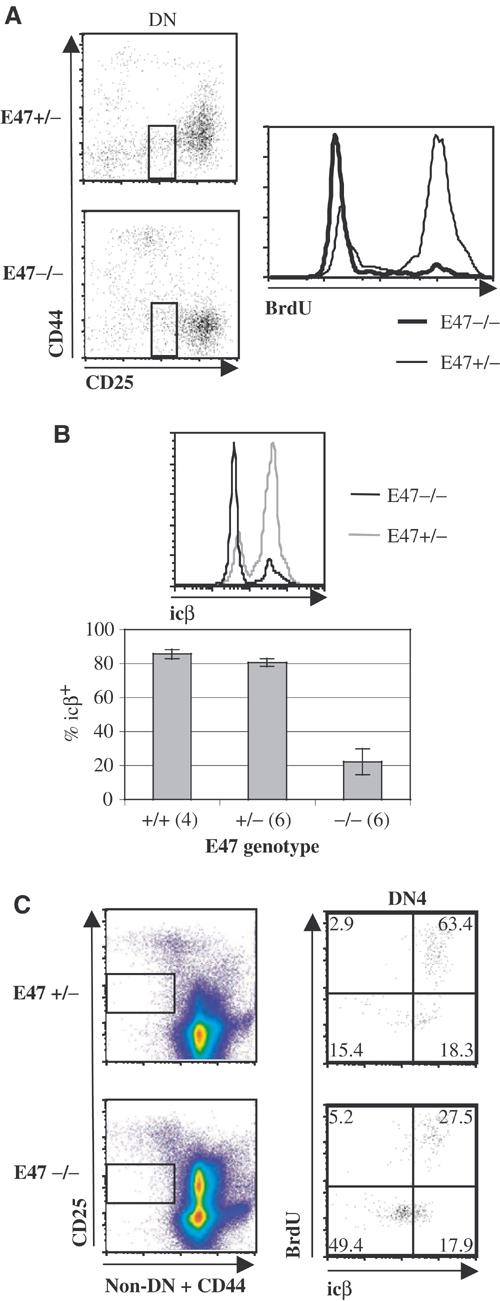
A high fraction of E2A-deficient DN4 thymocytes do not express TCRβ. (A) BrdU incorporation in E47−/− and +/− DN4 thymocytes. Left: dot plots of the CD25 and CD44 expression profiles of DN thymocytes from an E47+/− (top plot) and E47−/− (bottom plot) littermate pair pulse labeled with BrdU, with boxes depicting the electronic gate used to define the population assessed for BrdU incorporation and icβ expression. Right: histogram of BrdU incorporation within the gated cell subset for this littermate pair. Thick tracing: E47−/−. Thin tracing: E47+/−. Gates were drawn around CD44low, CD25low cells, rather than the CD44low, CD25− cells usually defined as DN4, because the latter population contained significant numbers of Thy 1low cells of unclear lineage (data not shown). Inclusion of the CD44low, CD25− cells decreased the fractions of both BrdU-incorporating and icβ+ cells in all samples, but did not affect the magnitude of the differences between E47−/− and E47+/− or +/+ thymocytes (data not shown). (B) Expression of icβ in E47−/−, +/− and +/+ DN4 thymocytes. Top histogram: cells from littermates gated as described in Figure 4A were stained and analyzed for icβ expression. Black tracing: E47−/−. Gray tracing: E47+/−. Bottom bar graph: percentages of icβ+, DN4-gated cells (as defined in Figure 4A) from E47−/− and +/− mice obtained from three separate litters, as well as from age- and strain-matched wild-type mice. The number of mice represented for each genotype is indicated in parentheses. (C) Simultaneous analysis of icβ expression and BrdU incorporation in DN4 thymocytes from a littermate pair of E47+/− and E47−/− mice. Left: pseudo-color plots depicting the definition of the DN4 subset. See Supplementary Figure S2 and Supplementary Materials and Methods for additional details. Right: dot plots showing icβ expression and BrdU incorporation within the defined gates. Numbers depict the percentage of cells within each quadrant.
