Abstract
Dopamine-modulated behaviors, including information processing and reward, are subject to behavioral plasticity. Disruption of these behaviors is thought to support drug addictions and psychoses. The plasticity of dopamine-mediated behaviors, for example, habituation and sensitization, are not well understood at the molecular level. We show that in the nematode Caenorhabditis elegans, a D1-like dopamine receptor gene (dop-1) modulates the plasticity of mechanosensory behaviors in which dopamine had not been implicated previously. A mutant of dop-1 displayed faster habituation to nonlocalized mechanical stimulation. This phenotype was rescued by the introduction of a wild-type copy of the gene. The dop-1 gene is expressed in mechanosensory neurons, particularly the ALM and PLM neurons. Selective expression of the dop-1 gene in mechanosensory neurons using the mec-7 promoter rescues the mechanosensory deficit in dop-1 mutant animals. The tyrosine hydroxylase-deficient C. elegans mutant (cat-2) also displays these specific behavioral deficits. These observations provide genetic evidence that dopamine signaling modulates behavioral plasticity in C. elegans.
Keywords: C. elegans, dopamine, habituation, mechanosensation, receptor
Introduction
The dopamine system in the vertebrate central nervous system plays a critical role in motor control, reward and cognition. Dysfunction of this system is associated with various disorders and behaviors, including Parkinson's disease, schizophrenia, Tourette's syndrome, attention deficit hyperactivity disorder and addictions. Studies on the role of dopamine in reward and the mechanisms of addiction have revealed that this system is involved in behavioral plasticity. For example, dopamine D1 receptor stimulation is thought to play a central role in behavioral plasticity as seen in psychostimulant intake, including sensitization, tolerance and drug dependence (Nader et al, 1997; Berke and Hyman, 2000). Dysfunction of the molecular mechanisms underlying these forms of plasticity of the dopamine system has also been postulated to contribute to the development of schizophrenia (e.g. Beninger and Miller, 1998; Berke and Hyman, 2000). While much progress has been made in the understanding of dopamine signaling and its role in behavioral plasticity in mammalian organisms, the molecular basis for dopamine-induced plasticity is not well understood. To obtain more insight into this process, we studied the role of dopamine and a specific dopamine receptor, DOP-1, in behavioral plasticity in a simple, genetically tractable organism, the nematode Caenorhabditis elegans.
C. elegans is a well-studied genetic model organism for which cell lineage, neuronal network and genomic sequence have been well described (Sulston et al, 1983; White et al, 1986; The C. elegans Sequencing Consortium, 1998), resulting in unprecedented insight into the nervous system of a single animal species (Bargmann, 1998). In C. elegans, dopamine is a prominent neurotransmitter synthesized in eight sensory neurons: two anterior deirid neurons (ADEs), two posterior deirid neurons (PDEs) and four cephalic neurons (CEPs). In addition, three pairs of sex-specific dopaminergic neurons, namely the A neurons of rays 5, 7 and 9, were identified in the male tail of C. elegans (Sulston et al, 1975; for a review, see Wintle and Van Tol, 2001). Few major behavioral abnormalities have been reported for the tyrosine hydroxylase (TH)-deficient C. elegans mutant cat-2(e1112). However, recently it was shown that cat-2 mutants are deficient in food sensing, in that well-fed worms failed to slow upon entry into a bacterial lawn. The dopamine-mediated slowing of locomotor activity in bacteria appears to be a tactile response to the bacteria. The relationship between dopamine and mechanosensory locomotor responses is not yet elucidated, but it appears to underlie an important adaptive mechanism to maximize foraging strategies (Sawin et al, 2000).
Based on the observation made in TH-deficient mice that dopamine synthesis might be rescued through alternative routes of synthesis involving tyrosinases (Rios et al, 1999), we speculate that cat-2 mutants may not be completely dopamine-deficient. Indeed, we find that cat-2 mutants contained significant amounts of dopamine (this manuscript), and thus we argue that some dopamine-modulated behaviors may not be readily revealed in such mutants. We therefore pursued genetic analysis of dopamine-mediated behavioral plasticity in C. elegans by elucidating post-synaptic molecular components of the dopamine system. To date, little is known about the receptors through which dopamine mediates these physiological effects.
Here we describe the functional characterization of a dopaminergic receptor gene (dop-1) of C. elegans, previously identified by Suo et al (2002). The dop-1 gene resembles the mammalian D1-like receptor family. Loss of the dop-1 gene results in altered mechanosensory (Rose and Rankin, 2001) habituation. This behavior is considered to be a simple form of learning (Rose and Rankin, 2001) and is also affected in the TH-defective cat-2 (e1112) mutant strain. These findings further support a general role of dopamine in behavioral plasticity and an involvement of the D1 receptor subtype in some of these behaviors (e.g. Sawin et al, 2000; Rothenfluh and Heberlein, 2002; McNamara et al, 2003). This work also provides a genetic and molecular link between dopamine signaling and the mechanosensory neural circuits in C. elegans, serving as a simple genetic system for further elucidating the molecular mechanisms of dopamine receptor function with potential relevance to behaviors seen in vertebrates.
Results
Dopamine in C. elegans
While previous work showed that dopamine serves as a neurotransmitter in C. elegans (Sulston et al, 1975; for a review, see Wintle and Van Tol, 2001), levels of various amines and their metabolites are unknown. By using high-performance liquid chromatography (HPLC) analysis coupled to electrochemical detection, we analyzed the content of amines in wild-type (N2), cat-1(ok411), cat-2(e1112) and cat-4(e1141) strains. Thus, we detected serotonin (0.010±0.002 ng/wet weight (ww), n=8), dopamine (0.012±0.002 ng/ww, n=8), as well as several of their metabolites, including 5-hydroxyindole acetic acid (5-HIAA; 0.008±0.002 ng/ww) and homovanillic acid (HVA; 0.016±0.04 ng/ww) in crude C. elegans homogenates. We could not detect (nor)epinephrine or their metabolites, as reported previously by Horvitz et al (1982). The lack of (nor)epinephrine in C. elegans is congruent with the apparent absence of the biosynthetic enzymes and candidate receptors for these compounds in the genome. Mutant strains deficient for the vesicular monoamine transporter (cat-1), TH (cat-2) and GTP-cyclohydroxylase (cat-4) have reduced levels of dopamine (cat-1: 0.0059±0.0007 ng/ww, n=7; cat-2: 0.0070±0.0016 ng/ww, n=7; cat-4: 0.0073±0.0017 ng/ww, n=6). This indicates that these mutant animals can synthesize about 40% of wild-type levels of dopamine by means not involving TH, as seen for TH-deficient mutant mice (Rios et al, 1999).
dop-1 is a C. elegans D1-like dopamine receptor
To identify putative dopamine receptor genes in C. elegans, we performed an in silico analysis of the C. elegans genome database using known mammalian dopamine receptor sequences. We isolated the cDNAs for 13 of these receptors using RT–PCR. In a functional screen based on dopamine receptor-induced GDP–GTP exchange, we identified two cDNAs, dop-1s and dop-1l, which responded positively to dopamine. These two different cDNAs, both derived from the F15A8.5 genomic locus of C. elegans, were generated through alternative splicing of exon 5 (Supplementary data).
