Abstract
Several chemically modified tetracycline analogs (CMTs), which were chemically modified to eliminate their antibacterial efficacy, were unexpectedly found to have antifungal properties. Of 10 CMTs screened in vitro, all exhibited antifungal activities, although their efficacies varied. Among these compounds, CMT-315, -3, and -308 were found to be the most potent as antifungal agents. The MICs of CMT-3 against 47 strains of fungi in vitro were determined by using amphotericin B (AMB) and doxycycline as positive and negative controls, respectively. The MICs of CMT-3 were generally found to be between 0.25 and 8.00 μg/ml, a range that approximates the blood levels of this drug when administrated orally to humans. Of all the yeast species tested to date, Candida albicans showed the greatest sensitivity to CMT-3. The filamentous species most susceptible to CMT-3 were found to be Epidermophyton floccosum, Microsporum gypseum, Pseudallescheria boydii, a Penicillium sp., Scedosporium apiospermum, a Tricothecium sp., and Trichophyton rubrum. Growth inhibition of C. albicans by CMT-3, determined by a turbidity assay, indicated a 50% inhibitory concentration of 1 μg/ml. Thirty-nine strains, including 20 yeasts and 19 molds, were used to measure viability (the ability to grow after treatment with a drug) inhibition by CMT-3 and AMB. CMT-3 exhibited fungicidal activity against most of these fungi, especially the filamentous fungi. Eighty-four percent (16 of 19) of the filamentous fungi tested showed more than 90% inhibition of viability by CMT-3. In contrast, AMB showed fungicidal activity against all yeasts tested. However, most of the filamentous fungi (16 of 19) showed less than 50% inhibition of viability by AMB, indicating that AMB is fungistatic against most of these filamentous fungi. To begin to identify the sites in fungal cells affected by CMT-3, C. albicans and a Penicillium sp. were incubated with the compound at 35°C, and then the fluorescence of CMT-3 was observed by confocal laser scanning electron microscopy. CMT-3 appeared to have widespread intracellular distribution throughout C. albicans and the Penicillium sp. The mechanisms of the antifungal activity of CMT-3 are now being explored.
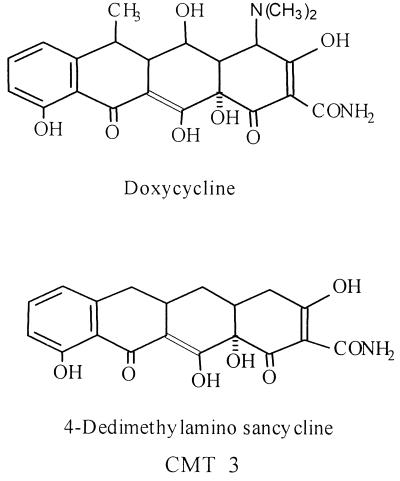
The first chemically modified tetracycline (CMT) was described in 1987 (13), soon after it was discovered that tetracyclines (TETs) could inhibit mammal-derived matrix metalloproteinases (MMPs) in vitro and in vivo (11, 14) by mechanisms which were independent of the antibacterial efficacy of these drugs. Since then, more than 30 different CMTs, in which the 4-dimethylamino group has been deleted, have been developed. The most prominent characteristic of these CMTs is their loss of antibacterial activity, accompanied by retention (or even enhancement) of their efficacy as inhibitors of MMPs. The CMTs do not appear to result in the side effect of antibacterial TETs, namely, the emergence of antibiotic-resistant bacteria (10). They have been tested for their efficacy as inhibitors of connective tissue breakdown, including the preservation of bone and cartilage, in a variety of animal models of diseases, including (but not limited to) arthritis, osteoporosis, aortic aneurysms, periodontitis, and cancer. In fact, one of these nonantibacterial TET analogs, CMT-3 (6-demethyl 6-deoxy 4-dedimethylamino TET, or 4-dedimethylamino sancycline) (Fig. 1), is currently in phase I and II clinical trials on humans with various types of cancer (20).
FIG. 1.
Structures of doxycycline and CMT-3. Note that for CMT-3, the dimethylamino group at the A ring has been removed, resulting in the loss of antibacterial properties.
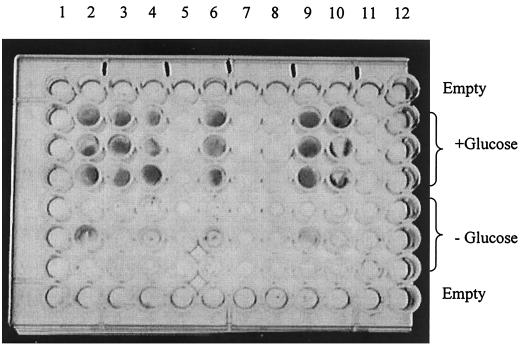
In a recent experiment designed to evaluate the potential ability of CMTs to inhibit nonenzymatic glycation in vitro, it was found that some CMTs (but not doxycycline) inhibited the growth of a mold that was contaminating the test. The contaminant was later identified as a Penicillium sp. (Fig. 2). This unexpected finding suggested that modifying the TET molecule by removing the dimethylamino group at position 4 (Fig. 1), thereby eliminating its antibacterial activity, conferred a new property to the compound, namely, antifungal activity.
FIG. 2.
Discovery of the antifungal activity of CMT-3 on a Matrigel culture. The contaminating fungus (dark color) was identified as a Penicillium sp. (wells 1 and 12 are empty; wells 2 through 11 contain DMSO, doxycycline, minocycline, and CMT-1, -2, -3, -308, -5, -6, and -8, respectively).