Ectopic expression of E47 and Bcl-2 induces cell-cycle arrest in E2A-deficient lymphomas
E2A-deficient mice are highly susceptible to T-cell lymphoma, indicating that the E2A proteins act as tumor suppressors (Bain et al, 1997a; Yan et al, 1997). The observations described above indicate that E47 acts to inhibit cell-cycle progression prior to the onset of pre-TCR expression. Taken together, these data raise the possibility that the aberrant proliferation in E2A-deficient mice ultimately leads to the development of lymphoma. We have previously observed that introduction of E47 or E12 caused the death of cell lines adapted from lymphomas that developed in E2A-deficient mice (Engel and Murre, 1999). Lymphomas from E2A-deficient mice typically express very low levels of endogenous Bcl-2 (data not shown). In contrast, DN thymocytes prior to β selection express high levels of Bcl-2 (Veis et al, 1993; Voll et al, 2000). To determine whether enforced expression of Bcl-2 would perturb the apoptotic ability of E47, we introduced Bcl-2 into E2A-deficient lymphoma lines by retroviral transduction. Subsequently, Bcl-2-transduced E2A-deficient cells were transduced with retrovirus encoding E47. Interestingly, ectopic expression of Bcl-2 blocked E47-mediated death (Figure 5A). In addition, cell cultures transduced with both E47 and Bcl-2 grew poorly, and cells expressing both Bcl-2 and E47 for 48 h became smaller in size (Figure 5B and data not shown). These observations suggest that the combination of Bcl-2 and E47 expression led to cell-cycle arrest. To test this hypothesis, we examined the cell-cycle profiles of transduced lymphomas by DNA content analysis. Transduction of Bcl-2 alone resulted in variable effects on cell-cycle profiles. In some experiments we found that ectopic Bcl-2 expression resulted in a moderate increase in the fraction of cells in the G0 or G1 phase of the cell cycle, while in other experiments little or no effect of Bcl-2 expression on cell-cycle profiles was observed (Figure 5C and data not shown). However, we consistently found that transduction of both Bcl-2 and E47 resulted in an increase in the G0 or G1 fraction that was much greater than the effect of Bcl-2 alone (Figure 5C). These data indicate that E47 and Bcl-2 synergize to inhibit cell-cycle progression in E2A-deficient lymphoma lines.
Figure 5.
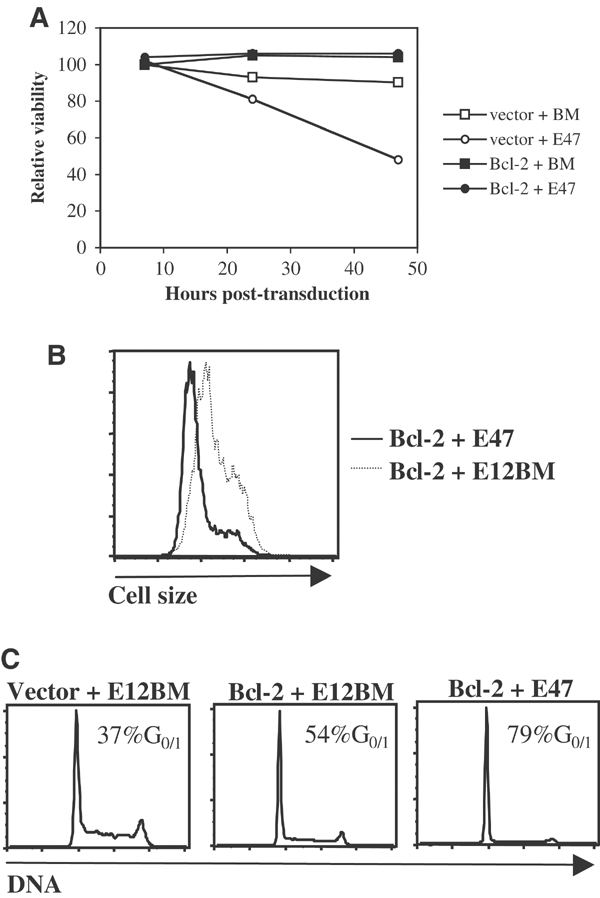
Bcl-2 blocks cell death and promotes cell-cycle arrest in E2A-deficient lymphoma lines transduced with E47. (A) Inhibition of E47-mediated death by Bcl-2. The 1.F9 E2A-deficient lymphoma line was transduced with retrovirus encoding Bcl-2 or ‘empty vector' retrovirus, cultured for 2 days, and then infected with retrovirus encoding E47 or a mutant form of E12 that does not bind DNA (E12BM). Cells were harvested 7, 24 or 48 h after transduction with E2A and the percentage of viable cells was determined by flow cytometry on the basis of forward and side scatter. The relative viability of each cell population (normalized to the viability of the BM-transduced population at 7 h post-transduction) is plotted against the time of harvest. Transduction efficiency was 90% or greater in all populations tested (data not shown). (B) Size reduction induced by E47 transduction into Bcl-2-expressing, E2A-deficient lymphoma lines. 1.F9 cells sequentially transduced with Bcl-2 and either E47 or E12BM retrovirus were fixed and permeabilized 48 h after E2A transduction, stained for ectopic E2A protein, and analyzed by flow cytometry. Plotted are the forward scatter profiles of the E2A+, live-gated cells within each population. (C) G0 or G1 arrest induced by transduction of both Bcl-2 and E47 into E2A-deficient lymphomas. 1.F9 cells treated as described in Figure 5B were analyzed for DNA content. Also included are cells sequentially transduced with empty vector and E12BM. Plots depict the DNA content profiles of the E2A-expressing cells within each population.
