Abstract
We analyzed the role of integrons in the dissemination of antibiotic resistance in a recent multiresistant clinical isolate, Serratia marcescens SCH88050909 (SCH909). This isolate harbors three integrons, all on a 60-kb conjugative plasmid. By PCR, hybridization, and sequencing analyses, we found that integron 1 has the dfrA1 and ant(3")-Ia cassettes. The first cassette in integron 2 contains the ant(2")-Ia gene, separated from its attC site (59-base element) by a 1,971-bp insert containing a group II intron; this intron codes for a putative maturase-reverse transcriptase on the complementary strand and is the first such intron to be found associated with an integron. The attC site is followed by a novel aminoglycoside resistance gene, ant(3")-Ii-aac(6′)-IId, which has been characterized for its bifunctional ANT(3")-I and AAC(6′)-II activities. DNA sequence analysis of this fused cassette suggests that insertion and excision due to the integrase activity could have an important role in the evolution of aminoglycoside resistance genes. This gene is followed by an unknown open reading frame with a typical attC site and a partial cassette composed of the beginning of the blaOXA-10 cassette interrupted by IS1. The sequence downstream of IS1 revealed that the blaOXA-10 cassette is incomplete and that the 3′ conserved segment of this integron is absent. Integron 3 is in a Tn1696-like transposon with the aac(3)-Ia cassette followed by three unknown cassettes and ant(3")-Ia. The presence of the group II intron and the relationship of group II introns in eubacteria with mobile elements suggest a possible role of this element in events such as cassette formation and/or plasmid evolution.
The spread of antibiotic resistance genes among bacterial strains is an increasing problem in nosocomial infections (7). Over 40 antimicrobial resistance genes are located within integrons as mobile DNA elements called cassettes and account for a significant proportion of the antibiotic resistance genes found in gram-negative bacteria (26). Integrons are often located in plasmids or transposons, thus enabling the rapid spread of the gene cassettes among a wide variety of bacterial species (2, 3, 23). In fact, several aminoglycoside resistance proteins, such as ANT(3")-I and AAC(6′)-I, which are common in the multiresistant gram-negative bacterial population, are coded for by gene cassettes [ant(3")-Ia, ant(3")-Ib, and aac(6′)-Ib] (14). Their expression depends on their insertion into the variable region of integrons, where the genes are transcribed from a common promoter in the 5′ conserved segment (13).
The origins of the cassette-associated genes are not known (9, 27). It has been proposed that cassettes may have originated from transcripts that were converted to DNA, involving the activity of an as-yet-unidentified reverse transcriptase (RT) (27). The palindromic attC sites (59-base elements) found at the end of each cassette either may have been present within the original transcripts, for example, as transcription terminators, or may have been added at a later stage (27). It has been also speculated that secondary sites for IntI1, defined as the degenerate pentanucleotide GWTMW, can facilitate the association of new genes into integrons and may have been the substrate for the creation of attC sites during evolution (6, 9).
The goal of our work is to elucidate the role of the antimicrobial resistance mechanisms carried on integrons in a multiresistant Serratia marcescens strain. We describe a novel ant(3")-Ii-aac(6′)-IId gene cassette, inserted into the variable region of a class I integron, that codes for a bifunctional ANT(3")-I-AAC(6′)-II mechanism. The role of the integrase activity in the formation of this fused cassette is discussed. The role of a group II intron located between the ant(2")-Ia resistance gene and its attC site is discussed.
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. marcescens SCH88050909 is a clinical isolate from Greece that was collected because of its multiple aminoglycoside resistance at Schering-Plough Corporation in 1988. Here, we use only the last three numbers of the Schering strain designation (SCH909) (14). A nalidixic acid-resistant (Nalr) derivative of Escherichia coli C600 was used as a recipient in conjugation experiments, and E. coli NM522 was used as a recipient in transformation studies. Plasmid pTZ19 (Pharmacia-LKB Biotechnology, Uppsala, Sweden) was used for cloning of DNA fragments.
Media and culture conditions.
Bacterial strains were grown on Luria-Bertani agar and in Luria-Bertani broth supplemented with one or more of the following antibiotics, as appropriate: ampicillin (50 μg/ml), gentamicin (25 μg/ml), nalidixic acid (50 μg/ml), or streptomycin (25 μg/ml).
Antibiotic susceptibility.
Determination of the aminoglycoside resistance profile (AGRP) (30) was done by using aminoglycoside disks (Schering-Plough Corporation, Kenilworth, N.J.) (17) on Mueller-Hinton agar. The 11 aminoglycosides included fortimycin, amikacin, tobramycin, apramycin, dibekacin, gentamicin, isepamicin, netilmicin, 5-episisomicin, 2′-N-ethylnetilmicin, and 6′-N-ethylnetilmicin. Assignment of enzymatic mechanisms to each strain was based on the unique pattern of resistance to these aminoglycosides (17). In addition, the MICs in liquid medium were determined with an inoculum of 105 to 106 CFU/ml.
DNA techniques.
Preparation of DNA was performed as described by Sambrook et al. (29). Plasmid DNA was prepared by using a Wizard purification kit supplied by Promega. DNA was digested with various restriction enzymes, and the fragments were separated in horizontal gels of 0.8 to 1.0% (wt/vol) agarose dissolved in 0.4 M Tris-acetate-0.01 M EDTA. Gels were stained with ethidium bromide, and the DNA was visualized by UV transillumination at 302 nm. Transformation procedures were performed as described by Sambrook et al. (29). Conjugation was carried out with brain heart infusion agar for 4 h at 37°C, and the transconjugants were selected on Mueller-Hinton agar medium containing ampicillin (50 μg/ml).