It is well known that fungi cause many diseases of plants, animals, and humans and often acquire drug resistance during treatment. Fungal infections are becoming increasingly common in humans, in part due to the extensive use of antibiotics that suppress the normal biota and promote fungal overgrowth. Antimicrobial chemotherapy, including the treatment of infections associated with AIDS, cancer, and organ transplantation, places humans at risk for acquiring opportunistic fungal infections. Moreover, compared to the repertoire of antibiotics used in the management of bacterial diseases, far fewer drugs are currently available to treat fungal infections. This limited choice of antifungal drugs makes it more difficult to avoid drug resistance. As an example, prolonged use of amphotericin B (AMB) in AIDS patients induces drug resistance in fungal strains, a common cause of death in these immunosuppressed patients (5).
Of the limited number of antifungal agents available clinically, most can trigger serious side effects. For example, recommended doses of AMB may produce adverse effects, such as fever, nausea, hypokalemia, nephrotoxicity, hepatotoxicity, leukocytopenia, thrombocytopenia, anemia, chills, and even death (15, 16, 26). It has also been reported that prolonged use of AMB can induce disseminated candidiasis in humans (4). Azole antifungal agents also have clinical limitations, such as poor absorption and a tendency to produce resistance (7). Clearly, the development of new classes of antifungal agents is of increasing importance.
Our current study attempted to assess the antifungal properties of CMT-3, to compare its efficacy to that of AMB, and to begin to explore its therapeutic potential.
MATERIALS AND METHODS
Fungal strains and reagents.
Forty-seven fungal strains were tested for their susceptibility to CMT-3 and AMB. Among these fungi, 11 strains were purchased from the American Type Culture Collection (Manassas, Va.) and included Candida parapsilosis (ATCC 22019), Candida tropicalis (ATCC 750), Candida krusei (ATCC 6258), Paecilomyces variotii (ATCC 22319), Trichophyton rubrum (ATCC 10218), Cryptococcus albidus (ATCC 34140), Aspergillus fumigatus (ATCC 1022), and Candida albicans (ATCC 24433, ATCC 76615, ATCC 18804, and ATCC 90028). Thirty-six clinical isolates were obtained from the Clinical Microbiology Laboratory, Department of Laboratories, University Hospital and Medical Center, State University of New York at Stony Brook.
CMTs and doxycycline were provided by Collagenex Pharmaceutical, Inc. (Newtown, Pa.). Potato dextrose broth (PDB) and SABHI agar were purchased from Difco Laboratories, Inc. (Detroit, Mich.). AMB, potato dextrose agar (PDA), and reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Screening of CMTs as antifungal agents.
Strains of four fungal species (which are usually found as clinical isolates), i.e., a Penicillium sp., A. fumigatus (ATCC 1022), a Rhizopus sp., and C. albicans, were cultured on PDA slants aerobically at 35°C for 48 h. A sterile cotton-tipped applicator was moistened with sterile 0.9% saline and rolled over the surface of each PDA slant of a fungus demonstrating copious conidiogenesis. The conidia were suspended in 0.9% saline, and the turbidity was adjusted to match a 0.5 MacFarland standard, which is equivalent to approximately 1.5 × 108 cells/ml. C. albicans was suspended in saline and adjusted to a 0.5 MacFarland standard in a similar manner. These suspensions were diluted 1:100 in sterile 0.9% saline.
SABHI agar at pH 7.0 was prepared in 100-ml aliquots and sterilized at 121°C for 15 min. The sterile SABHI agar was allowed to cool to 50°C, at which time 10 ml of each of the CMTs tested (prepared in 10% dimethyl sulfoxide [DMSO] at a concentration of 250 μg/ml) was added to produce a final concentration of 25 μg of CMT/ml incorporated into the agar base. After mixing, 20 ml of the SABHI agar solution with or without CMT was poured into a petri dish and allowed to solidify. A plate with 1% DMSO served as a control. Ten microliters of each of the conidial suspensions and 10 μl of C. albicans suspension were inoculated onto the agar plates, which were then incubated aerobically at 35°C overnight. The fungal growth on plates containing the different CMTs was compared to the control (containing 1% DMSO alone) as follows: growth of the fungus at levels observed in the control cultures was scored as +4; no detectable fungal growth was scored as 0; and +3, +2, and +1 represented 75, 50, and 25% the fungal growth observed in the control cultures, respectively.
Determination of fungal susceptibility to CMT-3 in vitro.
Among the 10 CMTs screened, CMT-3 was chosen for this study not only because it exhibited potent antifungal activity but also because it has been recently used in phase I clinical trials as a potential drug in humans with cancer. However, in our initial experiments, we found that the high phosphate concentration (0.8 mg/ml; 10 times higher than levels in serum) in RPMI 1640 cell culture medium severely interfered with the antifungal activity of CMT-3. Therefore, we decided to use a new assay to determine the MICs of CMT-3 and AMB; this assay has been demonstrated to be an accurate and practical method for antifungal susceptibility testing in vitro (18). AMB was used as a positive control and doxycycline was used as a negative control to assess the relative efficacy of CMT-3 in vitro.
Briefly, filamentous fungi were grown on PDA slants at 35°C for 48 h or until copious conidiogenesis was reached. The yeast strains were cultured at 35°C for 2 days. The conidial and yeast cell suspensions were prepared as described above and diluted to final concentrations of between 5 × 103 and 2 × 104 CFU/ml for inoculation. PDA was sterilized at 121°C for 15 min and cooled to 55°C. In a water bath at 55°C, 1.0 ml (for 24-well plates) or 0.5 ml (for 48-well plates) of still-molten PDA that had been mixed with CMT-3 or AMB in DMSO was poured into each well to yield different final concentrations of each drug (i.e., 0, 0.06, 0.12, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml). After the agar had cooled to room temperature, 10 μl of conidial or yeast cell suspension was inoculated into each well of the 24-well plate; 5 μl/well was used for the 48-well plate. The plates were then covered and incubated aerobically at 35°C for 48 h or until good growth was apparent on the control plates. The growth of the fungus in each well was scored as described earlier, and the MICs of both CMT-3 and AMB were determined by detecting the minimum drug concentration at which fungal growth was completely inhibited.