Discussion
Lck, Fyn, LAT and E47 activities are linked into a common pathway
E2A proteins play key roles in the control of gene expression during the early stages of thymocyte development. Both pre-Tα and RAG gene expression are regulated by E-protein activity (Schlissel et al, 1991; Herblot et al, 2000; Reizis and Leder, 2001; Takeuchi et al, 2001). E-protein activity has also been implicated in the control of TCR V(D)Jβ rearrangement (Barndt et al, 2000; Romanow et al, 2000; Ghosh et al, 2001). Additionally, we have demonstrated that E2A proteins are required for the developmental arrest observed in thymocytes with defects in TCRβ chain expression, and that pre-TCR signaling acts to inhibit E-protein DNA-binding activity (Engel et al, 2001). These observations allowed us to propose that E2A proteins prevent the differentiation of DN thymocytes to the DP stage, and that the expression of a complete pre-TCR complex promotes differentiation largely through the inhibition of E2A proteins. Previous in vitro studies have also suggested that signaling mediated by the tyrosine kinase Lck results in modulation of E2A DNA binding (Bain et al, 2001). We have now extended these studies to demonstrate that the block in thymocyte development in LAT−/− or Lck- and Fyn-null mice can be abrogated by a deficiency in E47. These data confirm that the activities of Lck, Fyn, LAT and E47 are linked into a common pathway (Figure 6). We also provide direct evidence that E2A proteins inhibit cell-cycle progression in thymocytes just prior to β selection, and that ectopic E47 expression can block the proliferation of T lymphoma lines that express Bcl-2. Taken together with previous observations, our data suggest that initiation of the pre-TCR signaling cascade promotes thymocyte proliferation at least in part through the inhibition of E2A-protein activity. Thus, these data provide additional support for a model in which E47 inhibits differentiation and proliferation prior to pre-TCR expression, whereas inhibition of E2A activity by pre-TCR signaling promotes both developmental progression and cellular expansion.
Figure 6.
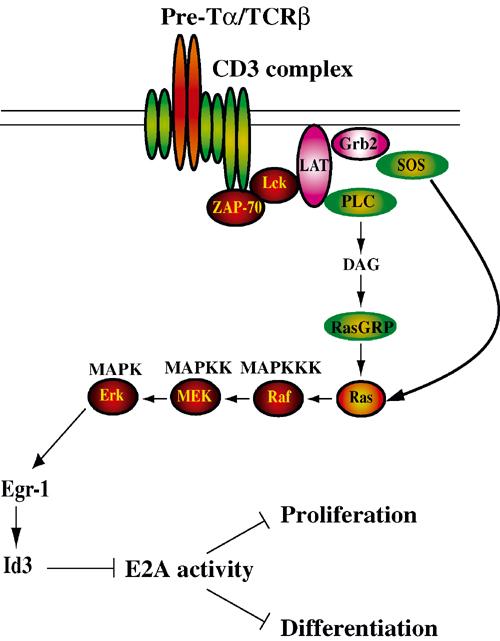
Model depicting how signaling from the pre-TCR complex leads to the inhibition of E2A activity, resulting in the release of blocks on thymocyte differentiation and proliferation.
E2A proteins and cell-cycle arrest
Ectopic expression of E2A proteins has been reported both to promote and inhibit cell-cycle expression in recipient cell lines (Peverali et al, 1994; Park et al, 1999; Zhao et al, 2001). Furthermore, enforced E2A expression can also cause cell death (Engel and Murre, 1999; Park et al, 1999). The apparent discrepancies with regard to the effects of E2A expression are likely explained by a number of factors, including differences in the cell lines used in the various studies. We have now shown that Bcl-2 can change the fate of cells transduced with E2A proteins from death to growth arrest. Enforced Bcl-2 expression induces a similar change in the fate of IL-3-dependent cell lines after lymphokine withdrawal (Lind et al, 1999). It is also interesting to note the correlations between the effect of E47 and Bcl-2 on E2A-deficient lymphoma lines and the regulation of Bcl-2 expression and E-protein activity in DN thymocytes. Resting DN3 cells express high levels of Bcl-2 and E-protein DNA-binding activity, both of which are rapidly downregulated after pre-TCR signaling (Voll et al, 2000; Engel et al, 2001). Our data thus suggest that E2A proteins and Bcl-2 may collaborate to regulate both viability and cell-cycle status in DN3 thymocytes.