PCR amplifications were carried out with 100-μl volumes containing 10 ng of DNA, 10 μl of 10× PCR buffer, 10 μl of 10× deoxynucleoside triphosphate mix (2 mM each dATP, dCTP, dGTP, and dTTP), 10 μl of each primer stock solution (2.5 pmol of each primer per μl), and 60 μl of sterile distilled water. Each reaction mixture was covered with 75 μl of mineral oil. Taq DNA polymerase from Promega was added (1 μl of a 3-U/μl diluted solution) after 12 min at 94°C (hot-start method). To amplify the DNA in the thermocycler (Perkin-Elmer Cetus, Emeryville, Calif.), we used a three-step profile described previously (14). The gene probes and/or primers used for PCRs were specific for the following nucleotide sequences: β-lactam resistance genes blaCTX-M-2 (ATGACTCAGAGCATTCGC and TCACTTTATCGGGACCAC), blaPER-2 (1), and blaTEM (14); aminoglycoside resistance genes aac(6′)-Ib, ant(3")-Ia, aac(3)-Ia, aac(3)-IIa, and ant(2")-Ia (14); trimethoprim resistance gene dfrA1 (14); and the 5′ conserved segment (5′ CS) and the integrase gene of class I integrons (Sulpro3) (14). DNA was transferred to nylon filters by Southern blotting and hybridized with appropriate probes as described by Sambrook et al. (29). Autoradiography was done with Kodak X-Omat AR film.
The 5.0-kb HindIII fragment of pSm909 containing integron 3 and Tn1696-related sequences, the 2.3-kb BamHI fragment of pSm909 containing the integrase gene, the ant(2")-Ia gene, and part of the group II intron from integron 2, and the 4.2-kb HincII fragment containing the remainder of the group II intron, ant(3")-Ii-aac(6′)-IId, open reading frame (ORF) O, blaOXA-10Δ, IS1, and unknown sequences from pSm909 were cloned into the corresponding sites of pUC19.
DNA sequencing and analysis.
DNA was purified by using a QIAquick kit according to the manufacturer's instructions (Qiagen Inc., Studio City, Calif.). Sequencing was done on both DNA strands by using an ABI 373 sequencer. Internal oligonucleotide primers were used when necessary to ensure that both strands were sequenced. The nucleotide sequences were analyzed by using Genetics Computer Group programs.
Nucleotide sequence accession numbers.
The nucleotide sequences of the variable regions of integrons 2 and 3 in S. marcescens SCH909 have been deposited in GenBank under accession numbers AF453998 and AF453999, respectively.
RESULTS
Characterization of the antimicrobial resistance genes of S. marcescens strain SCH909.
The MICs for multiresistant S. marcescens SCH909 and its transconjugant in E. coli C600 are shown in Table 1. Strain SCH909 showed susceptibility to cefoperazone, aztreonam, imipenem, norfloxacin, ciprofloxacin, nalidixic acid, netilmicin, and amikacin; reduced susceptibility to cefoxitin, cefotaxime, and ceftazidime; and resistance to gentamicin, tobramycin, neomycin, streptomycin, ampicillin, cephalothin, piperacillin, trimethoprim, sulfamethoxazole, and chloramphenicol. We tested for the presence of blaSHV, blaTEM, blaCTX-M-2, blaPER-2, cmlA, catB2, and Tn3 (where blaTEM is usually located) by PCR and the aac(3)-IIa gene by dot blot hybridization in this strain. The blaTEM gene and Tn3 were harbored by this strain. Sequence analysis of the blaTEM PCR product revealed that the gene was blaTEM1, conferring resistance to ampicillin but not to third-generation cephalosporins, such as cefotaxime. The genetic determinants for the intermediate susceptibility and/or resistance to cefoxitin, cefotaxime, ceftazidime, gentamicin, tobramycin, neomycin, streptomycin, ampicillin, cephalothin, piperacillin, trimethoprim, sulfamethoxazole, and chloramphenicol were transferable and carried on a 60-kb conjugative plasmid (Table 1).
TABLE 1.
MICs of antibiotics for S. marcescens SCH909, E. coli C600 transconjugant, and control E. coli ATCC 25922
| Antibiotic | MIC (μg ml−1) for:
|
||
|---|---|---|---|
| E. coli C600 | S. marcescens SCH909 | E. coli C600 transconjugant | |
| Gentamicin | 1 | 64 | 128 |
| Tobramycin | 0.5 | 128 | 128 |
| Amikacin | 1 | 2 | 2 |
| Netilmicin | 0.5 | 8 | 8 |
| Streptomycin | 1 | 64 | 2 |
| Neomycin | 0.5 | 64 | 64 |
| Ampicillin | 4 | >256 | >256 |
| Cefoxitin | 2 | 4 | 16 |
| Imipenem | 0.25 | 0.5 | 0.5 |
| Cephalothin | 4 | 256 | 256 |
| Cefotaxime | 0.06 | 16 | 16 |
| Cefoperazone | 0.12 | 8 | 8 |
| Ceftazidime | 0.25 | 16 | 16 |
| Piperacillin | 1.0 | >128 | >128 |
| Azthreonam | 0.12 | 4 | 4 |
| Norfloxacin | 0.125 | 0.25 | 0.125 |
| Ciprofloxacin | 0.0625 | 0.0625 | 0.0625 |
| Nalidixic acid | >64 | 4 | >64 |
| Chloramphenicol | 4 | 32 | 32 |
| Trimethoprim | 1 | 16 | 16 |
| Sulfamethoxazole | 16 | >512 | >512 |
Evidence for the presence of three integrons of class I in S. marcescens strain SCH909.