Inhibition of fungal cell viability by CMT-3 and AMB. (i) Time course study.
In this study, fungal cell viability is described as the ability of fungal cells to grow under the in vitro conditions of the experiment. We measured the viability of fungal cells after they were incubated with a drug, and then the drug was removed or diluted to an ineffective level. The inhibition of fungal cell viability reflects the irreversible growth inhibition of the fungal cells, i.e., the fungicidal activity. To determine the time needed for the inhibition of fungal cell viability by CMT-3 and AMB, freshly collected conidia of A. fumigatus and C. albicans cells were used. Both fungal cells and conidia were suspended individually in Tris-NaCl buffer (75 mM Tris, 140 mM NaCl, 11 mM KCl [pH 7.0]) at a concentration of between 5 × 105 and 2 × 106 CFU/ml. CMT-3 and AMB at 100 times their final concentrations in DMSO were added to the suspensions to yield a final drug concentration of 10 μg/ml. The controls were prepared by adding DMSO to the fungal suspensions to yield a final concentration of 1% (vol/vol). The control and drug-treated fungal suspensions were incubated at 35°C for 0, 1, 4, 8, 12, and 24 h. At the end of the incubation, each fungal suspension was diluted 1:1,000 with the same buffer to yield an ineffective drug concentration (0.01 μg/ml) and a fungal concentration of between 5 × 102 and 2 × 103 CFU/ml. One hundred microliters of each diluted fungal suspension was evenly inoculated onto PDA in a sterilized petri dish (100 by 15 mm) and then incubated at 35°C for 48 h or until the control colonies were clearly visible to be counted.
(ii) Viability assay.
Thirty-nine fungal strains were grown on PDA slants at 35°C for 2 days or until the conidiogenesis phase. The conidia or cells of each fungus were collected and suspended in Tris-NaCl buffer, preincubated with 10 μg of CMT-3 or AMB/ml at 35°C for 12 h, and then diluted and inoculated onto PDA petri dishes as described above. The viability of each fungal strain after exposure to CMT-3 or AMB was calculated as a percentage of the colony count on the control cultures. Three parallel assays for each fungal strain were carried out to obtain a mean value for percent fungal viability.
Inhibition of C. albicans growth by CMT-3.
Determination of C. albicans growth inhibition by CMT-3 was carried out by a modified turbidity assay (21). A series of tubes containing PDB (5 ml) and different concentrations of CMT-3 (0, 0.125, 0.25, 0.5, 1.0, and 2.0 μg/ml) were each inoculated with a 100-μl suspension of C. albicans (isolate 2730) in late log phase to yield a final cell concentration of 106/ml. The tubes were aerobically incubated at 35°C, and at each time point (0, 1, 2, 4, 6, 12, and 24 h), the turbidity in each tube was determined spectrophotometrically (Spectronic 70; Bausch & Lomb) at 600 nm.
CLSM.
C. albicans (2730) and a Penicillium sp. were grown on PDA slants at 35°C for 48 h. At the end of incubation, the conidia and the yeast cells were collected and washed with Tris-NaCl buffer twice and resuspended in the same buffer to yield a concentration of 106/ml. Aliquots (0.5 ml) of the suspensions were incubated with different concentrations of CMT-3 (0.5, 5, and 10 μg/ml) at 35°C for different times (0, 1, 6, and 12 h). After incubation, the fungal suspensions were washed twice with the same buffer to remove CMT-3, resuspended in the same volume of distilled water (containing 0.1% glycerol), and stored at −20°C for later examination with confocal laser scanning microscopy (CLSM) to determine the fluorescence of CMT-3 in the fungi.
Aliquots of each type of fungi were placed individually on clean glass slides. Slow Fade Antifade reagent in glycerol-phosphate buffer (The Slow Fade Antifade Kit; Molecular Probes, Eugene, Oreg.) was applied to each sample to prevent fading of any fluorescent compound. Each glass slide containing a sample was covered by a coverslip and sealed around the edges with fingernail polish to keep the sample contents and reagents confined and the cells hydrated. The samples were sectioned optically with emission and excitation wavelengths of 520 and 380 nm, respectively, by using a model 510 confocal laser scanning microscope (Zeiss Optical Systems, Inc., Thornwood, N.Y.).
RESULTS
Antifungal activity of CMT-3.
Preliminary screening with selected CMTs, including CMT-3, -4, -7, -8, -302, -303, -306, -308, -309, and -315, showed that all 10 compounds tested exhibited antifungal activity with various potencies; CMT-315 and -3 were among the most effective, followed by CMT-308, -7, and -4. Of the four fungal strains initially tested, the Rhizopus sp. was the most resistant to CMTs and the Penicillium sp. was the most sensitive (Table 1).
TABLE 1.
Initial screening of 10 different CMTs as antifungal agents in vitro
| Organisma | Scoreb for the following CMT:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 7 | 8 | 302 | 303 | 306 | 308 | 309 | 315 | None (DMSO) | |
| Penicillium sp. (CI) | 0 | 0 | 0 | 0 | 0 | 0 | 1+ | 0 | 0 | 0 | 4+ |
| A. fumigatus (ATCC 1022) | 0 | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 0 | 3+ | 0 | 4+ |
| Rhizopus sp. (CI) | 3+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 1+ | 4+ |
| C. albicans (CI) | 1+ | 1+ | 0 | 4+ | 4+ | 3+ | 3+ | 4+ | 4+ | 0 | 4+ |
CI, clinical isolate.