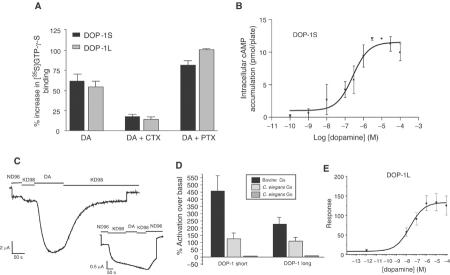
Both dop-1 isoforms mediated dopamine-stimulated [35S]-GTP-γ-S binding in COS-7 cells in a concentration-dependent manner (EC50 ∼0.8 μM) (Figure 1). Among the other amines tested, only (nor)epinephrine could measurably stimulate the receptor, albeit at a 10-fold lower potency than dopamine (Figure 1). The five different mammalian dopamine receptors are classified into two groups, the D1- and D2-receptor families, that couple either through the stimulatory G protein Gαs or the inhibitory family Gαi/o (Missale et al, 1998). Both splice forms of DOP-1 showed cholera toxin sensitivity in dopamine-stimulated [35S]GTP-γ-S binding assays and stimulated cAMP production (DOP-1L EC50: 20±10 nM; DOP-1S EC50: 28±13 nM) (Figure 2). Furthermore, coexpression of DOP-1 and the G protein-activated inwardly rectifying potassium channel Kir3.2 with bovine or C. elegans Gαs subunits in Xenopus oocytes facilitated robust channel activation by DOP-1 (DOP-1S EC50: 11.3±1.0 nM; DOP-1L: 23.0±1.0 nM), while coexpression of the receptor and channel with the C. elegans Gαo ortholog goa-1 did not.
Figure 1.

Catecholamines stimulate DOP-1. (A) Stimulation of DOP-1L by various biogenic amines as measured by [35S]GTP-γ-S binding in COS-7 cells. Each bar represents the normalized mean±s.e.m. of triplicate values after subtracting mean baseline levels. (B, C) Dopamine-, epinephrine- and norepinephrine-mediated [35S]GTP-γ-S binding in COS-7 cells expressing DOP-1L (B) and DOP-1S (C). Data points represent normalized mean±s.e.m. of triplicate values.
Figure 2.
DOP-1 functionally couples to Gαs in heterologous expression systems. Top panels: (A) Dopamine-mediated stimulation of [35S]GTP-γ-S binding in DOP-1-expressing COS-7 cells was inhibited by preincubating cells with 0.1 μg/ml CTX but not by 0.1 μg/ml PTX for 24 h post-transfection. Each bar represents the mean±s.e.m. (n=3). (B) Dopamine increases intracellular cAMP levels in a concentration-dependent manner in CHO-K1 cells expressing dop-1l or dop-1s. The graph represents a typical observation for DOP-1S. Each data point represents the mean±s.e.m. of triplicate measurements. Similar curves are obtained for DOP-1L (not shown). (C) Stimulation of Kir3.2 by DOP-1 in Xenopus oocytes requires Gsα expression. Shown are the current traces of oocytes expressing dop-1l, Kir3.2 (human) and Gsα (bovine) or goa-1 (inset). DA is 1 μM dopamine in KD98. The recording solution is KD98 (98 mM KCl, 1 mM MgCl2, 5 mM K-HEPES, pH 7.5). (D) Summary of the experiments for the expression of the channel with the receptor and the different G protein subunits (Gsα (bovine), gsa-1 and goa-1 (C. elegans)). The data are expressed as percentage activation over basal (mean±s.e.m.; n⩾10). (E) Dose–response curve for DOP-1L-mediated activation of Kir3.2 by dopamine. Each data point is the mean±s.e.m. (n⩾5). Similar curves are obtained for DOP-1S (not shown).
We performed a pharmacological analysis of the receptor, including an assessment of whether the DOP-1 receptor displays ligand-independent activity (Lefkowitz et al, 1993) (Figure 3 and Supplementary data). Unlike the mammalian D1-like receptors, DOP-1 could not bind to the specific mammalian D1 receptor antagonist [3H]SCH23390. However, at high concentrations (1 μM), this ligand acts as an agonist on DOP-1. SKF38390, another benzazepine with potent agonist effects on mammalian D1 receptors, also acts like an agonist for DOP-1 (Figure 3), although its potency is three orders of magnitude lower than dopamine on DOP-1. In this study, the mammalian D2 antagonists, (+)-butaclamol and haloperidol, the mixed D1/D2 antagonist cis-flupenthixol and the neurotransmitter octopamine displayed clear inverse agonist effects at DOP-1 by reducing basal levels of intracellular cAMP (Figure 3). The presence of cis-flupenthixol (10 μM) in dopamine dose–response experiments shifted the EC50 to a lower potency, while maximum dopamine-mediated stimulation was enhanced (Figure 3). These data are consistent with an interpretation that the DOP-1 receptors have ligand-independent activity. It is unknown whether DOP-1 displays similar functional characteristics in its native environment or whether this receptor can couple effectively to other G proteins.
Figure 3.