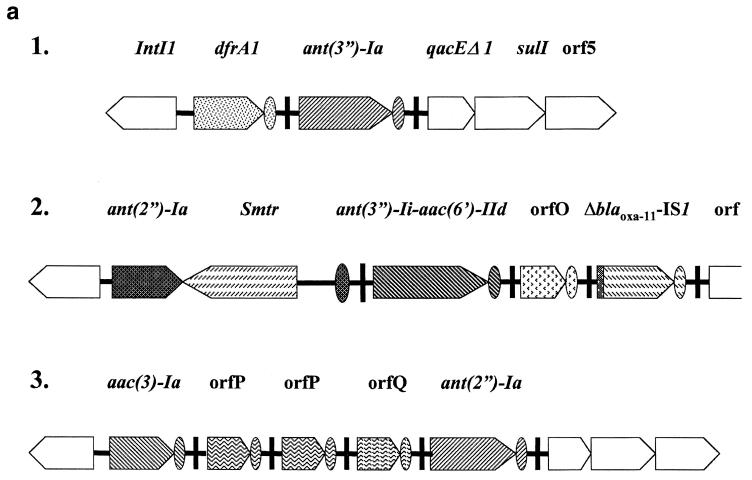
By PCR with primers 5′-CS and 3′-CS from the 5′ and 3′ conserved segments of the integrons, we amplified the variable regions of the integrons. With this pair of primers, there was only one PCR product with a 1,500-bp length, suggesting the presence of only one integron in SCH909. Sequencing of this PCR product revealed the presence of two cassettes, dfrA1 and ant(3")-Ia, in that order, within the integron. However, PCR mapping with primers from the 5′ conserved segment of the integron in conjunction with primers from various resistance gene cassettes showed that there were three different cassettes, dfrA1, ant(2")-Ia (aadB), and aac(3)-Ia (aacC1), each immediately adjacent to an integron 5′ conserved segment (Fig. 1). Therefore, PCR mapping of integrons (14) must not be limited to the use of primers from the 5′ and 3′ conserved sequences. The PCR bias toward smaller products may mask larger integrons in the same strain, or the 3′ conserved segment may be missing (see below).
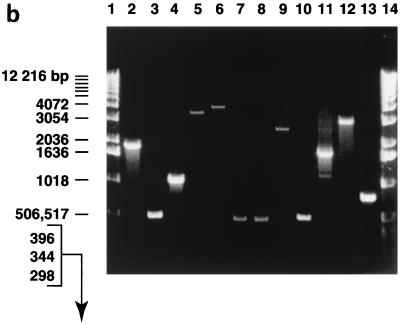
FIG. 1.
Mapping of integrons of S. marcescens SCH909. (a) General structures of integrons 1, 2, and 3 found in S. marcescens SCH909. The arrows show the direction of transcription. (b) PCR amplification from SCH909. The PCR products were separated by electrophoresis through 1.0% agarose. Lanes 1 and 14, 1-kb DNA ladder; lanes 2 to 13, SCH909 with the following primers: lane 2, Sulpro3 and 3′-CS; lane 3, Sulpro3 and dfrA1; lane 4, Sulpro3 and ant(3")-Ia; lane 5, Sulpro3 and ant(3")-Ii; lane 6, Sulpro3 and aac(6′)-Ib; lane 7, Sulpro3 and ant(2")-Ia; lane 8, Sulpro3 and aac(3)-Ia; lane 9, aac(3)-Iacooh and 3′-CS; lane 10, ant(3")-Iacooh and 3′-CS; lane 11, aac(3)-Iacooh and ant(3")-Ia; lane12, ant(2")-Iacooh and aac(6′)-Ib; and lane 13, tnpR and BLATEM. Primer Sulpro3 is a rightward primer in the 5′ conserved segment. Primers with gene names are leftward primers near the beginning of their respective cassettes. Primers with “cooh” are rightward primers near the end of their respective cassettes. Primer 3′-CS is a leftward primer in the 3′ conserved sequence. These primers are used to determine gene order in integrons (14).
The finding of three integrons by PCR analysis was corroborated by hybridization to the whole genome of SCH909 and to the transconjugant digested with HindIII. Copies of the intI1 gene were found in the 15.0-, 13.0-, and 5.0-kb HindIII fragments by use of the Sulpro3 and 5′-CS probes. The 3′ conserved segment of the integrons was found in the 15.0- and 5.0-kb HindIII DNA fragments, suggesting that it may be missing in one integron.
Analysis of the three variable regions of the integrons.
The nucleotide sequences of the 1.5-, 5.2-, and 3.1-kb regions from the variable regions of the three integrons found in SCH909 were determined (see Materials and Methods). Integron 1 contains the dfrA1 and ant(3")-Ia genes, and its variable region is identical to that of pLMO229 (32).