Role of E47 in enforcing the developmental block at the pre-TCR checkpoint
We found that the majority of E47-deficient DN4 thymocytes do not express TCRβ. These data provide additional evidence for a role for E2A proteins in the efficient elimination of TCRβ− cells at the β selection checkpoint. It should be noted that DN4 cells that fail to express clonotypic TCR chains have been reported to be eliminated via apoptosis (Falk et al, 2001). Our data, taken together with previous observations that E2A activity can promote cell death, suggest that the apoptosis of TCRβ− DN4 cells may be partially dependent upon E2A proteins (Engel and Murre, 1999; Park et al, 1999). Such a model would predict that the increased percentage of TCRβ− DN4 thymocytes is due to a delay in the apoptotic elimination of these cells. We detect only marginally higher fractions of TCRβ− cells in ISP and DP thymocytes from E47-null mice relative to heterozygote or wild-type controls (data not shown). The marked decline in the fraction of TCRβ− cells in E47–/– thymocytes during maturation from the DN4 to the DP stage is at least partly the result of the outgrowth of rapidly proliferating TCRβ+ cells. However, it is possible that a delayed apoptotic process also contributes to the removal of these cells.
Regulation of cellular expansion by pre-TCR-mediated signaling independent of E47
Although our data suggest that pre-TCR expression can promote cell-cycle progression through the inhibition of E2A-protein activity, it is clear that much of the effect of pre-TCR signaling on cell cycle is independent of E2A. First of all, while E47−/−, LAT−/− ISP cells clearly exhibit cell-cycle activity, the fraction of BrdU+ cells within this population are lower than that found in ISP cells from mice that expressed LAT. Furthermore, a deficiency in E2A proteins does not affect the frequency of BrdU incorporation within the TCRβ−, DN4 subset. However, it is important to note that thymocytes also express E proteins encoded by the HEB and E2-2 genes. Although deletion of each of these genes has different effects on thymocyte development, the fact that the E proteins share similar DNA-binding specificities and transcriptional activation properties suggests that they are likely to have redundant functions (Barndt et al, 1999; Bergqvist et al, 2000). In fact, replacement of E2A with HEB can correct defects in B-cell development and neonatal viability observed in E2A-deficient mice (Zhuang et al, 1998).
Thus, our analyses of the effects of E47 deficiency on pre-TCR-mediated initiation of cell-cycle progression may underestimate the extent to which E proteins function downstream of the pre-TCR to regulate proliferation and suppress lymphoma genesis.
Aberrant proliferation and thymic lymphoma in E2A-deficient mice
Our observations indicate that E47 acts at the DN3 compartment to block cell-cycle progression. Interestingly, this effect of E2A proteins is specific to the DN3 stage. Deficiencies in E2A proteins lead to the development of rapidly proliferating thymic lymphomas (Bain et al, 1997a; Yan et al, 1997). However, the proliferating DN3 cells reported in this paper are distinguished from the lymphomas that eventually develop in E2A-deficient mice by a number of criteria. We observe the increased proliferation in E2A-deficient mice of 4–6 weeks of age, while E2A-deficient mice rarely become ill from lymphoma before 13 weeks of age (Bain et al, 1997a). None of the animals used in this study exhibited any evidence of thymic lymphoma (data not shown). In addition, lymphomas isolated from ill E2A-deficient mice generally express high levels of CD4 and CD8, and so would not be included in analyses of the DN3 subset (Bain et al, 1997a; Engel and Murre, 2002). Finally, we note that while heterozygosity for E2A alone does not lead to increased susceptibility to lymphoma, we often observe increased BrdU incorporation within the DN3 compartment of E47+/− mice. Thus, it is unlikely that the cycling cells we detect indicate the presence of completely transformed lymphoma cells already present within the analyzed thymuses. Nevertheless, it is quite conceivable that the aberrant cell-cycle activity observed within the DN3 subset of E2A-deficient thymocytes represents an early stage in the eventual development of thymic lymphoma, and that the tumor suppressor activity of E2A is directly related to its role as a cell-cycle inhibitor.