See the text for definitions of scores.
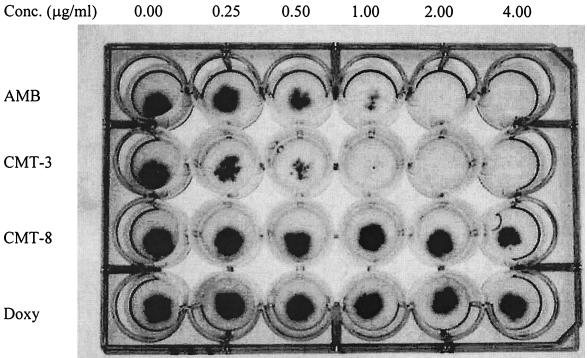
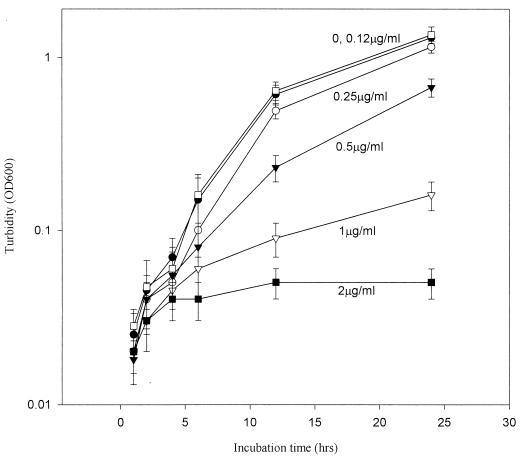
Antifungal susceptibility testing for CMT-3 and AMB showed clear end points in the PDA method (Fig. 3). The MICs of CMT-3 and AMB against 47 fungal strains are listed in Table 2. Interestingly, CMT-3 appeared to function much better as an antifungal agent against filamentous fungi than AMB, especially against a Cunninghamella sp., Epidermophyton floccosum, a Fonsecaea sp., Pseudallescheria boydii, Phialophora verrucosa, Scedosporium apiospermum, and a Tricothecium sp., whereas AMB appeared to be ineffective against these fungi. Conversely, AMB showed better efficacy than CMT-3 against yeast strains; different strains of C. albicans exhibited highly variable susceptibility to CMT-3 (at 0.25 to >8.0 μg/ml). CMT-3 showed no activity against some strains of C. krusei, C. parapsilosis, Candida glabrata, and C. tropicalis at concentrations of up to 8 μg/ml. However, in PDB, the growth of C. albicans (isolate 2730) was noticeably inhibited by CMT-3 (Fig. 4); the MIC and the 50% inhibitory concentration were found to be 2 and 1 μg/ml, respectively. This result is consistent with the MICs determined with the PDA method, as shown in Table 2.
FIG. 3.
MICs of AMB, CMT-3, CMT-8, and doxycycline against A. fumigatus, determined by the PDA method. The four drugs were individually integrated into PDA, 10 μl of fungal suspension was inoculated onto the agar in each well, and incubation was done at 35°C for 48 h. Wells in the first lane (drug free) were used as negative controls, and MIC end points were determined when complete inhibition of fungal growth was observed visually. The MICs of AMB and CMT-3 were determined to be 2.00 μg/ml, and CMT-8 and doxycycline (Doxy) showed no antifungal activity.
TABLE 2.
MICs of CMT-3 and AMB
| Organism | Sourcea | MIC (μg/ml) of:
|
No. of strains | |
|---|---|---|---|---|
| CMT-3 | AMB | |||
| C. albicans | ATCC 24433, ATCC 76615, ATCC 18804, ATCC 90028, CI | 0.25->8 | 0.12-4.0 | 17 |
| C. albidus | ATCC 34140 | 2.0 | 1.0 | 1 |
| C. glabrata | CI | 4.0-8.0 | 0.5-2.0 | 2 |
| C. krusei | ATCC 6258, CI | 4.0->8.0 | 1.0-2.0 | 3 |
| C. parapsilosis | ATCC 22019, CI | >8.0 | 1.0-2.0 | 2 |
| C. tropicalis | ATCC 750, CI | 4.0->8.0 | 0.5-2.0 | 3 |
| Absidia sp. | CI | 4.0 | 4.0 | 1 |
| A. flavus | CI | 2.0 | 1.0 | 1 |
| A. fumigatus | ATCC 1022 | 2.0 | 2.0 | 1 |
| Cunninghamella sp. | CI | 8.0 | >8.0 | 1 |
| E. floccosum | CI | 0.5 | >8.0 | 1 |
| Fonsecaea sp. | CI | 4.0 | >8.0 | 1 |
| M. canis | CI | 2.0 | 0.5 | 1 |
| M. gypseum | CI | 1.0 | 4.0 | 1 |
| P. boydii | CI | 0.25 | >8.0 | 1 |
| Penicillium sp. | CI | 0.5 | 0.25 | 1 |
| P. variotii | ATCC 22319 | 1.0 | 0.5 | 1 |
| P. verrucosa | CI | 8.0 | >8.0 | 1 |
| Rhizopus sp. | CI | 1.0 | 0.5 | 1 |
| S. apiospermum | CI | 0.5 | >8.0 | 1 |
| T. mentagrophytes | CI | 1.0 | 4.0 | 1 |
| T. tonsurans | CI | 2.0 | 0.25 | 1 |
| Tricothecium sp. | CI | 0.5 | >8.0 | 1 |
| T. rubrum | ATCC 10218 | 0.5 | 1.0 | 1 |
| Ulocladium sp. | CI | 1.0 | 2.0 | 1 |
CI, clinical isolate.
FIG. 4.