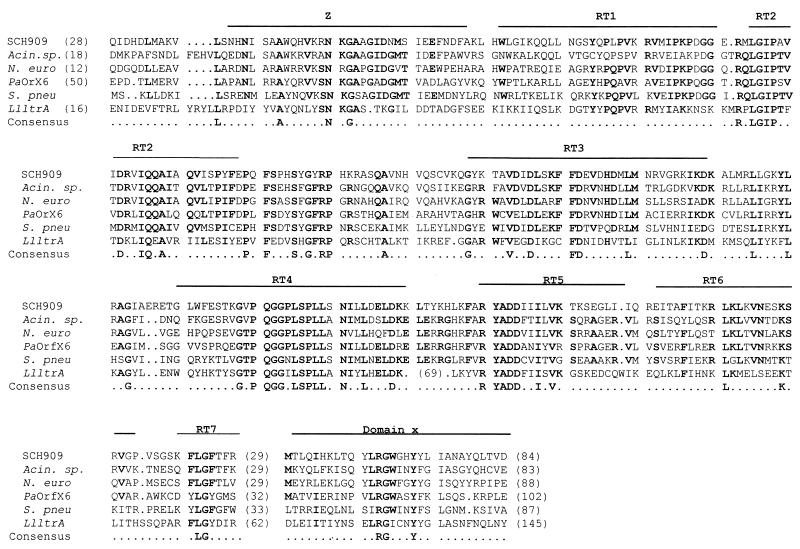
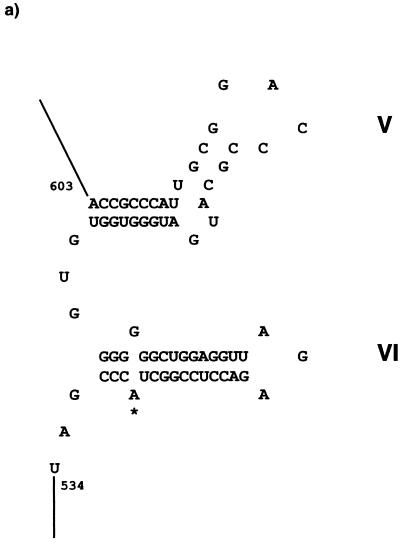
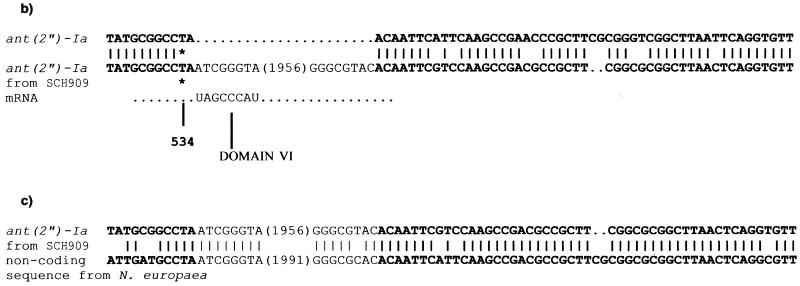
Integron 2 (GenBank accession no. AF453998) contains an ant(2")-Ia gentamicin resistance gene which is separated from its attC site by a 1,971-bp insert containing an ORF on the complementary strand (Fig. 1a). While the ant(2")-Ia gene was identical, the ant(2")-Ia attC site possessed only 84.5% identity with that of the ant(2")-Ia gene already described (4). The complementary strand of the insert showed 48.1% identity with a group II intron from Pseudomonas alcaligenes (36). The product of this ORF, called Smtr, from the bottom strand showed 39.2% identity with the maturase of this intron, although the ORF length for the putative maturase and/or RT differed. The putative RT from SCH909 possessed all seven polymerase-like domains characteristic of RT activity (Fig. 2). In addition to the seven RT-like domains and like the maturase from P. alcaligenes (intron xln6), a domain, z, which is characteristic of non-long terminal repeat retroelements (19), was detected at the N-terminal end of the RT from SCH909. Also, domain x was found downstream of the seven RT domains in Smtr. Domain x has been suggested to be involved in binding of the intron RNA during reverse transcription and splicing. A noncoding region immediately downstream of the putative RT could fold into typical V and VI RNA secondary structures (Fig. 3). 3 Domain V is the only component other than domain I that is absolutely essential for group II ribozyme activity (16). A 1,971-bp sequence (from bp 534 to bp 2504 of integron 2) could fold into the typical RNA secondary structure of a central wheel with six spokes that define the six major ribozyme domains of group II introns (data not shown).
FIG. 2.
Protein alignment of the putative maturase, Smtr, in SCH909 with eubacterial group II intron-encoded proteins from an Acinetobacter sp. (Acin.sp.) (J. H. Yum et al., unpublished; GenBank accession number AF369871; ORF II), N. europaea (N. euro) (JGI, unpublished), P. alcaligenes (PaOrX6) (36), Streptococcus pneumoniae (S. pneu) (5), and Lactococcus lactis (LlltrA) (18). Consensus amino acids (four or more identical in the six sequences) are in bold letters. Domains conserved among intron-encoded proteins are denoted by the lines above the alignment. RT1 through RT7, RT-like domains (19); z, domain of undetermined function in non-long terminal repeat retroelements; x, maturase-specific domain (19). Numbers in parentheses indicate numbers of amino acids not shown.
FIG. 3.
General features of Smtr and its insertion site. (a) Secondary structure model of domains V and VI of the group II intron, Smtr, within integron 2 from SCH909. The boundary of the intron and the ant(2")-Ia attC site is indicated by a vertical line. The bulging adenine residue involved in lariat formation is indicated by an asterisk. (b) Nucleotide sequence alignment of the ant(2")-Ia stop codon and its attC site (59-base element) (4) with the ant(2")-Ia gene and Smtr-intron boundary from SCH909). ant(2")-Ia stop codons are indicated by asterisks. Exon sequences are shown in bold letters. (c) Nucleotide sequence alignment of the group II introns of SCH909 and N. europaea. The latter is adjacent to an attC site on one end; there is no recognizable ORF on the other end.
This region is followed by a novel aminoglycoside resistance gene [ant(3")-Ii-aac(6′)-IId] which codes for a fusion protein involved in streptomycin, spectinomycin, and gentamicin resistance [ANT(3")-I-AAC(6′)-II profile; see below]. This gene is followed by an ORF (ORF O) whose product has weak similarity to qacE multidrug transporters, with a typical attC site [95% identity to aac(6′)-IIa 59-base element] and the beginning of a blaOXA-10 β-lactamase cassette interrupted by IS1. At the other end of IS1, the sequence does not return to that of blaOXA-10, indicating possible recombination between two copies of IS1. Also, PCR analysis with internal and specific primers for the blaOXA-10 gene revealed that the whole gene is not present in strain SCH909. Therefore, the 60-kb plasmid from SCH909 has evolved by several insertions and recombinations.
Integron 3 (GenBank accession no. AF453999) contains another aminoglycoside resistance gene cassette, aac(3)-Ia, followed by two identical ORF P cassettes, a different ORF Q cassette, and ant(3")-Ia (Fig. 1). The sequence to the left of the integrase of integron 3 shows that this integron is part of a Tn1696-like transposon (data not shown). ORF P and ORF Q are identical to ORF X(A) and ORF X(B) of Klebsiella oxytoca plasmid pACM1 (24), which has only one copy of the former.