Mechanism of Id regulation of proliferation and oncogenesis
Recently accumulated evidence has implicated the Id proteins as performing essential roles in promoting cell-cycle progression. Specifically, the Id2 gene product has the ability to reverse cell-cycle arrest by the retinoblastoma protein, and Id1 has been shown to inhibit E-protein- and Ets-protein-mediated activation of the cdk inhibitor p16/INK4a (Lasorella et al, 2000; Alani et al, 2001; Ohtani et al, 2001). Furthermore, Id genes are potential proto-oncogenes, since their expression is activated in a large variety of malignancies (Asp et al, 1998; Rockman et al, 2001; Nishimori et al, 2002; Wang et al, 2002). Overexpression of Id1 and Id2 in the thymocyte compartment leads to the rapid development of T-cell lymphoma with similar kinetics and phenotypes as described for tumor development in E2A-deficient mice (Kim et al, 1999; Morrow et al, 1999). Our data, when taken together with previous studies, suggest that the effects of Id proteins on cell homeostasis and tumorigenesis may be mediated in large part through the inhibition of E-protein function. Alternatively, the possibility that E proteins act by preventing the inhibitory interactions of Id proteins with Rb and Ets must also be considered. We also note that Ras-mediated signaling can activate Id3 gene expression (Bain et al, 2001). Thus, regardless of whether E proteins act upstream or downstream of Id to suppress tumorigenesis, it is conceivable that Ras-mediated transformation may, at least in some cell types, act in large part through altering the ratio of Id and E proteins, which in turn could allow for aberrant mitotic activity at specific developmental stages such as those defined in this report.
Materials and methods
Mice
To generate mice deficient for E47 and LAT, mice carrying the E47-null mutation in an FVB background were bred to mice carrying the LAT mutation in a mixed genetic background (Bain et al, 1997b; Zhang et al, 1999). Mice deficient for E47, Lck and Fyn were generated by interbreeding mice carrying null mutations for E47 or Lck on a C57Bl/6J background and the Fyn null mutation in a mixed genetic background (Appleby et al, 1992; Molina et al, 1992). E47-mutant mice were from our own colony, Fyn- and Lck-mutant mice were purchased from Jackson Laboratories (Bar Harbor, ME), and LAT-mutant mice were obtained from Dr Lawrence Samelson (NICHD, Bethesda, MD). Genotyping was performed via PCR of genomic DNA prepared from tail sections; primer sequences and PCR protocols are available upon request. The E47−/− and +/− mice used for BrdU and icβ analyses were littermates generated by interbreeding E47-null males and E47+/− females that had been backcrossed for 10 or more generations into a C57Bl/6J genetic background. The wild-type mice included in these comparisons were C57Bl/6J mice purchased from Jackson Laboratories that were age matched to the E47-mutant mice.
Flow cytometric analyses
Thymuses were dissected, suspended in buffered saline solutions and pressed through 40 μm cell strainers (Falcon) to generate thymocyte suspensions. Thymocyte suspensions were washed once and then counted. Thymocytes (1–4 × 106) were stained for surface antigens as previously described (Engel et al, 2001). For DN thymocyte analysis, cells were stained with biotinylated antibody conjugates specific for mouse CD4, CD8a, CD3ɛ, TCRβ, TCRγδ, TER-119, B220, CD11b, GR-1 and NK1.1, followed by streptavidin-peridinin chlorophyll protein (PerCP), anti-CD25 (clone PC61)-phycoerythrin (PE) and anti-CD44-allophycocyanin (APC). DN thymocytes were then defined by excluding PerCP+ cells during the subsequent analysis. For icβ staining, cells were fixed in 0.25% paraformaldehyde, permeabilized with 0.2% Tween-20 and stained with anti-TCRβ-FITC or -APC conjugates as previously described (Schmid et al, 1991). Staining of transduced lymphoma lines for Bcl-2 was performed as previously described, using an anti-human Bcl-2 antibody (Engel et al, 2001). Staining for transduced E47 and E12BM was performed as described (Engel and Murre, 1999). For DNA content determination, transduced lymphoma lines were first stained for human E2A, washed and incubated for at least 30 min in PBS containing 10 μg/ml propidium iodide and 100 μg/ml Rnase A. All samples were analyzed using a FACScalibur (Becton-Dickinson) with Cellquest (Becton-Dickinson) and FlowJo (Tree Star) software. Doublet discrimination was used for DNA content analysis. All antibodies were purchased from Pharmingen/Becton-Dickinson or eBiosciences.