CMT-3 inhibits the growth of C. albicans. The yeast was incubated with different concentrations of CMT-3 in PDB at 35°C, and growth (turbidity) was monitored at each time point; OD600, optical density at 600 nm. Three parallel assays were done to obtain means; error bars show standard deviations. The MIC of CMT-3 was 2.0 μg/ml, and the 50% inhibitory concentration was about 1.0 μg/ml.
Inhibition of fungal viability by CMT-3 and AMB was generally consistent with the MIC results. That is, CMT-3 appeared to be more effective than AMB as a fungicidal agent against filamentous fungi but was less effective than the latter on yeasts (Table 3). At a concentration of 10 μg/ml, CMT-3 showed efficacy against most of the tested fungi; i.e., the viability of 33 of the 39 tested strains (85%) was diminished by more than 50% in the presence of CMT-3. Particularly potent fungicidal activity of CMT-3 was observed against filamentous fungi; i.e., the viability of 84% (16 of 19) of the tested filamentous fungi (including P. boydii, a Penicillium sp., S. apiospermum, a Tricothecium sp., T. rubrum, Microsporum canis, Trichophyton mentagrophytes, and Trichophyton tonsurans) was diminished by more than 90% in the presence of CMT-3. In contrast, AMB (at 10 μg/ml) eliminated the viability of all yeasts tested by 100%, but the viability of only 15% (3 of 19) of the filamentous fungi was diminished by more than 50% by this drug.
TABLE 3.
Inhibition of fungal viability by CMT-3 and AMB
| Organism | Sourcea | Control CFU | Result obtained with:
|
|||
|---|---|---|---|---|---|---|
| CMT-3
|
AMB
|
|||||
| CFU | % Viability | CFU | % Viability | |||
| C. albidus | ATCC 34140 | 96 | 85 | 88 | 0 | 0 |
| C. albicans | ATCC 24433 | 137 | 9 | 7 | 0 | 0 |
| C. albicans | ATCC 76615 | 116 | 6 | 5 | 0 | 0 |
| C. albicans | ATCC 18804 | 148 | 11 | 7 | 0 | 0 |
| C. albicans | ATCC 90028 | 109 | 2 | 2 | 0 | 0 |
| C. albicans | 45881 (CI) | 91 | 1 | 1 | 0 | 0 |
| C. albicans | 2730 (CI) | 165 | 0 | 0 | 0 | 0 |
| C. albicans | 3533 (CI) | 76 | 13 | 17 | 0 | 0 |
| C. albicans | 42420 (CI) | 89 | 5 | 6 | 0 | 0 |
| C. albicans | 14053 (CI) | 114 | 51 | 45 | 0 | 0 |
| C. albicans | 57187 (CI) | 108 | 47 | 43 | 0 | 0 |
| C. albicans | 56344 (CI) | 121 | 110 | 91 | 0 | 0 |
| C. albicans | 44231 (CI) | 92 | 19 | 21 | 0 | 0 |
| C. glabrata | CI | 102 | 28 | 27 | 0 | 0 |
| C. krusei | ATCC 6258 | 72 | 3 | 4 | 0 | 0 |
| C. parapsilosis | ATCC 22019 | 98 | 85 | 86 | 0 | 0 |
| C. parapsilosis | 47903 (CI) | 89 | 81 | 91 | 0 | 0 |
| C. tropicalis | ATCC 750 | 114 | 5 | 4 | 0 | 0 |
| C. tropicalis | 66029 (CI) | 78 | 2 | 2 | 0 | 0 |
| C. tropicalis | 57421 (CI) | 89 | 3 | 3 | 0 | 0 |
| Fonsecaea sp. | CI | 155 | 5 | 3 | 142 | 92 |
| P. boydii | CI | 96 | 0 | 0 | 93 | 97 |
| Cunninghamella sp. | CI | 91 | 73 | 80 | 95 | 100 |
| Ulocladium sp. | CI | 106 | 67 | 63 | 110 | 100 |
| A. fumigatus | ATCC 1022 | 102 | 4 | 4 | 92 | 90 |
| A. flavus | CI | 82 | 3 | 4 | 75 | 91 |
| Penicillium sp. | CI | 98 | 1 | 1 | 46 | 47 |
| P. verrucosa | CI | 112 | 3 | 2 | 107 | 96 |
| Rhizopus sp. | CI | 85 | 2 | 2 | 63 | 74 |
| Absidia sp. | CI | 101 | 42 | 42 | 104 | 100 |
| S. apiospermum | CI | 84 | 0 | 0 | 77 | 92 |
| Tricothecium sp. | CI | 77 | 1 | 1 | 72 | 94 |
| T. rubrum | ATCC 10218 | 92 | 1 | 1 | 68 | 74 |
| P. variotii | ATCC 22319 | 84 | 8 | 10 | 9 | 23 |
| M. canis | CI | 78 | 1 | 1 | 33 | 42 |
| M. gypseum | CI | 103 | 3 | 3 | 74 | 72 |
| T. mentagrophytes | CI | 72 | 0 | 0 | 65 | 90 |
| E. floccosum | CI | 110 | 2 | 2 | 98 | 89 |
| T. tonsurans | CI | 79 | 0 | 0 | 45 | 57 |
CI, clinical isolate.