Sequencing of the 5′ conserved segments of integrons 1 and 2 revealed integrase genes identical to that of Tn21 (13), while that of integron 3 is like that of Tn1696. Unlike the situation in Tn1696, the 19-bp duplication which permits the expression of the aac(3)-Ia gene as a translational fusion which begins before the attI site (35) is absent. In SCH909, the aac(3)-Ia gene probably uses an initiation codon internal to the cassette.
Characterization of a new aminoglycoside resistance gene, the ant(3")-Ii-aac(6′)-IId gene cassette.
Plasmid DNA from strain SCH909 was digested with HincII. The fragments were ligated to the HincII site of pTZ19r and introduced by transformation into E. coli NM522. The transformants selected on ampicillin-gentamicin were screened for inserts by agarose gel electrophoresis, and a plasmid containing an insert of 4.2 kb was chosen (pGM172). The fragment from a Sau3AI partial digest was subcloned into the BamHI site of pTZ19r to yield plasmid pGM172-7, which contained a 2.3-kb insert that conferred resistance to gentamicin, streptomycin, and spectinomycin on E. coli NM522. The AGRP of pGM172 and pGM172-7 revealed the combination of ANT(3")-I plus AAC(6′)-II mechanisms (Tables 2 and 3). We found an ORF spanning 1,392 nucleotides from the start codon ATG at position 2563 to the stop codon TAA at positions 3952 to 3954 in the sequence of integron 2 (GenBank accession number AF453998). The first 789 nucleotides of this ORF possessed 99.2 and 82.7% identities, respectively, with the novel ant(3")-If (aadA6) (22) and ant(3")-Ig (aadA7) (15) over the entire length and 76.4 and 77.1% identities, respectively, with ant(3")-Ia and ant(3")-Ib over a 785-bp length. There is no stop codon at the position corresponding to the ant(3")-Ia and ant(3")-Ib stop codons. We called this part of this ORF ant(3")-Ii (aadA9).
TABLE 2.
Disk susceptibility of host strain, clones, and S. marcescens parent straina
| Antibiotic | Zone diam (mm) for:
|
|||
|---|---|---|---|---|
| E. coli NM522-pTZ19 | SCH909 | pGM172 | pST2 | |
| Neomycin | 19 | 8 | 18 | 18 |
| Kanamycin | 24 | 6 | 10 | 26 |
| Tobramycin | 22 | 7 | 10 | 24 |
| 5′-Episisomicin | 22 | 12 | 12 | 22 |
| Gentamicin | 25 | 8 | 12 | 24 |
| Amikacin | 26 | 22 | 22 | 26 |
| Isepamicin | 30 | 26 | 28 | 30 |
| Netilmicin | 26 | 18 | 14 | 26 |
| 2′-N-Ethylnetilmicin | 30 | 12 | 12 | 30 |
| Apramycin | 28 | 28 | 28 | 30 |
| 6′-N-Ethylnetilmicin | 24 | 26 | 30 | 28 |
| Fortimycin A | 30 | 6 | 30 | 30 |
Disk susceptibility data show a typical AAC(6′)-II profile for the clone of the entire fusion protein but not for that of the ant(3")-Ii moiety. The sequence of the ant(3")-Ii gene is 84% similar to those of the known ant(3")-Ia gene from Tn21 and ant(3")-Ib from pSa. aac(6′)-IId is a point mutation of aac(6′)-Ib resulting in the amino acid change L119→S, which changes the specificity from Akr Gms to Aks Gmr.
TABLE 3.
Susceptibility to streptomycin and spectinomycina
| Antibiotic | MIC (μg/ml) for:
|
|||
|---|---|---|---|---|
| E. coli NM522-pTZ19 | SCH909 | pGM172 | pST2 | |
| Streptomycin | 4 | 64 | 64 | 64 |
| Spectinomycin | 8 | >512 | >512 | >512 |
MIC data show a typical ANT (3") profile of resistance to streptomycin and spectinomycin for the clones including ant(3")-Ii.
To test the AGRP of the ant(3")-Ii sequence alone, two oligonucleotide primers were used for subcloning the fragment that possessed significant homology with the ant(3")-I genes (the upper primer was the M13 15-base sequencing primer, 5′ to 3′, CCCAGTCACGACGTT; the lower primer was aadA9L, 5′ to 3′, CGCGGATCCTTAGGCACCAAGCAATTTAGT). Plasmid pGM172 was used as a template for the PCR, which yielded a product of 2,112 bp. A BamHI site was located in the PCR product 945 bp upstream of the ant(3")-Ii initiation codon. A stop codon (TAA), at the location corresponding to the ant(3")-Ia and ant(3")-Ib stop codons, and a second BamHI site were included in the lower primer. The PCR product was digested with BamHI, yielding a 1,731-bp BamHI fragment; this fragment was ligated to the BamHI site of pTZ19r, and the clone was introduced by transformation into E. coli NM522. Transformants selected on streptomycin-ampicillin were screened for inserts by agarose gel electrophoresis. A hybrid plasmid (named pST2) containing the 1,731-bp insert was chosen. The AGRP determination of pST2 corresponded, as expected, to an ANT(3")-I mechanism. The ant(3")-Ii-aac(6′)-IId gene cassette is the second in the variable region of the integron and is expressed from a promoter directed toward it from within the class II intron. We are attempting to locate this promoter by primer extension.
Downstream of the ant(3")-Ii sequence, there is a sequence from positions 3457 to 3954 constituting a gene that was previously called aac(6′)-Ib′ but that we renamed (because of the AGRP) aac(6′)-IId, since the AGRP of the protein is that of an AAC(6′)-II, i.e., amikacin sensitive and gentamicin resistant. The ant(3")-Ii-aac(6′)-IId gene confers resistance to streptomycin, spectinomycin, and gentamicin, while the cloned ant(3")-Ii sequence confers resistance only to streptomycin and spectinomycin. Nucleotide sequence determination indicated total identity between the aac(6′)-IId sequence from SCH909 and the aac(6′)-Ib′ sequence from Pseudomonas aeruginosa BM2687 (12), which confers resistance to gentamicin but not to amikacin. Like other aac(6′)-Ib′ genes, the aac(6′)-IId sequence has a T-to-C transition that results in a leucine-to-serine substitution at position 90. This point mutation is responsible for the altered substrate specificity (25).