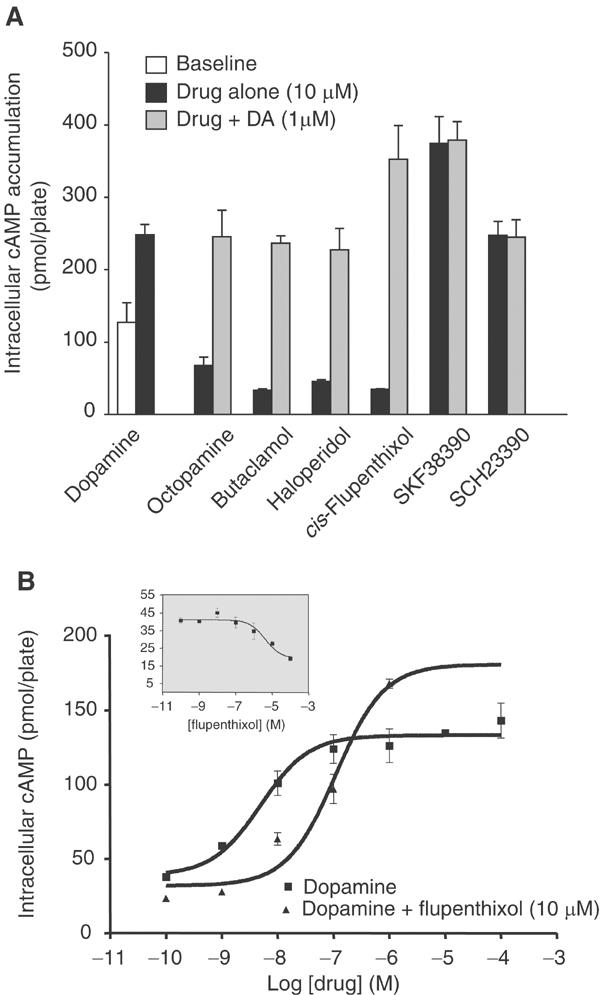
Pharmacological profile of DOP-1. (A) Effect of various mammalian dopamine receptor agonists and antagonists on DOP-1L-stimulated cAMP accumulation was evaluated alone or in the presence of 1 μM dopamine. (B) Dose–response curves for DOP-1L-mediated cAMP accumulation by dopamine in the presence or absence of cis-flupenthixol are shown in the lower panel. The inset in the lower panel shows the dose–response curve for cis-flupenthixol on DOP-1L. Each bar or data point on the curves represents mean±s.e.m. for triplicate measurements.
Molecular genetics of dop-1 in C. elegans
To date, only a role for dopamine in food sensing has been revealed through analysis of cat-2 mutants. Considering that cat-2 mutants are not completely dopamine-deficient and that dopamine receptors can display ligand-independent activity, dopamine may be involved in behaviors previously not identified. We addressed the question of dop-1 function by determining where the receptor is expressed, and by identifying and characterizing mutants of the dop-1 gene.
To determine the expression pattern of dop-1, we constructed a dop-1∷gfp fusion and generated transgenic lines that expressed the reporter from an extrachromosomal array. GFP fluorescence was detected in the transgenic animals consistently in the PLM and PHC mechanosensory neurons, and less frequently with lower intensity in the ALM mechanosensory neurons (Figure 4). In addition, we identified fluorescent signals in several neurons just behind the nerve ring (Figure 4). The relatively low intensity of fluorescence for dop-1∷gfp- expressing head neurons reduced the possibility for accurate assignment, although tentative assignments for AUA, RIB and RIM interneurons could be made. While the dop-1∷gfp construct included 1.7 kb of promoter sequence plus the large first intron of the gene, the expression pattern of the gene may be incomplete considering that the next predicted gene is about 15 kb upstream from dop-1. However, expression of dop-1∷gfp in the ALM, PHC and PLM mechanosensory neurons suggests a role of the DOP-1 receptor in mechanosensation (Rose and Rankin, 2001).
Figure 4.
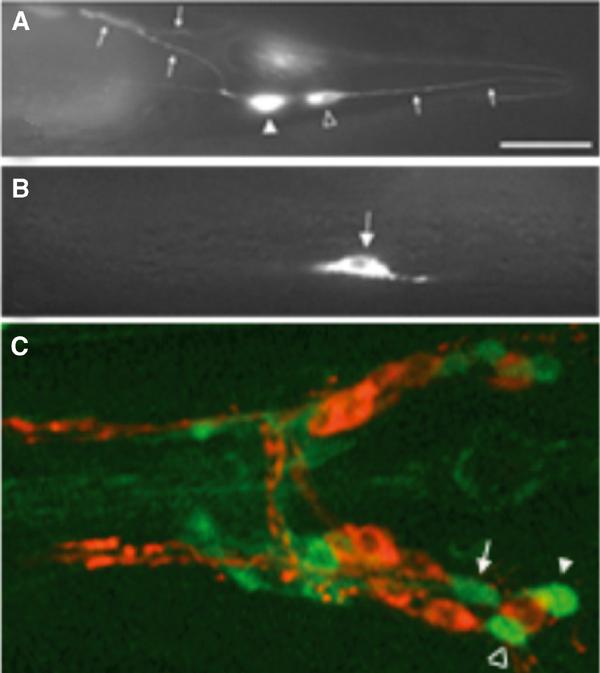
Expression of dop-1∷gfp in wild-type animals. The dop-1∷gfp construct and corresponding transgenic animals were made as described in Materials and methods. The tip of the head is to the left in all panels. (A) The PLM neuron (open arrowhead) has a process that extends into the tip of the tail (arrows to the right of the cell body). The PHC neuron (closed arrowhead) has axons (small arrows to the left) that extend into the ventral nerve cord. (B) ALM neuron expressing dop-1∷gfp. (C) The head of a dop-1∷gfp animal labeled with Di-I to stain certain amphid chemosensory sensory neurons. The most posterior of these neurons is ASI, which is just anterior to ASH. Three of the dop-1∷gfp-expressing neuron cell bodies in the head are found between ASI and ASH and are tentatively identified as RIM (arrow), AUA (open arrowhead) and RIB (closed arrowhead).
We identified a mutant line that is deleted for 2.4 kb of the dop-1 coding region. This deletion eliminates sequences from the 5′ promoter region through the translation initiation codon up to the end of the predicted fourth transmembrane α-helix of the receptor protein (nucleotide −533 to nucleotide +1872, as measured from the position of the initiation codon; see Supplementary data). Thus, the dop-1(ev748) deletion mutation is likely to render the gene nonfunctional.
Effect of the dop-1 mutation on mechanosensory responses
Since dop-1∷gfp is expressed in the mechanoreceptor neurons ALM and PLM, we reasoned that dop-1 might affect mechanosensory responses mediated by these neurons. However, dop-1(ev748) mutant animals appeared to be normal for light touch sensitivity (Chalfie et al, 1985). A related behavior shown previously to require these cells is the response to a nonlocalized mechanical stimulus, or taps, administered to the culture plate. Adult animals respond to tap by switching from forward to backward movement (‘reversal') mediated largely by the ALM neurons or, less frequently, by moving forward at a higher speed (‘acceleration') mediated largely by the PLM neurons. Repeated stimulation of wild-type animals results in a decrease in the frequency of tap-induced reversals as well as a reduction in the distance traveled during a reversal, a process known as tap habituation (Rose and Rankin, 2001).