BrdU incorporation analysis
Mice were administered two intraperitoneal injections of 1 mg of BrdU in 0.1 ml PBS over a 2 h interval, and then killed 30 min after the last injection. After preparation of thymocyte suspensions and surface antigen staining, cells were resuspended in 1% paraformaldehyde and 0.01% Tween-20 and incubated overnight at 4°C. Cells were then treated with Dnase I and stained with anti-BrdU-FITC (Becton-Dickinson) as previously described (Carayon and Bord, 1992; Penit et al, 1995). Simultaneous analysis of icβ expression and BrdU incorporation in DN thymocytes was performed as described in Supplementary Materials and Methods.
Retroviral transduction
Retroviral supernatants were prepared and spin transduction into the E2A−/− lymphoma line 1.F9 was performed as previously described (Engel and Murre, 1999). A Bcl-2 retroviral expression vector was prepared using a human Bcl-2 cDNA originally from the laboratory of Stanley Korsmeyer (Dana-Farber Cancer Institute); a sequence immediately upstream of the initial codon was changed by PCR-directed mutagenesis in order to improve expression. The modified Bcl-2 cDNA was subcloned into the retroviral vector LZRSpBMN-linker-IRES-EGFP (NotI-) (Heemskerk et al, 1997). The E47 and E12BM retroviral vectors were as previously described (Engel and Murre, 1999). For studies of the combined effect of ectopic E47 and Bcl-2 expression, 1.F9 cells were first transduced with Bcl-2 retrovirus, cells were cultured for 3–6 days, and the resulting Bcl-2-expressing line was transduced with E47 and E12BM virus. The lines used in these experiments were determined to be >90% positive for ectopic Bcl-2 by flow cytometry (data not shown).
Supplementary Material
Supplemental Materials and Methods
Acknowledgments
We thank Jessica Novak for technical assistance, and Connie Sommers and Lawrence Samelson for providing LAT–/– mice. This work was supported by grants from the NIH (CM) and Lymphoma Research Foundation (IE).
References
- Alani RM, Young AZ, Shifflett CB (2001) Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc Natl Acad Sci USA 98: 7812–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM (1992) Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell 70: 751–763 [DOI] [PubMed] [Google Scholar]
- Asp J, Thornemo M, Inerot S, Lindahl A (1998) The helix–loop–helix transcription factors Id1 and Id3 have a functional role in control of cell division in human normal and neoplastic chondrocytes. FEBS Lett 438: 85–90 [DOI] [PubMed] [Google Scholar]
- Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C (2001) Regulation of the helix–loop–helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol 2: 165–171 [DOI] [PubMed] [Google Scholar]
- Bain G, Engel I, Maandag ECR, Riele HPJt, Voland JR, Sharp LL, Chun J, Huey B, Pinkel D, Murre C (1997a) E2A deficiency leads to abnormalities in αβ T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol 17: 4782–4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G, Maandag E, Izon D, Amsen D, Kruisbeek A, Weintraub B, Krop I, Schlissel M, Feeney A, van Roon M, van der Valk M, te Riele H, Berns A, Murre C (1994) E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell 79: 885–892 [DOI] [PubMed] [Google Scholar]
- Bain G, Maandag ECR, Riele H, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C (1997b) Both E12 and E47 allow commitment to the B cell lineage. Immunity 6: 145–154 [DOI] [PubMed] [Google Scholar]
- Bain G, Quong MW, Soloff RS, Hedrick SM, Murre C (1999) Thymocyte maturation is regulated by the activity of the helix–loop–helix protein, E47. J Exp Med 190: 1605–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barndt R, Dai MF, Zhuang Y (1999) A novel role for HEB downstream or parallel to the pre-TCR signaling pathway during alpha beta thymopoiesis. J Immunol 163: 3331–3343 [PubMed] [Google Scholar]
- Barndt RJ, Dai M, Zhuang Y (2000) Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol 20: 6677–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist I, Eriksson M, Saarikettu J, Eriksson B, Corneliussen B, Grundstrom T, Holmberg D (2000) The basic helix–loop–helix transcription factor E2-2 is involved in T lymphocyte development. Eur J Immunol 30: 2857–2863 [DOI] [PubMed] [Google Scholar]
- Cantrell D (1996) T cell antigen receptor signal transduction pathways. Annu Rev Immunol 14: 259–274 [DOI] [PubMed] [Google Scholar]
- Carayon P, Bord A (1992) Identification of DNA-replicating lymphocyte subsets using a new method to label the bromodeoxyuridine incorporated into the DNA. J Immunol Methods 147: 225–230 [DOI] [PubMed] [Google Scholar]