The ant(3")-Ii-aac(6′)-IId gene cassette extends from positions 2563 to 3954. At the 3′ end of the ant(3")-Ii-aac(6′)-IId gene, there is a typical attC site that has a 74-nucleotide sequence (positions 3949 to 4022) with an approximate 20-base similarity at each end that is related to the attC site (59-base element) consensus sequence (31). This 74-bp sequence showed 91.9% identity with the aac(6′)-Ib′ attC site from P. aeruginosa BM2687 (12) and 89.2% identity with the attC site of aac(6′)-Ib from Tn1331 (33).
DISCUSSION
SCH909 has three class I integrons on a single plasmid, two containing integrase genes identical to that of pVS1 (2) and a third identical to that of Tn1696 (35). There are multiple mechanisms for resistance to some antibiotics, e.g., gentamicin [aac(3)-Ia, ant(2")-Ia, and ant(3")Ii-aac(6′)-Iid] and streptomycin [ant(3")Ii-aac(6′)-IId and two copies of ant(3")-Ia]. Integron 2 contains two unusual features: a fused aminoglycoside resistance gene, ant(3")-Ii-aac(6′)-IId, and a group II intron located precisely at the junction of the ant(2")-Ia gene and its attC site.
At least two hypotheses can be proposed for the origin of the fused protein: the bifunctional protein could have been created by cassette fusion, or it could have been encoded by an ancestral aminoglycoside resistance gene from which several others evolved. The recent finding of the ant(3")-If (aadA6) gene (22) favors the former possibility, since translation of this gene continues well into its attC site. Indeed, the part of the ant(3")-Ii structural gene before the (missing) attC site differs from ant(3")-If by only six nucleotides. The close relationship of ant(3")-Ia and ant(3")-Ib suggested that they have evolved either from one another or from a common ancestral gene after the formation of a cassette, since their attC sites are also very similar. However, the attC site of ant(3")-If is also very similar to those of ant(3")-Ia and ant(3")-Ib, but the ca. 23% difference in the structural genes points to separate events of cassette formation rather than evolution after acquisition of an attC site by an ancestral gene. The sequence downstream of ant(3")-Ii corresponds to the aac(6′)-IId gene, which has total identity with the aac(6′)-Ib′ gene from P. aeruginosa BM2687 (12). However, their attC sites are not identical (91.9% identity), again raising the possibility of independent events of cassette formation. The spacer sequence between the presumed mutation removing the ant(3")-Ii stop codon (positions 3352 to 3354) and a possible internal aac(6′)-IId start codon (position 3457) includes the sequence CTAAAACAAA, identical to 10 bases at the end of the attI site from the 5′ conserved segment of the integrons.
The fused cassette could have been created by an illegitimate integrase-mediated intermolecular recombination event involving the inverted core site of the attC site of ant(3")-Ii (CTAA′CAATT) and the end of an attI site (CTAA′AACAAAGTT) preceding aac(6′)-IId. An identical event might have been responsible for the creation of the fused ant(3")-Ia-blaOXA-9 cassette in Tn1331 (33). The crossing-over point in attI would correspond to a seven-nucleotide stagger with G′TT and cutting 3′ of two A residues, as seen with XerC-XerD recombination and as proposed for the integron integrase. A similar intramolecular excision event involving attI and the inverted core site of an attC site has been observed (F. Gagnon and P. H. Roy, unpublished results). The gene aac(6′)-IId is probably the ancestral gentamicin resistance gene from which the amikacin resistance gene aac(6′)-Ib emerged by the mutation resulting in Ser119Leu. aac(6′)-Ib often uses protein fusions for optimal expression: fusion with TEM-1 in Tn1331 (33), fusion with the catB4Δ product in Serratia sp. strain 45 (34), and fusion with the 5′ conserved sequence in pCFF04, which can use a 19-bp repeat in a manner identical to that used for the expression of aac(3)-Ia in Tn1696 (35).
An alternate explanation for the origin of the bifunctional protein is that ant(3")-Ii-aac(6′)-IId was an ancestral aminoglycoside gene from which aac(6′)-Ib, aac(6′)-Ib′, aac(6′)-IIa, aac(6′)-IIb, ant(3")-Ia, and ant(3")-Ib evolved by deletion and cassette formation due to selective pressure. There is another case of a bifunctional enzyme described in the aminoglycoside resistance protein family, that encoded by aac(6′)-Ie-aph(2") and confined to gram-positive species.
In integron 2, the ant(2")-Ia gene is separated from its attC site by a group II intron that we call Smtr. At least two possible routes that would lead to a group II intron within a cassette can be envisaged. The simplest explanation is a direct insertion of Smtr, mediated by one of the two retrotransposition pathways, retrohoming or ectopic insertion (8, 16, 18, 19), at the inverted core site at the junction of ant(2")-Ia and its attC site. The SCH909 group II intron Smtr begins with GTACG and ends with GAT, in accord with consensus sequences for the ends of group II introns. The target DNA needed for reverse splicing to occur in Ll.LtrB, the only bacterial group II intron that has been shown to be functional for splicing and mobility in vivo, is rather specific and contains the target site TG′GTTA (20). In contrast, although the complete recognition site of group II intron xln6 from P. alcaligenes has not been determined, it contains TTGT′TA (36). The SCH909 group II intron Smtr forms a subgroup with xln6 and also with a group II intron found in 14 copies in genomic DNA of Pseudomonas putida, with a site specificity of TTTTTGT′T. The complement of the consensus inverted core site of the attC site (59-base element) is AATTGT′TAGGC, where the prime corresponds to the point of intron insertion. Notably, in the integron context, the group II intron is “upside down,” so that the attC site is exon 1 while the ant(2")-Ia structural gene is exon 2. It is unknown what effect transcription arriving from exon 2, in contrast to the situation in Ll.LtrB, where external transcription occurs from exon 1, has on intron functions.