We subjected dop-1 mutant and wild-type animals to a series of 30 taps administered 10 s apart, and measured the frequency of reversals detected by our system immediately following the stimulus. We observed that dop-1 and wild-type animals both initially exhibited high rates of reversals in response to tap stimuli. Wild-type animals showed a significant decrease in reversal rate after repeated stimulation. This decline in reversal rate correlated with a decrease in reversal distance, an increase in accelerations and nonresponses, and showed significantly slower kinetics when the interstimulus interval was increased (see Supplementary data). Thus, our stimulus protocol could reliably measure tap habituation. We observed that the dop-1(ev748) mutant showed a much more rapid decline in reversal rate in response to habituation training than the wild-type strain (Figure 5A), although reversal distance was less affected suggesting that reversal rate and distance are distinct measurable responses to tap stimulation (see Supplementary data). These alterations in reversal rate in tap habituation were rescued by a wild-type dop-1 transgene expressed under its own promoter, indicating that the phenotype was indeed a consequence of the dop-1 loss-of-function mutation (χ2 plus Bonferroni, P<0.0005; Figure 5B). Moreover, cat-2(e1112) mutant animals, which contain reduced levels of dopamine, also exhibited more rapid habituation to tap than wild type (χ2 plus Bonferroni, P≪0.0001; Figure 5A). The cat-2 tap habituation phenotype could be rescued by acute administration of exogenous dopamine (χ2 plus Bonferroni, P<0.0005; Figure 5C). Thus, these experiments suggest that dopamine modulates tap habituation in C. elegans, and that the DOP-1 receptor is required for this modulation. dop-1 is expressed in both the ALM and PLM neurons; thus, we reasoned that dopamine might affect the tap response by modulating the functional properties of the mechanoreceptor neurons themselves. However, dop-1 is also expressed in other neurons, including PHC, a neuron with significant synaptic input to the interneurons in the touch avoidance circuit (White et al, 1986) that might also affect tap habituation. To address whether dop-1 functioned cell autonomously in the touch neurons to modulate tap responses, we constructed transgenic lines in which dop-1 was rescued by a wild-type dop-1(+) coding region expressed under the control of the touch neuron-specific mec-7 promoter evEx167[dop-1(ev748)] (Figure 5D). We observed that these lines did not exhibit the dop-1 precocious habituation phenotype; indeed they habituated at least as slowly as wild-type animals (χ2 plus Bonferroni, P<0.0005). Thus, expression of functional DOP-1 protein in the mechanosensory neurons was sufficient to rescue the dop-1 mutant phenotype. This result indicates that dopamine influences tap habituation by directly modulating the properties of the touch receptor neurons.
Figure 5.

Tap response and habituation in dop-1 mutants. For all the indicated genotypes, 40 hermaphrodites were subjected to tap stimulation with a 10 s interstimulus interval (ISI), and their behavioral responses were scored using an automated tracking system. (A) Reversal rate of wild-type, cat-2, dop-1 mutant strains. (B, D) Reversal rate for dop-1 and dop-1+Ex[Pdop-1∷dop-1] (B), and dop-1;him-5 and dop-1;him-5+Ex[Pmec-7∷dop-1] rescue (D). (C) Reversal rate for cat-2 and cat-2 rescued with exogenous dopamine. The graphs indicate the rate (out of the total number of trials) with which the indicated strain reversed in response to a tap stimulus. Error bars represent s.e.m. A χ2 test was performed on the fraction of reversals in wild type versus mutant or mutant versus rescue over the interval tap stimulus 2 to tap stimulus 13. With a Bonferroni correction for multiple comparisons, we found P-values ≪0.0001 for cat-2(e1112) versus wild type (N2), P<0.0005 for dop-1(ev748);him-5(e1490) versus dop-1(ev748);him-5(e1490)+Ex[Pmec-7∷dop-1] rescue, P<0.005 for dop-1(ev748) versus dop-1(ev748) +Ex[Pdop-1∷dop-1 and P≪0.0001 for cat-2(e1112) versus cat-2(e1112)+dopamine. (E) Diagram of the tap avoidance circuitry. Hypothesized humoral signaling between dopamine neurons and dop-1-expressing mechanosensory neurons is indicated.
dop-1 is not required for food sensing and pharmacologically induced dopamine behaviors
As indicated previously, in C. elegans the cat-2 gene modulates locomotor rate in response to its food, bacteria. Well-fed wild-type animals move more slowly in the presence of bacteria than in the absence of bacteria. The basal slowing response in C. elegans is mediated through a dopamine-containing neural circuit that senses a mechanical attribute of bacteria and is defective in cat-2(e1112) (Sawin et al, 2000). Well-fed dop-1(ev748) mutants did not show any significant difference compared to controls in dopamine-mediated slowing response upon encountering a bacterial lawn. In the food-sensing assay, a significant decrease in locomotor rate was observed for both the mutant and control worms when they were placed in a bacterial lawn as compared to those placed on plates containing no bacteria (Figure 6A).
Figure 6.

Behavioral analysis of dopamine-modulated behaviors in dop-1 C. elegans mutants. (A) Analysis of the slowing response, as measured by the number of body bends per min (Y-axis), of control animals (him-5; white bars) and dop-1 mutants (black bars) before (−bacterial lawn) and after entry (+bacterial lawn) into the bacterial lawn. (B) Block of serotonin-induced egg laying (2.5 mM) by dopamine (1 mM) in him-5 control animals (white bars) versus dop-1 mutants (black bars). The amount of eggs laid per animal after 20 min is plotted on the Y-axis. (C) Dopamine-induced immobilization of the control strains N2 (white bars) and him-5 (gray bars) compared to the dop-1 deletion strain (black bars). The concentrations of dopamine used in the analyses are indicated on the X-axis and the percentage of immobile animals on the Y-axis. Because the dop-1 mutant animals are in a him-5 genetic background, we used him-5 animals as control (see Materials and methods). The bars in the different experiments represent the mean±s.e.m.
While no other genetic data exist for dopamine-mediated behaviors, pharmacological data using exogenously supplied dopamine and/or dopaminergic agonists and antagonists have implicated the C. elegans dopamine system in egg-laying behavior and motility (Schafer and Kenyon, 1995; Weinshenker et al, 1995, 1999; for a review, see Wintle and Van Tol, 2001). Exogenously supplied serotonin stimulates egg laying in C. elegans whereas exogenously supplied dopamine inhibits both basal and serotonin-induced egg laying. We assessed whether dop-1 mutants display changed dopamine-mediated reduction of serotonin-induced egg laying. In our study, dopamine (1 mM) inhibited both basal and serotonin-induced (2.5 mM) egg laying in dop-1 mutants and in wild-type or him-5 controls. This indicates that DOP-1 receptors are not required for dopamine to exert its inhibitory effect on egg laying (Figure 6B).