- Engel I, Johns C, Bain G, Rivera RR, Murre C (2001) Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med 194: 733–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Murre C (1999) Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas. Proc Natl Acad Sci USA 96: 996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Murre C (2002) Disruption of pre-TCR expression accelerates lymphomagenesis in E2A-deficient mice. Proc Natl Acad Sci USA 99: 11322–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk I, Nerz G, Haidl I, Krotkova A, Eichmann K (2001) Immature thymocytes that fail to express TCRbeta and/or TCRgamma delta proteins die by apoptotic cell death in the CD44(-)CD25(-) (DN4) subset. Eur J Immunol 31: 3308–3317 [DOI] [PubMed] [Google Scholar]
- Ghosh JK, Romanow WJ, Murre C (2001) Induction of a diverse T cell receptor gamma/delta repertoire by the helix–loop–helix proteins E2A and HEB in nonlymphoid cells. J Exp Med 193: 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounari F, Aifantis I, Khazaie K, Hoeflinger S, Harada N, Taketo MM, von Boehmer H (2001) Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat Immunol 2: 863–869 [DOI] [PubMed] [Google Scholar]
- Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ (1996) Fyn can partially substitute for Lck in T lymphocyte development. Immunity 5: 417–428 [DOI] [PubMed] [Google Scholar]
- Heemskerk MHM, Blom B, Nolan G, Stegmann APA, Bakker AQ, Weijer K, Res PCM, Spits H (1997) Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix–loop–helix factor Id3. J Exp Med 186: 1597–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herblot S, Steff AM, Hugo P, Aplan PD, Hoang T (2000) SCL and LMO1 alter thymocyte differentiation: inhibition of E2A-HEB function and pre-T alpha chain expression. Nat Immunol 1: 138–144 [DOI] [PubMed] [Google Scholar]
- Hoffman ES, Passoni L, Crompton T, Leu TMJ, Schatz DG, Koff A, Owen MJ, Hayday AC (1996) Productive T-cell receptor β-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev 10: 948–962 [DOI] [PubMed] [Google Scholar]
- Jacobs H, Vandeputte D, Tolkamp L, Vries Ed, Borst J, Berns A (1994) CD3 components at the surface of pro-T cells can mediate pre-T cell development in vivo. Eur J Immunol 24: 934–939 [DOI] [PubMed] [Google Scholar]
- Kim D, Peng XC, Sun XH (1999) Massive apoptosis of thymocytes in T-cell-deficient Id1 transgenic mice. Mol Cell Biol 19: 8240–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Xu M, Nie L, Peng XC, Jimi E, Voll RE, Nguyen T, Ghosh S, Sun XH (2002) Helix–loop–helix proteins regulate pre-TCR and TCR signaling through modulation of Rel/NF-kappaB activities. Immunity 16: 9–21 [DOI] [PubMed] [Google Scholar]
- Kruisbeek AM, Haks MC, Carleton M, Michie AM, Zuniga-Pflucker JC, Wiest DL (2000) Branching out to gain control: how the pre-TCR is linked to multiple functions. Immunol Today 21: 637–644 [DOI] [PubMed] [Google Scholar]
- Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A (2000) Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407: 592–598 [DOI] [PubMed] [Google Scholar]
- Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K (1993) Restoration of early thymocyte differentiation in T-cell receptor beta-chain-deficient mutant mice by transmembrane signaling through CD3 epsilon. Proc Natl Acad Sci USA 90: 11401–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind EF, Wayne J, Wang QZ, Staeva T, Stolzer A, Petrie HT (1999) Bcl-2-induced changes in E2F regulatory complexes reveal the potential for integrated cell cycle and cell death functions. J Immunol 162: 5374–5379 [PubMed] [Google Scholar]
- Massari ME, Murre C (2000) Helix–loop–helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20: 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina TJ, Kishihara K, Siderovski DP, Ewijk WV, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann K-U, Davidson D, Mak TW (1992) Profound block in thymocyte development in mice lacking p56lck. Nature 357: 161–164 [DOI] [PubMed] [Google Scholar]
- Morrow MA, Mayer EW, Perez CA, Adlam M, Siu G (1999) Overexpression of the helix–loop–helix protein Id2 blocks T cell development at multiple stages. Mol Immunol 36: 491–503 [DOI] [PubMed] [Google Scholar]
- Nishimori H, Sasaki Y, Yoshida K, Irifune H, Zembutsu H, Tanaka T, Aoyama T, Hosaka T, Kawaguchi S, Wada T, Hata J, Toguchida J, Nakamura Y, Tokino T (2002) The Id2 gene is a novel target of transcriptional activation by EWS–ETS fusion proteins in Ewing family tumors. Oncogene 21: 8302–8309 [DOI] [PubMed] [Google Scholar]
- Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E (2001) Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409: 1067–1070 [DOI] [PubMed] [Google Scholar]
- Pan L, Hanrahan J, Li J, Hale LP, Zhuang Y (2002) An analysis of T cell intrinsic roles of E2A by conditional gene disruption in the thymus. J Immunol 168: 3923–3932 [DOI] [PubMed] [Google Scholar]
- Park ST, Nolan GP, Sun X-H (1999) Growth inhibition and apoptosis due to restoration of E2A activity in T cell acute lymphoblastic leukemia cells. J Exp Med 189: 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C, Lucas B, Vasseur F (1995) Cell expansion and growth arrest phases during the transition from precursor (Cd4(−)8(−)) to immature (Cd4(+)8(+)) thymocytes in normal and genetically modified mice. J Immunol 154: 5103–5113 [PubMed] [Google Scholar]
- Peverali F, Ramqvist T, Saffrich R, Pepperkok R, Barone M, Philipson L (1994) Regulation of G1 progression by E2A and Id helix–loop–helix proteins. EMBO J 13: 4291–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Leder P (2001) The upstream enhancer is necessary and sufficient for the expression of the pre-T cell receptor {alpha} gene in immature T lymphocytes. J Exp Med 194: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera RR, Johns CP, Quan J, Johnson RS, Murre C (2000) Thymocyte selection is regulated by the helix–loop–helix inhibitor protein, Id3. Immunity 12: 17–26 [DOI] [PubMed] [Google Scholar]
- Rockman SP, Currie SA, Ciavarella M, Vincan E, Dow C, Thomas RJ, Phillips WA (2001) Id2 is a target of the beta-catenin/T cell factor pathway in colon carcinoma. J Biol Chem 276: 45113–45119 [DOI] [PubMed] [Google Scholar]
- Romanow WJ, Langerak AW, Goebel P, Wolvers-Tettero IL, van Dongen JJ, Feeney AJ, Murre C (2000) E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol Cell 5: 343–353 [DOI] [PubMed] [Google Scholar]
- Schlissel M, Voronova A, Baltimore D (1991) Helix–loop–helix transcription factor-E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev 5: 1367–1376 [DOI] [PubMed] [Google Scholar]
- Schmid I, Uittenbogaart CH, Giorgi JV (1991) A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry 12: 279–285 [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Alt FW (1994) CD3ɛ-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/− mice in the absence of TCRβ chain expression. Int Immunol 6: 995–1001 [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Yamasaki S, Takase K, Nakatsu F, Arase H, Onodera M, Saito T (2001) E2A and HEB activate the pre-TCRalpha promoter during immature T cell development. J Immunol 167: 2157–2163 [DOI] [PubMed] [Google Scholar]
- van Oers NS, Lowin-Kropf B, Finlay D, Connolly K, Weiss A (1996) Alpha beta T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity 5: 429–436 [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ (1993) Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T-lymphocytes. J Immunol 151: 2546–2554 [PubMed] [Google Scholar]
- Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, Flavell RA, Ghosh S (2000) NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity 13: 677–689 [DOI] [PubMed] [Google Scholar]
- Wang X, Xu K, Ling MT, Wong YC, Feng HC, Nicholls J, Tsao SW (2002) Evidence of increased Id-1 expression and its role in cell proliferation in nasopharyngeal carcinoma cells. Mol Carcinogen 35: 42–49 [DOI] [PubMed] [Google Scholar]
- Yan W, Young AZ, Soares VC, Kelley R, Benezra R, Zhuang Y (1997) High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol Cell Biol 17: 7317–7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE (1998) LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92: 83–92 [DOI] [PubMed] [Google Scholar]
- Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE (1999) Essential role of LAT in T cell development. Immunity 10: 323–332 [DOI] [PubMed] [Google Scholar]
- Zhao F, Vilardi A, Neely RJ, Choi JK (2001) Promotion of cell cycle progression by basic helix–loop–helix E2A. Mol Cell Biol 21: 6346–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Barndt RJ, Pan L, Kelley R, Dai M (1998) Functional replacement of the mouse E2A gene with a human HEB cDNA. Mol Cell Biol 18: 3340–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Soriano P, Weintraub H (1994) The helix–loop–helix gene E2A is required for B cell formation. Cell 79: 875–884 [DOI] [PubMed] [Google Scholar]
- Zuniga-Pflucker JC, Lenardo MJ (1996) Regulation of thymocyte development from immature progenitors. Curr Opin Immunol 8: 215–224 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods