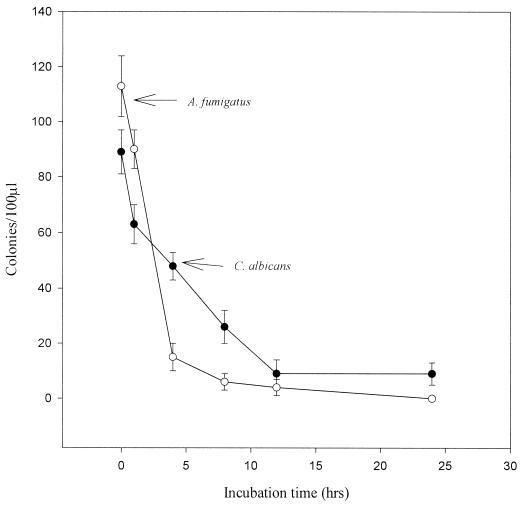
The time course study revealed that AMB at 10 μg/ml killed 100% of C. albicans in only 1 h, but this drug had no effect on the conidia of A. fumigatus (ATCC 1022), even after 24 h of exposure to the drug (data not shown). In contrast, 4 and 12 h of exposure to CMT-3 were required to kill 90% of A. fumigatus conidia and C. albicans, respectively (Fig. 5). These results indicated that CMT-3 was fungicidal against both of these fungi, whereas AMB was fungicidal against C. albicans but only fungistatic against A. fumigatus.
FIG. 5.
Time course test for the fungicidal activity of CMT-3 against A. fumigatus and C. albicans. The fungi were individually incubated with the drug (10 μg/ml) at 35°C for 0, 1, 4, 8, 12, and 24 h and then diluted 1:1,000; 100 μl of each fungal cell suspension was separately inoculated onto PDA plates and incubated at 35°C for 48 h. The colonies were counted, and the results of three parallel assays were combined to obtain the means and standard deviations.
CLSM observation of CMT-3 in fungal cells.
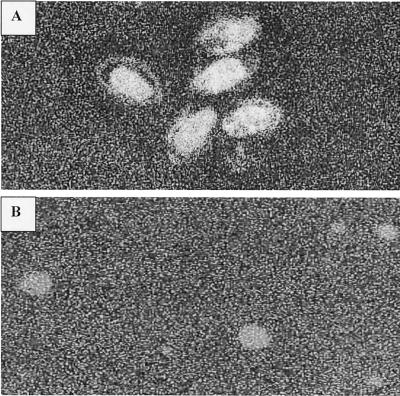
CMTs, like the parent compounds TET and doxycycline, show strong fluorescence at 520 nm with excitation at 380 nm. After 6 h of incubation (at 35°C) of C. albicans and a Penicillium sp. with 10 μg of CMT-3/ml, the fluorescence of CMT-3 was clearly observed inside both types of fungal cells (Fig. 6). Although fluorescence was mainly concentrated inside the fungal cells, some fluorescence was detected in the region of the cell wall (Fig. 6A).
FIG. 6.
CLSM of a Penicillium sp. (A) and C. albicans (B) showing the intracellular fluorescence of CMT-3. Note that the fluorescence was less intense in the region of the cell wall than in the intracellular area (A). Magnification, ×75.
DISCUSSION
The long-recognized antibacterial properties of the TET family of antibiotics is attributed to binding to the 30S subunit of bacterial ribosomes and inhibition of protein synthesis. However, these antibiotics are not considered to be toxic to eukaryotic cells, such as fungal cells, because they have different ribosome subunits. Therefore, TET and its semisynthetic analogs, doxycycline and minocycline, have not been considered antifungal agents and, in fact, have been found to promote the overgrowth of fungi due to the suppression of normal microbiota. Recently, a new group of TET analogs, CMTs, was described. These compounds were modified to eliminate their antibacterial activity (to reduce the development of antibiotic-resistant bacteria) by removal of the dimethylamino group from carbon 4 of the A ring of the 4-ring TET backbone (10, 13). CMTs, like antibacterial TETs, exhibit pleiotropic nonantibiotic properties, including, but not limited to, an ability to inhibit host-derived MMPs and inflammatory mediators, such as prostaglandin E2 and the cytokines interleukin 1β and tumor necrosis factor alpha (6, 12). One mechanism, among several identified, includes the ability of TETs and CMTs to bind to metal ions, such as Ca2+ and Zn2+, in the catalytic domain of an MMP molecule, thus blocking proteinase activity (12).
The discovery of the antifungal activity of the CMTs, however, was surprising and, to date, this activity has not been related to any of the properties associated with antibacterial TETs. In addition, only a subset of the CMTs, notably, CMT-3, exhibits this antifungal property; as an example, CMT-8, a very potent inhibitor of MMPs and bone resorption by nonantibacterial mechanisms, exhibited much less antifungal activity than CMT-3 (Table 1 and Fig. 3).
In most of the in vitro assays, CMT-3 was compared to AMB as a positive control and to doxycycline as a negative control (Fig. 3); at no time did doxycycline exhibit antifungal activity under the in vitro conditions used in this study. Compared to AMB, CMT-3 is an especially effective inhibitor of the growth and viability of filamentous fungi. Most of the MICs of CMT-3 against filamentous fungi were found to be between 0.25 and 8 μg/ml, and the inhibition of viability of these fungi by CMT-3 was routinely higher than 90%. However, its potency against yeast cells varied significantly with different strains, even within a single species, such as C. albicans. However, levels of CMT-3 in serum in both rats and humans after oral administration of this drug have been observed to reach a range of between 3 and 10 μg/ml (17, 23). These serum levels are significantly higher than most of the MICs of CMT-3 against the different fungal strains tested so far.
The antifungal mechanism of CMT-3 may be quite different from that of AMB. For example, at 10 μg of AMB/ml and 106 cells of C. albicans/ml, it took less than 1 h for AMB to kill 100% of this organism, reflecting its great potency as a fungicidal agent against yeast cells. However, CMT-3 exhibited much higher potency as a fungicidal agent against filamentous fungi (more than 90% inhibition of the viability of most filamentous fungi tested), but it took a longer time for CMT-3 than for AMB to kill fungal cells.