Exogenous dopamine also inhibits motility in C. elegans (Schafer and Kenyon, 1995). Using our experimental conditions, 20 mM dopamine is required for complete immobilization of worm populations when preincubated on NGM plates containing dopamine. The dop-1 mutant animals were completely immobilized when preconditioned for 1 h on dopamine-containing plates, as were the different controls. Detailed analysis demonstrated that there is no difference in the sensitivity in dopamine-induced immobility as determined by dose–response analysis (Figure 6C). Thus, dop-1 does not appear to be required for dopamine-induced paralysis.
Discussion
Dopamine in C. elegans
C. elegans is a unique genetic model system whose cell lineage, neuroanatomy and genomic sequence have been well described. Despite the relative simplicity of this system, it has the ability to display behavioral plasticity, which may underlie learning processes. Two well-documented examples in this regard in C. elegans are olfactory adaptation and habituation to a nonlocalized mechanical stimulus (Colbert and Bargmann, 1995; Rose and Rankin, 2001). In mammals the dopamine system is thought to play an important role in behavioral plasticity, particularly with regard to reward and cognition (Nader et al, 1997; Beninger and Miller, 1998; Berke and Hyman, 2000). The critical importance of dopamine in mammals is illustrated by the severe motor disabilities, aphagia and adipsia observed in dopamine-deficient mice, resulting in the death of newborn mice around the weaning period (Zhou and Palmiter, 1995; Szczypka et al, 2000). In contrast, C. elegans cat-2 mutants, which lack TH activity, are viable, which allows the use of this model system for studying dopamine-related behaviors. However the TH pathway is not the only potential source for dopamine synthesis. It has been shown that tyrosinase activity can substantially contribute to dopamine synthesis (Rios et al, 1999). Indeed, biochemical analysis showed that cat-2 mutants have only a 60–70% reduction in dopamine compared to wild-type animals. As seen in mice, it is possible that this dopamine is produced through the activity of tyrosinases, which are known to play a critical role in cuticle formation in C. elegans. The lack of an effect on food-sensing behavior after ablation of neurons postsynaptic to dopamine neurons (Sawin, 1996) shows that dopamine can act as a neurohormone in C. elegans, and thus synthesis of the neurotransmitter in the appropriate cell type may not be critical for all dopamine-mediated functions. Furthermore, C. elegans dopamine neurons contain the dopamine transporter CeDAT (Jayanthi et al, 1998); thus, dopamine synthesized at alternate locations may be taken up in these neurons and participate in regulated release.
dop-1 encodes a D1-like receptor displaying constitutive activity
It is possible that critical functional roles for dopamine in C. elegans have been overlooked because of the significant amount of dopamine present in cat-2(e1112) animals. Therefore, selective disruption of targets for dopamine signaling may be more appropriate to reveal the functional role of dopamine in C. elegans. In working towards this goal, we have described the isolation and characterization of a dopamine receptor gene, dop-1, which can give rise to two alternatively spliced forms of the receptor. While the observed alternative splicing is reminiscent of what is seen for the mammalian D2 receptor, the primary sequence does not point to a closer relationship with either the mammalian dopamine D1- or D2-receptor family. These clones are similar to the two splice variants of exon 5 described by Suo et al (2002). A third splice variant has been described by Suo et al (2002), which has no exon 5 either but employs an alternative downstream 3′ splice junction site for exon 8, shortening this exon by three codons. Pharmacological analyses using [125I]iodolysergic acid diethylamide showed that dopamine bound with high affinity to this receptor. We have made similar observations (not shown). Our extended functional analyses establish that for both spliced forms of dop-1, dopamine is the most likely endogenous ligand for this receptor, particularly since we could not detect endogenous (nor)epinephrine in C. elegans. Furthermore, because this receptor appears to be preferentially coupled to Gαs over Gαi/o and can stimulate adenylyl cyclase activity, it is functionally related to the mammalian dopamine D1-receptor subclass (Missale et al, 1998). The ALM and PLM neurons express gsa-1, goa-1 and gpa-16 (PLM only) (Jansen et al, 1999), which supports the possibility that DOP-1 also couples to GSA-1 in vivo.
The observation that several ligands suppress the basal activity of DOP-1 indicates that the receptor displays constitutive activity. The reduced potency but enhanced maximal stimulation of DOP-1 by dopamine in the presence of the inverse-agonist cis-flupenthixol likely reflects the block of constitutive receptor desensitization, thereby increasing the pool of receptors available for agonist activation (Leurs et al, 1998). Constitutive activity is a feature that has been observed for the mammalian D1-receptor class (Charpentier et al, 1996). While the pharmacological and functional profile of DOP-1 does not precisely mimic the mammalian D1 receptor, it is noteworthy that a single amino-acid change in the mammalian receptor can enhance constitutive activity and make the mammalian D1 antagonist SCH23390 behave like a partial agonist as it does for DOP-1 (Cho et al, 1996). It is unknown whether DOP-1 has similar functional characteristics in its native environment.
Role of DOP-1 receptors in nematode behaviors
Genetic and pharmacological studies have clearly demonstrated a role for dopamine in regulating several behaviors in C. elegans, including egg laying, locomotor activity and food sensing. The mechanisms underlying these dopamine-modulated behaviors, however, remain largely unknown. As might be expected from the lack of dop-1∷gfp expression in egg-laying neurons or muscles, the dop-1 mutants displayed no overt abnormalities in the dopamine-mediated inhibitory response on serotonin-induced egg laying (Weinshenker et al, 1995, 1999; Wintle and Van Tol, 2001). Likewise, neither food-sensing (Sawin et al, 2000) nor dopamine-induced immotility (Schafer and Kenyon, 1995) appeared to be defective in the dop-1 mutants under our experimental conditions. These behavioral data suggest that there may be additional dopamine receptor types, besides dop-1, through which dopamine can mediate these behaviors. Alternatively, the high concentrations of drugs that are required for penetration of the C. elegans cuticle in the egg laying and immotility assays might result in activation of nondopaminergic receptors, for example octopamine receptors, that are also known to inhibit egg-laying (Horvitz et al, 1982). Given that cat-2(e1112) animals are also defective in the food-sensing response (Sawin et al, 2000), perhaps a more parsimonious explanation for these behaviors is that they are mediated through alternative dopamine receptors. Indeed, recently another dopamine receptor (dop-2) has been described (Suo et al, 2003) that may be involved in these behaviors.