Taking into account the special features of mobility of group II introns, splicing (to remove the intron), retrohoming (in which RNA invades double-stranded DNA), and ectopic insertion (in which RNA invades RNA) (5a, 8, 16, 18, 19), an alternative explanation for the presence of Smtr could involve a role in one or more steps in the formation of cassettes from preexisting structural genes and attC recombination sites. It was proposed that cassettes could be formed by reverse transcription (10) mediated by RT activity (27), although no role for introns was mentioned by these authors. A possible mechanism for cassette formation would involve two independent transpositional events placing one intron immediately downstream of a structural gene (such as a resistance gene) and another adjacent to an attC site. The next event would involve recombination, possibly recA mediated, between the two introns. The next step would involve splicing out of the intron, followed by final reverse transcription to conserve the newly formed cassette at the DNA level. An interesting example of one of these possible intermediates was found in the partial genome sequence of Nitrosomonas europaea (Joint Genome Institute [JGI], unpublished data), in which one copy of a novel group II intron which we call Netr is precisely inserted at the inverted core site of an attC site, in the same manner as in SCH909 (Fig. 3c). The other end of this element is not associated with the stop codon of a structural gene; thus, it may represent the intron-attC intermediate. A second copy of Netr is adjacent to a putative gene cassette, and there is no attC site at its other end; thus, it may be an example of a gene-intron intermediate. The target specificity of the intron, as mentioned above, may explain the precise juxtaposition in many cassettes in integrons of the structural gene stop codon and the inverted core site of the attC site. In SCH909, the ant(2")-Ia gene is identical to that of pDGO100 (4),whereas the ant(2")-Ia attC site shows only 84.5% identity with ant(2")-Ia attC sites already described (3). The SCH909 sequence may represent an unspliced intermediate in an independent event of ant(2")-Ia cassette formation.
Recently, three integron sequences submitted to GenBank and containing a gene for β-lactamase VIM-2 from Acinetobacter spp. (J. H. Yum et al., unpublished data; accession number AF369871), P. aeruginosa (K. Lee et al., unpublished data; accession number AY029772), and S. marcescens (K. Lee et al., unpublished data; accession number AY030343) indicated the presence of a group II intron (identical among these three but distinct from that of SCH909) between ant(3")-Ia and its attC site (in the first two) or between the qacF gene and an attC site identical to that of ant(3")-Ia rather than that of qacF (in the third). These may be further examples of independent events of cassette formation.
A few scattered attC sites (59-base elements), apparently not associated with structural genes and not making up parts of integrons, are found in some partially sequenced genomes, such as those of Shewanella putrefaciens (The Institute for Genomic Research, unpublished data) and N. europaea (JGI, unpublished). These genomes also contain functional integron integrases (70; G. Léon and P. H. Roy, unpublished data), and closely related integrases have been found in other strains of these species (28). These environmental organisms may serve as reservoirs of integron components.
Group II introns have been described, until now, as being inserted close to mobile elements in eubacteria (11, 21). Moreover, as mobile elements by themselves and located, in the case of SCH909, within antimicrobial resistance gene cassettes, they contribute to DNA rearrangements leading to R plasmid evolution. The elucidation of the mechanism of the formation of integron cassettes remains a key to the understanding of their relationship with the group II introns and their role in aminoglycoside resistance gene evolution and in the accumulation of resistance genes by integrons. We are attempting to determine whether the intron RNA can be spliced to yield a template in which cassette formation could be completed by reverse transcription. If splicing occurs, intron mobility will be tested to determine whether it can be targeted to an attC site within a cassette, e.g., ant(2")-Ia (retrohoming), to an attC site not associated with a cassette, and to the region of the stop codon of a resistance gene. In N. europaea, the two copies of a group II intron are in the latter two contexts, which may be earlier intermediates in cassette formation. Recombination between them would produce a putative later intermediate like that in SCH909, and this process is also being attempted.
Acknowledgments
We thank the JGI for communication of the partial genome sequence of N. europaea and The Institute for Genomic Research for P. putida and S. putrefaciens sequence information prior to publication.
This work was supported by grant MT-13564 from the Medical Research Council of Canada to P.H.R. D.C. was a CONICET postdoctoral fellow from Argentina.
REFERENCES
- 1.Bauernfeind, A., I. Stemplinger, R. Jungwirth, P. Mangold, S. Amann, E. Akalin, O. Ang, C. Bal, and J. M. Casellas. 1996. Characterization of beta-lactamase gene blaPER-2, which encodes an extended-spectrum class A beta-lactamase. Antimicrob. Agents Chemother. 40:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissonnette, L., and P. H. Roy. 1992. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J. Bacteriol. 174:1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunny, K. L., R. M. Hall, and H. W. Stokes. 1995. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob. Agents Chemother. 39:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, F. H., D. J. Groot Obbink, V. P. Ackerman, and R. M. Hall. 1986. Nucleotide sequence of the AAD(2") aminoglycoside adenylyltransferase determinant aadB. Evolutionary relationship of this region with those surrounding aadA in R538-1 and dhfrII in R388. Nucleic Acids Res. 14:8625-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 5a.Cousineau, B., S. Lawrence, D. Smith, and M. Belfort. 2000. Retrotransposition of a bacterial group II intron. Nature 404:1018-1021. [DOI] [PubMed] [Google Scholar]
- 6.Davies, J. 1994. Inactivation of antibiotics and dissemination of resistance genes. Science 264:375-381. [DOI] [PubMed] [Google Scholar]
- 7.Davies, J. 1996. Origins and evolution of antibiotic resistance. Microbiologia 12:9-16. [PubMed] [Google Scholar]
- 7a.Drouin, F., and P. H. Roy. 2002. The IntI-like tyrosine recombinase of Shewanella oneidensis is functional as an integron integrase. J. Bacteriol. 184:1811-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunny, G. M., and L. L. Mc Kay. 1999. Group II introns and expression of conjugative transfer functions in lactic acid bacteria. Antonie Leeuwenhoek 76:77-88. [PubMed] [Google Scholar]
- 9.Francia, M. V., P. Avila, F. de la Cruz, and J. M. García Lobo. 1997. A hot spot in plasmid F for site-specific recombination mediated by Tn21 integron integrase. J. Bacteriol. 179:4419-4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall, R. M., D. E. Brookes, and H. W. Stokes. 1991. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol. Microbiol. 5:1941-1959. [DOI] [PubMed] [Google Scholar]
- 11.Knoop, V., and A. Brennicke. 1994. Evidence for a group II intron in Escherichia coli inserted into a highly conserved reading frame associated with mobile DNA sequences. Nucleic Acids Res. 22:1167-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert, T., M. C. Ploy, and P. Courvalin. 1994. A spontaneous point mutation in the aac(6′)-Ib′ gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas aeruginosa strain. FEMS Microbiol. Lett. 115:297-304. [DOI] [PubMed] [Google Scholar]
- 13.Lévesque, C., S. Brassard, J. Lapointe, and P. H. Roy. 1994. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene 142:49-54. [DOI] [PubMed] [Google Scholar]
- 14.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1998. A distinctive class of integron in the Vibrio cholerae genome. Science 280:605-608. [DOI] [PubMed] [Google Scholar]
- 16.Michel, F., and J. L. Ferat. 1995. Structure and activities of group II introns. Annu. Rev. Biochem. 64:435-461. [DOI] [PubMed] [Google Scholar]
- 17.Miller, G. H., F. J. Sabatelli, R. S. Hare, and J. A. Waitz. 1980. Survey of aminoglycoside resistance patterns. Dev. Ind. Microbiol. 21:91-104. [Google Scholar]
- 18.Mills, D. A., L. L. Larry, and G. M. Dunny. 1996. Splicing of a group II intron involved in the conjugative transfer of pRS01 in lactococci. J. Bacteriol. 178:3531-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohr, G., S. Perlman, and A. M. Lambowitz. 1993. Evolutionary relationships among group II intron-encoded proteins and identification of a conserved domain that may be related to maturase function. Nucleic Acids Res. 21:4991-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohr, G., D. Smith, M. Belfort, A. M. Lambowitz. 2000. Rules for DNA target-site recognition by a lactococcal group II intron enable retargeting of the intron to specific DNA sequences. Genes Dev. 1:559-573. [PMC free article] [PubMed] [Google Scholar]
- 21.Mullany, P., M. Pallen, M. Wilks, J. R. Stephen, and S. Tabaqchali. 1996. A group II intron in a conjugative transposon from the Gram-positive bacterium, Clostridium difficile. Gene 174:145-150. [DOI] [PubMed] [Google Scholar]
- 22.Naas, T., L. Poirel, and P. Nordmann. 1999. Molecular characterisation of In51, a class 1 integron containing a novel aminoglycoside adenylyltransferase gene cassette, aadA6, from Pseudomonas aeruginosa. Biochim. Biophys. Acta 1489:445-451. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen, I. T., T. G. Littlejohn, P. Rådström, L. Sundström, O. Sköld, G. Swedberg, and R. A. Skurray. 1993. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob. Agents Chemother. 37:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston, K. E., C. C. Radomski, and R. A. Venezia. 1999. The cassettes and 3′ conserved segment of an integron from Klebsiella oxytoca plasmid pACM1. Plasmid 42:104-114. [DOI] [PubMed] [Google Scholar]
- 25.Rather, P. N., H. Munayyer, P. A. Mann, R. S. Hare, G. H. Miller, and K. J. Shaw. 1992. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J. Bacteriol. 174:3196-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 27.Recchia, G. D., and R. M. Hall. 1997. Origins of the mobile gene cassettes found in integrons. Trends Microbiol. 5:389-394. [DOI] [PubMed] [Google Scholar]
- 28.Rowe-Magnus, D. A., A.-M. Guerout, P. Ploncard, B. Dychinco, J. Davies, and D. Mazel. 2001. The evolutionary history of chromosomal super-integrons provides an ancestry for multiresistant integrons. Proc. Natl. Acad. Sci. USA 98:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes, H. W., D.B. O'Gorman, G. D. Recchia, M. Parsekhian, and R. M. Hall. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26:731-745. [DOI] [PubMed] [Google Scholar]
- 32.Sundström, L., and O. Sköld. 1990. The dhfrI trimethoprim resistance gene of Tn7 can be found at specific sites in other genetic surroundings. Antimicrob. Agents Chemother. 34:642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolmasky, M. E., and J. H. Crosa. 1993. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid 29:31-40. [DOI] [PubMed] [Google Scholar]
- 34.Toriya, M., M. Sakakibara, K. Matsushita, and T. Morohoshi. 1992. Nucleotide sequence of aminoglycoside 6′-N-acetyltransferase [AAC(6′)] determinant from Serratia sp. 45. Chem. Pharm. Bull. 40:2473-2477. [DOI] [PubMed] [Google Scholar]
- 35.Wohlleben, W., W. Arnold, L. Bissonnette, A. Pelletier, A. Tanguay, P. H. Roy, et al. 1989. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I (AAC(3)-I), another member of the Tn21-based expression cassette. Mol. Gen. Genet. 217:202-208. [DOI] [PubMed] [Google Scholar]
- 36.Yeo, C. C., J. M. Tham, M. W. C. Yap, and C. L. Poh. 1997. Group II intron from Pseudomonas alcaligenes NCIB 9867 (P25X): entrapment in plasmid RP4 and sequence analysis. Microbiology 143:2833-2840. [DOI] [PubMed] [Google Scholar]