The antifungal mechanism of AMB has been extensively studied. The most recognized mechanism is the formation of pores in the cell membrane. Increased membrane permeability causes leakage of small molecules (1, 3, 8, 22, 24). The primary action site of AMB on fungal cells is believed to be the membrane component, ergosterol. AMB binds to this component, resulting in a loss of the permeability barrier to small metabolites. The fact that fungal membranes contain ergosterol while mammalian cell membranes contain cholesterol may help explain the greater toxicity of AMB to fungi, even though this drug is also toxic to mammals. On the other hand, the azole antifungal agents act by inhibition of P-450DM, which causes depletion of ergosterol in the fungal membrane, resulting in inhibition of fungal growth.
CMT-3 may exert its fungicidal activity through mechanisms different from those of current antifungal drugs, such as AMB or the azoles, because CMT-3 exhibits a different antifungal spectrum. That is, CMT-3 exhibits more efficacy against filamentous fungi, and AMB and azoles display more efficacy against yeasts. The longer incubation time required for CMT-3 to inhibit the viability of fungal cells (compared to AMB) and the observation of strong CMT-3 fluorescence inside both filamentous and yeast cells indicate that CMT-3 may exert its activity within fungal cells by, for example, acting on subcellular organelles or affecting some aspect of metabolism. Based on the current experimental results, we hypothesize that CMT-3 may inhibit fungal viability mainly through actions on intracellular organelles, such as the mitochondria, nucleus, and endoplasmic reticulum. The reaction of CMT-3 with these organelles may disturb intracellular membranes, resulting in inhibition of some metabolic steps, such as oxidative phosphorylation or protein synthesis in fungal cells. Recently, CMT-3 and some of its derivatives were found to be able to cause depolarization of mitochondrial membranes of tumor cell lines in vitro (19), suggesting a possible analogous antifungal mechanism for CMTs.
AMB is known to be the most efficacious drug in the treatment of deep-seated and systemic mycoses (9). Unfortunately, severe adverse effects, such as fever, vomiting, nausea, nephrotoxicity, and others, have greatly limited its use, especially for kidney transplant patients (2). Several lipid formulations of AMB, such as ABLC (AMB lipid complex; The Liposome Co., Princeton, N.J.), ABCD (AMB colloidal dispersion; Sequus Pharmaceuticals, Menlo Park, Calif.), and L-AMB (AMBisome; NeXstar Pharmaceuticals/Fujisawa, San Dimas, Calif.), have been developed to reduce its nephrotoxicity (30). However, the high cost of these new formulations creates an additional limitation. Therefore, they are recommended for use only in patients with severely impaired renal function and invasive fungal infections that cannot be treated with other antifungal agents (28). Moreover, AMB has no effect on some filamentous fungi, such as P. boydii (29).
Azole antifungal agents, such as ketaconazole, fluconazole, and itraconazole, were developed during the 1970s and 1980s. This class of antifungal agents was shown to be less toxic than AMB yet effective for immunocompetent patients with non-life-threatening, nonmeningeal forms of fungal infections. However, the significant limitations of these azoles should not be neglected, such as the development of resistance, slow clearance from the host, narrow spectrum of activity, and negative effects on hepatic microsomal enzyme activity. A severe disadvantage of the use of azoles is that they decrease the susceptibility of fungi to AMB (25, 27) due to their inhibition of P-450DM. This action results in the depletion of membrane ergosterol, to which AMB binds. Therefore, in addition to improvement of existing antifungal agents, the development of new antifungal drugs is an important way to overcome the current difficulties in the treatment of fungal infections.
The significance of the discovery of several CMTs as antifungal agents lies not only in the identification of the apparently novel antifungal activity of this class of compounds but also in the potential of the CMTs to play a complementary role in the chemotherapy of fungal infections in combination with other antifungal agents, such as AMB (note that CMT-3 has an antifungal spectrum complementary to that of AMB). It is obviously beneficial to patients with fungal infections when there are more antifungal agents available for alternative use, so that the development of drug resistance may be reduced or prevented during treatment.
REFERENCES
- 1.Bolard, J. 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864:257-304. [DOI] [PubMed] [Google Scholar]
- 2.Clements, J. S., and J. R. Peacock. 1990. AMB revisited: reassessment of toxicity. Am. J. Med. 88:5-22N-5-27N. [PubMed] [Google Scholar]
- 3.Cohen, B. E. 1992. A sequential mechanism for the formation of aqueous channels by AMB in liposomes. The effect of sterols and phospholipid composition. Biochim. Biophys. Acta 1108:49-58. [DOI] [PubMed] [Google Scholar]
- 4.Conly, J., R. Rennie, J. Johnson, S. Farah, and L. Hellman. 1992. Disseminated candidiasis due to AMB-resistant Candida albicans. J. Infect. Dis. 165:761-764. [DOI] [PubMed] [Google Scholar]
- 5.Dupont, B., J. R. Graybill, D. Armstrong, R. Laroche, J. E. Touze, and L. J. Wheat. 1992. Fungal infections in AIDS patients. J. Med. Vet. Mycol. 30(Suppl. 1):19-28. [DOI] [PubMed] [Google Scholar]
- 6.Eklund, K. K., and T. Sorsa. 1999. Tetracycline derivative CMT-3 inhibits cytokine production, degranulation, and proliferation in cultured mouse and human mast cell. Ann. N. Y. Acad. Sci. 878:689-692. [DOI] [PubMed] [Google Scholar]
- 7.Fan-Havard, P., D. Capano, S. M. Smith, A. Mangia, and R. H. Eng. 1991. Development of resistance in Candida isolates from patients receiving prolonged antifungal therapy. Antimicrob. Agents Chemother. 35:2302-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale, E. F. 1974. The release of potassium ions from Candida albicans in the presence of polyene antibiotics. J. Gen. Microbiol. 80:451-465. [DOI] [PubMed] [Google Scholar]
- 9.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. AMB: 30 years of clinical experience. Rev. Infect. Dis. 12:308.. [DOI] [PubMed] [Google Scholar]
- 10.Golub, L. M., R. A. Greenwald, N. S. Ramamurthy, T. F. McNamara, and B. R. Rifkin. 1991. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit. Rev. Oral Biol. Med. 2:297-322. [DOI] [PubMed] [Google Scholar]
- 11.Golub, L. M., H. M. Lee, G. Lehrer, A. Nemiroff, T. F. McNamara, R. Kaplan, and N. S. Ramamurthy. 1983. Minocycline reduces gingival collagenolytic activity during diabetes: preliminary observations and a proposed new mechanism of action. J. Periodontal Res. 18:516-526. [DOI] [PubMed] [Google Scholar]
- 12.Golub, L. M., H. M. Lee, and M. E. Ryan. 1998. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 12:12-26. [DOI] [PubMed] [Google Scholar]
- 13.Golub, L. M., T. F. McNamara, G. D'Angelo, R. A. Greenwald, and N. S. Ramamurthy. 1987. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J. Dent. Res. 66:1310-1314. [DOI] [PubMed] [Google Scholar]
- 14.Golub, L. M., N. S. Ramamurthy, T. F. McNamara, J. B. Gomes, M. Wolff, A. Casino, A. Kapoor, J. J. Zambon, S. Ciancio, M. Schneir, and H. D. Perry. 1984. Tetracyclines inhibit tissue collagenase activity: a new mechanism in the treatment of periodontal disease. J. Periodontal Res. 19:651-655. [DOI] [PubMed] [Google Scholar]
- 15.Koren, G., A. Lau, C. F. Kenyon, D. Kroppert, and J. Klein. 1990. Clinical course and pharmacokinetics following a massive overdose of AMB in a neonate. J. Toxicol. Clin. Toxicol. 28:371-378. [DOI] [PubMed] [Google Scholar]
- 16.Kreft, B., C. De Wit, R. Marre, and K. Sack. 1991. Experimental studies on the nephrotoxicity of AMB in rats. J. Antimicrob. Chemother. 28:271-281. [DOI] [PubMed] [Google Scholar]
- 17.Liu, Y., N. S. Ramamurthy, J. Marecek, H. M. Lee, J. L. Chen, M. E. Ryan, B. R. Rifkin, and L. M. Golub. 2001. The lipophilicity, pharmacokinetics, and cellular uptake of different chemically-modified tetracyclines (CMTs). Curr. Med. Chem. 8:243-252. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y., G. Tortora, M. E. Ryan, H.-M. Lee, and L. M. Golub. 2002. Potato dextrose agar antifungal susceptibility testing for yeasts and molds: evaluation of phosphate effect on antifungal activity of CMT-3. Antimicrob. Agents Chemother. 46:1455-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lokeshwar, B. L., E. Escatel, and B. Zhu. 2001. Cytotoxic activity and inhibition of tumor cell invasion by derivatives of a chemically modified tetracycline CMT-3 (COL-3). Curr. Med. Chem. 8:271-279. [DOI] [PubMed] [Google Scholar]
- 20.Lokeshwar, B. L., H. L. Houston-Clark, M. G. Selzer, N. L. Block, and L. M. Golub. 1998. Potential application of a chemically modified non-antimicrobial tetracycline (CMT-3) against metastatic prostate cancer. Adv. Dent. Res. 12:149-151. [DOI] [PubMed] [Google Scholar]
- 21.Pollock, J. J., L. Denepitiya, B. J. MacKay, and V. J. Iacono. 1984. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect. Immun. 44:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos, H., E. Valdivieso, M. Gamargo, F. Dagger, and B. E. Cohen. 1996. AMB kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J. Membr. Biol. 152:65-75. [DOI] [PubMed] [Google Scholar]
- 23.Rudek, M. A., W. D. Figg, V. Dyer, W. Dahut, M. L. Turner, S. M. Steinberg, D. J. Liewehr, D. R. Kohler, J. M. Pluda, and E. Reed. 2001. Phase I clinical trial of oral COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J. Clin. Oncol. 19:584-592. [DOI] [PubMed] [Google Scholar]
- 24.Saha, A. K., T. Mukherjee, and A. Bhaduri. 1986. Mechanism of action of AMB on Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 19:195-200. [DOI] [PubMed] [Google Scholar]
- 25.Schaffner, A., and P. Frick. 1985. The effect of ketoconazole on AMB in a model of disseminated aspergillosis. J. Infect. Dis. 151:902-910. [DOI] [PubMed] [Google Scholar]
- 26.Tasset, C., V. Preat, A. Bernard, and M. Roland. 1992. Comparison of nephrotoxicities of different polyoxyethylene glycol formulations of amphotericin B in rats. Antimicrob. Agents Chemother. 36:1525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez, J., M. Arganoza, and J. Vaishampayan. 1996. In vitro interaction between amphotericin B and azoles in Candida albicans. Antimicrob. Agents Chemother. 40:2511-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viscoli, C., E. Castagnola, and M. Machetti. 1997. Antifungal treatment in patients with cancer. J. Intern. Med. 242(Suppl. 740):89-94. [PubMed] [Google Scholar]
- 29.Walsh, M., L. White, K. Atkinson, and A. Enno. 1992. Fungal Pseudoallescheria boydii lung infiltrates unresponsive to AMB in leukaemic patients. Aust. N. Z. J. Med. 22:265-268. [DOI] [PubMed] [Google Scholar]
- 30.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of AMB: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]