dop-1 and behavioral plasticity
The behavioral analysis of the dop-1(ev748) deletion mutant clearly implicates DOP-1 in the control of habituation to nonlocalized mechanical stimulation (tap). Tap habituation (Rankin et al, 1990) is a well-characterized paradigm for behavioral plasticity in C. elegans, and is amenable to analysis at the molecular and cellular levels. These effects of the dop-1 mutation are mimicked by a cat-2 mutation and rescued by a wild-type dop-1 transgene, indicating a role for dopamine in modulating mechanosensory plasticity. Importantly, behavioral plasticity itself is not eliminated in dop-1 mutants, but rather proceeds with an altered time course relative to wild-type animals, placing dopamine signaling in a critical position to modulate multiple sensory inputs contributing to whole animal behavior. Tap avoidance behavior is thought to reflect a combination of two antagonistic reflexes: a reversal response mediated by the anterior mechanoreceptor neurons ALML, ALMR and AVM, and a forward acceleration response mediated by the posterior mechanoreceptor neurons PLML and PLMR (Wicks and Rankin, 1995) (see Figure 5E). Habituation training results in a progressive decrease in the magnitudes of both the ALM/AVM-dependent reversal response and the PLM-dependent acceleration response. Since the ALM/AVM component appears to habituate faster than the PLM-dependent component, this also leads to a decrease in reversal frequency (Wicks and Rankin, 1996). The decrement in reversal frequency was enhanced by dop-1 and cat-2 loss-of-function mutations, and the dop-1 mutant defect was rescued by expression of functional receptor in the touch receptor neurons. Thus, the DOP-1 receptor may function within the ALM neurons to regulate habituation of the reversal response negatively, perhaps by decreasing its touch sensitivity and/or synaptic output. Since the rate of habituation of the PLM-dependent acceleration component would also affect changes in reversal frequency, it is possible that the dop-1 phenotype could also involve modulatory actions of dopamine on processes taking place within the PLM neurons. In the future, optical imaging studies of neuronal activity during habituation (Kerr et al, 2000) should provide a deeper insight into the cellular basis for the dop-1 phenotypes.
ALM and PLM are not postsynaptic to the dopamine neurons; however, the dopamine-containing CEP and PDE neurons receive significant synaptic input from ALM and PLM, respectively, and their processes are closely apposed in the nerve ring and ventral nerve cord. Previous laser ablation studies (Sawin, 1996) support the notion that dopamine can function as a neurohumoral agent in C. elegans. Moreover, it is possible that the dopamine transporter CeDAT in the dopamine neurons may mediate extrasynaptic secretion of dopamine, in a manner similar to that recently observed for the mammalian dopamine transporter (Falkenburger et al, 2001). Thus, we hypothesize that dopamine could be released humorally from the CEPs to modulate ALM function and from the PDEs to modulate PLM function. Synaptic input from the touch neurons could control the release of dopamine from the CEP and PDE neurons, which could in turn serve as a feedback loop to modulate the activity and habituation properties of the touch circuit (see Figure 5E). Additionally, the CEP and PDE dopamine neurons themselves have been implicated in sensing the tactile presence of bacteria (Sawin et al, 2000); thus, dopamine neurotransmission through DOP-1 receptors could serve to mediate functional interactions between these two distinct mechano-sensory modalities.
In conclusion, the demonstration that dop-1 plays a role in the kinetics of habituation without affecting habituation itself indicates that habituation to ‘tap' is not simply the result of desensitization of a single molecular component, but rather an active process, which includes DOP-1 signaling. The role of dop-1 in the habituation of mechanosensory and olfactory responses (WMNuttley and DvdKooy, unpublished) may be part of a logical behavioral circuit to optimize food-foraging strategies without directly affecting food sensing per se (Sawin et al, 2000). The results presented here provide evidence that dopamine can modulate behavioral plasticity in C. elegans and raise the possibility that dopamine acts as a neurohumoral agent that regulates habituation in C. elegans. This may be a general principle that is applicable to more complex nervous systems. For example, the involvement of dopamine in behavioral plasticity and learning has been well documented in vertebrates. The parallels between dopamine's role in the behavioral plasticity processes of such phylogenetically diverse animals suggests that the analysis of DOP-1's role in habituation may provide important general insights into the molecular basis of dopaminergic modulation of behavioral plasticity.
Materials and methods
Nematode strains, cell culture and transfection
All C. elegans strains were cultured by standard methods (Brenner, 1974). cat-1(ok411), cat-2(e1112) and cat-4(e1141) were obtained from the Caenorhabditis Genetics Centre (University of Minnesota, St Paul, MN, USA).
COS-7 and CHO-K1 cells were cultured in alpha-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and with 2.5% FBS, 2.5% horse serum, respectively. cDNAs encoding receptors were subcloned into the expression vector pRSV/Rc (Invitrogen Life Technologies, Burlington, Ont., Canada) and transfected into the cells using LipofectAMINE™ according to recommended protocols (Invitrogen Life Technologies, Burlington, Ont., Canada).
High-performance liquid chromatography
Dopamine, serotonin and their metabolites were measured in mixed-stage populations of C. elegans by a coulometric method using HPLC (for details, see Supplementary data).
Pharmacological and functional studies
[35S]GTP-γ-S binding assays were performed as described previously (Oldenhof et al, 1998) on membrane preparations of COS-7 cells harvested 48 h after transfection with the different DNA constructs. To determine the effect of cholera toxin (CTX) and pertussis toxin (PTX) on agonist-induced [35S]GTP-γ-S stimulation, transfected cells were preincubated with either CTX (100 ng/ml) or PTX (100 ng/ml) for 24 h prior to harvesting.
The ability of the cloned receptors to increase intracellular cAMP accumulation was assessed 24 h after transfection of the DNA constructs as described by us previously (Asghari et al, 1995). Functional coupling of dop-1 to Kir3.2 channels was assessed in Xenopus oocytes as described previously (Schoots et al, 1999). cRNA was synthesized in vitro from dop-1, human Kir3.2, goa-1 and gsa-1 (kind gift from Dr RH Plasterk, The Hubrecht Laboratory and Center for Biomedical Genetics, Utrecht, The Netherlands). Isolated oocytes were injected with approximately 10 ng of the desired cRNAs. Currents were recorded 48–72 h after injection. The dop-1 receptors expressed on the oocytes were exposed to 1 μM dopamine or (nor)epinephrine in the recording solution.
Derivation of dop-1 deletion mutants
A dop-1 deletion mutant was detected in a C. elegans deletion library (Ginzburg et al, 2002) generated by UV-TMP mutagenesis (Gengyo-Ando and Mitani, 2000). Two sets of nested PCR primers, located at 845 bp upstream from the initiation codon and the TM5 region of the dop-1 receptor gene, spanning 3.6 kb of genomic DNA, were used to detect a deletion that generated a 750 bp product. The corresponding mutant was isolated as a homozygote by standard sib-selection methods. The PCR product from this mutant was sequenced and revealed a deletion of 2.4 kb spanning from upstream of the translation initiation codon to the end of TM4 in the dop-1 gene (nucleotide −533 to nucleotide +1872, as measured from the position of the initiation codon). The dop-1 mutant homozygous for the deletion was backcrossed into wild type (N2) and twice into him-5(e1490) V to generate NW1583 dop-1(ev748) X; him-5(e1490) V.
dop-1∷gfp and dop-1 rescue constructs
A 3.6 kb genomic DNA fragment, containing the promoter (1.7 kb upstream of translation initiation methionine) up to and including most of exon II, was inserted in frame into the XmaI–KpnI site of a GFP vector, pPD95.79 (kindly provided by A Fire, Carnegie Institution of Washington, Baltimore, MD). Cell bodies of neurons in the head that express dop-1∷gfp were localized relative to chemosensory amphid neurons that take up lipophilic dyes such as DiI, DiO, FITC and rhodamine (Hedgecock et al, 1985). A rescue construct containing the entire dop-1 gene (4.5 kb) plus the 1.7 kb promoter sequence was created by replacing the GFP portion of the construct with a BamHI–KpnI fragment containing the remainder of the dop-1 gene (exon 2–exon 9) (pVTDOP1.R1). Another dop-1 rescue construct using the mec-7 promoter (pmec-7) was created by cloning a PCR-amplified pmec-7 fragment (nucleotide −14 to −852 from the initiation codon) in the SalI/ApaI restriction sites of pD95.79 followed by insertion of dop-1 gene sequences downstream from nucleotide −47 (ApaI/KnpI fragment) (pVTDOP1.R2).
Derivation of transgenic lines
Extrachromosomal arrays of dop-1∷gfp and rol-6(su1006) were generated by comicroinjection of the DNAs into wild type (N2) or him-5(e1490) hermaphrodite gonads containing oogonia (Mello and Fire, 1995). F1 and F2 transformants were selected based on the dominant rolling phenotype induced by rol-6(su1006). Similarly, the dop-1 rescue strains NW1584 dop-1(ev748) X;him-5(e1490) V;evEx153[sur-5∷gfp pVTDOP1.R1] and NW1632 dop-1(ev748) X;him-5(e1490) V;evEx153[sur-5∷gfp pVTDOP1.R2] were generated by coinjection of the pVTDOP1.R1 and pVTDOP1.R2 rescue constructs with sur-5∷gfp into dop-1(ev748) X;him-5(e1490) V. Transformants were selected based on positive GFP expression.
Microscopy
GFP-expressing transgenic animals were immobilized using 1 mM levamisole and transferred to a 1% agarose pad on a glass slide. GFP was visualized with a Leica DMRXE microscope (Leica Microsystems Inc., Bannockburn, IL) equipped with a broad-pass I3 FITC filter. In some cases, serial optical sections were acquired with an Olympus Inverted IX-70 microscope equipped with fluorescence optics and Deltavision Deconvolution Microscopy hardware and software (Applied Precision Inc., Issaquah, WA). Images were deconvolved and then 3D reconstructions were made using the Deltavison software.
Behavioral studies
Analysis of tap responses. To assay the tap response, hermaphrodite worms at the fourth larval stage were selected and placed on NGM plates containing OP50 for approximately 24 h. Next, each worm was transferred to new NGM plates. These plates were prepared by adding 60 ml saturated OP50 culture solution to the surface of the plate and allowing it to dry for 1 h at room temperature. Plates were secured to a computer-controlled tapping and tracking system, which administered taps of 6 mJ at selected time intervals (for details, see Supplementary data). Prior to the training sessions, the worms were allowed to adjust to the plate for 1 h. In the next 20 min the animals were assessed for behavior. Recordings of spontaneous reversal behavior under these conditions did not reveal significant differences between mutant and control animals.
Reversal frequency was defined as the number of reversals at a given stimulus number divided by total number of data points at that stimulus. Missing data points occurred if the worm was already reversing at the time of the stimulus or if the tracker failed to record the animal's position accurately due to a twisted or coiled body posture. The average number of data points per stimulus (out of 40 possible) were: wild type (N2)—38.3, dop-1(ev748) X;him-5(e1490) V;evEx153[sur-5∷gfp pVTDOP1.R1]—38.2, dop-1(ev748) X;him-5(e1490) V;evEx153[sur-5∷gfp pVTDOP1.R2]—36.9, dop-1(ev748) X;him-5(e1490) V—38.2. On graphs error bars represent s.e.m.; for reversal frequency, s.e.m. was calculated using the Bernoulli random variable method. The χ2 test was performed on the fraction of reversals in wild type versus mutant or mutant versus rescue over the interval of tap stimuli 2–13.
Egg-laying assays. Three separate drops (40 μl each) of M9 media alone, or M9 containing drugs (2.5 mM 5-HT, 1 mM dopamine), were placed on a 100 mm petri dish. All drugs were made fresh in M9 buffer immediately prior to assay. Three adult worms (24–48 h post L4 stage) were picked and placed into each drop of media and the number of eggs laid per drop was determined after 20, 40 or 60 min.
Dopamine-induced immotility assay. Assay plates were made by adding dopamine at a final concentration of 0–20 mM to 1.5% agar-noble in 10 mM HEPES, pH 7.2 (melted and cooled to 50°C). As has been documented extensively by others, the relatively high concentrations of drugs are required to elicit a response in the behavioral assays because the cuticle is relatively impermeable to these substances. Approximately 10 adult worms from a synchronized population were transferred to a marked center location of the plate. After an incubation period of 40 min at 21°C, the animals were scored as mobile, immobile or unresponsive. The worms that were able to complete sinusoidal body motions and move from their position during the 10–20 s observation period were considered mobile. Worms were considered immobile if they were motionless but responded to a gentle touch, or if the tail region of the animal moved but there was very little positional change on the plate. Only animals that were responsive to a gentle touch were calculated in the % immotility. The nonresponsive rate (unresponsive:responsive) was 0.089 for wild type (N2), 0.081 for him-5 and 0.088 for dop-1;him-5. The agar plates were not used beyond 4 h post dopamine addition.
Food-sensing behavior assay. The food-sensing behavior assays were performed as described in Sawin et al, (2000).
Supplementary Material
Acknowledgments
We thank Mr N Santos, Mr W Kawczynski, Mrs K Shannak and Ms Ordog for technical assistance. DNA sequencing was supported by the Centre for Applied Genomics, The Hospital for Sick Children, Toronto. Special thanks to Rajesh Ranganathan in Dr Robert Horvitz's group for his assistance with the C. elegans food-sensing behavioral (MOD) assay. SS and RFW contributed equally to this study. Our studies have been supported by Canadian Institutes of Health Research (Grants # MT-14573 and MGC-36040 to HHMVT; Grant FE-27881 to RFW; Grant MOP36379 to TEH and Grants MOP-9990 and MME-38332 to JGC), NARSAD (HHMVT), the NIH (Grants NS41397 to JGC and DA15823 and DA16445 to WRS), the Natural Sciences and Engineering Research Council of Canada (DvdK), the Ontario Mental Health Foundation (fellowship support to SS) and the Heart and Stroke Foundation of Québec (TEH). TEH is a MacDonald Scholar of the Heart and Stroke Foundation of Canada. HHMVT holds a Canadian Research Chair in Neurobiology.
References
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH (1995) Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65: 1157–1165 [DOI] [PubMed] [Google Scholar]
- Bargmann CI (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282: 2028–2033 [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Miller R (1998) Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev 22: 335–345 [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515–532 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S (1985) The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 5: 956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier S, Jarvie KR, Severynse DM, Caron MG, Tiberi M (1996) Silencing of the constitutive activity of the dopamine D1B receptor. Reciprocal mutations between D1 receptor subtypes delineate residues underlying activation properties. J Biol Chem 271: 28071–28076 [DOI] [PubMed] [Google Scholar]
- Cho W, Taylor LP, Akil H (1996) Mutagenesis of residues adjacent to transmembrane prolines alters D1 dopamine receptor binding and signal transduction. Mol Pharmacol 50: 1338–1345 [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI (1995) Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14: 803–812 [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM (2001) Dendrodendritic inhibition through reversal of dopamine transport. Science 293: 2465–2470 [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando K, Mitani S (2000) Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem Biophys Res Commun 269: 64–69 [DOI] [PubMed] [Google Scholar]
- Ginzburg VE, Roy PJ, Culotti JG (2002) Semaphorin 1a and semaphorin 1b are required for correct epidermal cell positioning and adhesion during morphogenesis in C. elegans. Development 129: 2065–2078 [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Thomson JN, Perkins LA (1985) Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev Biol 111: 158–170 [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD (1982) Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014 [DOI] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RHA (1999) The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet 21: 414–419 [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, Blakely RD (1998) The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol 54: 601–609 [PubMed] [Google Scholar]
- Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR (2000) Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26: 583–594 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Cotecchia S, Samama P, Costa T (1993) Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci 14: 303–307 [DOI] [PubMed] [Google Scholar]
- Leurs R, Smit MJ, Alewijnse AE, Timmerman H (1998) Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem Sci 23: 418–422 [DOI] [PubMed] [Google Scholar]
- McNamara FN, Clifford JJ, Tighe O, Kinsella A, Drago J, Croke DT, Waddington JL (2003) Congenic D1A dopamine receptor mutants: ethologically based resolution of behavioral topography indicates genetic background as determinant of knockout phenotype. Neuropsychopharmacology 28: 86–99 [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A (1995) DNA transformation. Methods Cell Biol 48: 451–482 [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78: 189–225 [DOI] [PubMed] [Google Scholar]
- Nader K, Bechara A, van der Kooy D (1997) Neurobiological constraints on behavioral models of motivation. Annu Rev Psychol 48: 85–114 [DOI] [PubMed] [Google Scholar]
- Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, von Zastrow M, Van Tol HH (1998) SH3 binding domains in the dopamine D4 receptor. Biochemistry 37: 15726–15736 [DOI] [PubMed] [Google Scholar]
- Rankin CH, Beck CD, Chiba CM (1990) Caenorhabditis elegans: a new model system for the study of learning and memory. Behav Brain Res 37: 89–92 [DOI] [PubMed] [Google Scholar]
- Rios M, Habecker B, Sasaoka T, Eisenhofer G, Tian H, Landis S, Chikaraishi D, Roffler-Tarlov S (1999) Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase. J Neurosci 19: 3519–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Rankin CH (2001) Analyses of habituation in Caenorhabditis elegans. Learn Mem 8: 63–69 [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Heberlein H (2002) Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Curr Opin Neurobiol 12: 639–645 [DOI] [PubMed] [Google Scholar]
- Sawin ER (1996) Genetic and cellular analysis of modulated behaviors in Caenorhabditis elegans, PhD thesis, Massachusetts Institute of Technology, Cambridge, Massachusetts
- Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631 [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ (1995) A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375: 73–78 [DOI] [PubMed] [Google Scholar]
- Schoots O, Wilson JM, Ethier N, Bigras E, Hebert TE, Van Tol HH (1999) Co-expression of human Kir3 subunits can yield channels with different functional properties. Cell Signal 11: 871–883 [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163: 215–226 [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100: 64–119 [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S (2002) Identification of a dopamine receptor from Caenorhabditis elegans. Neurosci Lett 319: 13–16 [DOI] [PubMed] [Google Scholar]
- Suo S, Sasawaga N, Ishiura S (2003) Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem 86 (4): 869–878 [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA, Palmiter RD (2000) Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet 25: 102–104 [DOI] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium (1998) Genome sequence of the nematode C. elegans: a platform for investigating biology. The C. elegans Sequencing Consortium. Science 282: 2012–2018 [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH (1995) Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci 15: 6975–6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Wei A, Salkoff L, Thomas JH (1999) Block of an ether-a-go-go-like K(+) channel by imipramine rescues egl-2 excitation defects in Caenorhabditis elegans. J Neurosci 19: 9831–9840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode C. elegans. Philos Trans R Soc London 314B: 1–340 [DOI] [PubMed] [Google Scholar]
- Wicks SR, Rankin CH (1995) Integration of mechanosensory stimuli in Caenorhabditis elegans. J Neurosci 15: 2434–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks SR, Rankin CH (1996) The integration of antagonistic reflexes revealed by laser ablation of identified neurons determines habituation kinetics of the Caenorhabditis elegans tap withdrawal response. J Comp Physiol [A] 179: 675–685 [DOI] [PubMed] [Google Scholar]
- Wintle RF, Van Tol HH (2001) Dopamine signaling in Caenorhabditis elegans-potential for parkinsonism research. Parkinsonism Relat Disord 7: 177–183 [DOI] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83: 1197–1209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.