Abstract
Tenofovir diphosphate (PMPApp) is a weak inhibitor of DNA polymerases (pol) α, δ, and ɛ*, with values for the Ki for PMPApp (PMPAppKi) relative to the Km for dATP (dATPKm) of 10.2, 10.2, and 15.2, respectively. Its incorporation into DNA was about 1,000-fold less efficient than that of dATP, with PMPAppKm values 350-, 2,155-, and 187-fold higher than dATPKm values for pol α, δ, and ɛ*, respectively.
Tenofovir {9-R-[2-(phosphonomethoxy)propyl]adenine;PMPA} is an acyclic nucleoside phosphonate (ANP) (12, 14) whose lipophilic prodrug, tenofovir disoproxil fumarate (Viread), was recently approved (18) for the treatment of human immunodeficiency virus (HIV) infection. In cells, PMPA is phosphorylated by AMP kinase (23, 28) and subsequently by nucleoside diphosphate kinase to tenofovir diphosphate (PMPApp) (Fig. 1). PMPApp is a competitive inhibitor (with respect to dATP) and a substrate of HIV type 1 (HIV-1) reverse transcriptase (RT) (9, 30). Diphosphates of several ANPs have been identified as good substrates as well as potent inhibitors of cellular replicative DNA polymerases (pol) in vitro (5, 6, 19-21). This activity correlates well with the cytostatic effects of their parental compounds (1, 2, 14, 25, 31), suggesting that the interaction of phosphorylated analogs with cellular DNA synthesis may significantly contribute to their cellular toxicity.
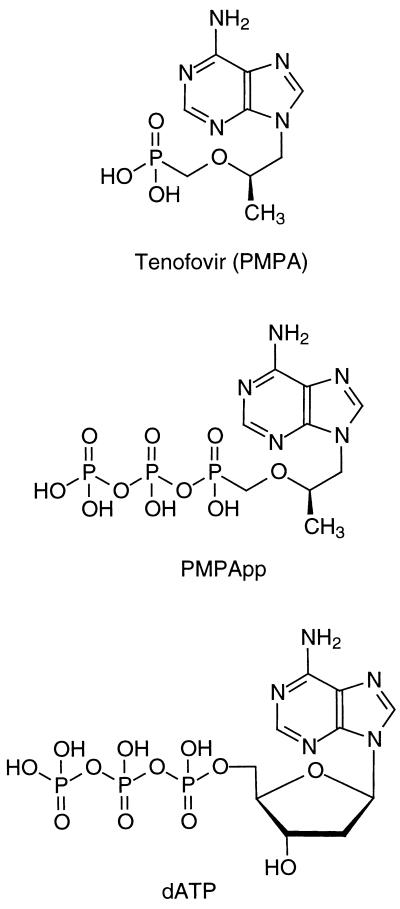
FIG. 1.
Structures of tenofovir, PMPApp, and dATP.
In this study, we examined the substrate affinity and inhibitory efficiency of PMPApp with respect to rat pol α, δ, and ɛ*. PMPApp was synthesized from PMPA (16) by the standard procedure (17). The enzymes were isolated from Sprague-Dawley rat compact transplantable lymphomas by previously described procedures (7, 22) but omitting glycerol gradient centrifugation. The human recombinant proliferating cell nuclear antigen was purified from BL21(DE3) cells by using a published protocol (15). Enzyme activities on the template-primer DNA40/18 (5′-GAGATCTCCTAGGGGCCC, 3′-CTCTAGAGGATCCCCGGGTACCGAGCTCGAATTCGTAATC-5′; molar ratio, 1:1.5) were measured under the following reaction conditions: (i) for pol α, 40 mM HEPES-KOH (pH 7.0), 25 mM KCl, and 10 mM MgCl2; (ii) for pol δ, 40 mM HEPES-KOH (pH 7.0), 50 mM KCl, 10 mM MgCl2, and proliferating cell nuclear antigen (18 μg ml−1); and (iii) for pol ɛ*, 40 mM HEPES-KOH (pH 7.5), 100 mM KCl, and 10 mM MgCl2. All reaction mixtures also contained 1 mM dithiothreitol, 200 μg of BSA ml−1, and 10% glycerol. The experiments using poly(dT)-oligo(dA)12-18 as a template primer were conducted as previously published (21). One unit of DNA pol activity is defined as the amount of enzyme that catalyzes the incorporation of 1 nmol of dATP into the template primer poly(dT)-oligo(dA)12-18 after 30 min of incubation under previously described reaction conditions (21).
Efficiencies of PMPApp and dATP incorporation catalyzed by examined DNA pol were compared by measurement of kinetic constants (Km for PMPApp [PMPAppKm], PMPAppVmax, dATPKm, dATPVmax) using the template-primer DNA40/18 with 0.5 μM 32P-labeled primer. The experiments using the reaction mixture (25 μl) were carried out at 37°C in the presence of eight different concentrations of either dATP or PMPApp by using the reaction conditions described above. After incubation over four different time intervals, the products were processed as described previously (6) and separated by polyacrylamide gel electrophoresis. The amounts of extended and unextended primer were assessed by using a PhosphoImager (Molecular Dynamics, Sunnyvale, Calif.). Kinetic experiments to determine PMPAppKi and dATPKm values for the enzymes on the poly(dT)-oligo(dA)12-18 template-primer were performed as outlined previously by Kramata et al. (21).
Our results show a very poor capability of all three enzymes for incorporation of the analog into DNA (Fig. 2 and 3); the incorporation efficiency (fins) (8) was approximately 3 orders of magnitude lower for PMPApp than for dATP, with PMPAppKm values being 350-, 2,155-, and 187-fold higher than those for dATPKm for pol α, δ, and ɛ*, respectively (Table 1). In contrast, HIV RT incorporates PMPApp with only sixfold lower efficiency than dATP (30). The relative incorporation efficiencies for several other ANPs, as determined in our earlier studies for pol α, δ, and ɛ*, were in the range of 0.35 to 120% (5, 6, 19, 20) (Table 2). The values for PMPApp as measured in this study (0.03 to 0.11%; Table 1) clearly demonstrate a significantly lower substrate affinity of this analog toward examined DNA pol compared to those of other ANPs. Relative incorporation efficiency values determined for PMPApp and human DNA pol α by Cihlar and Chen (13) were approximately 10-fold higher than our values, owing to different PMPAppKm values in the two studies. This discrepancy might reflect differences in reaction conditions, template-primer, and/or biological origin of the enzymes used.
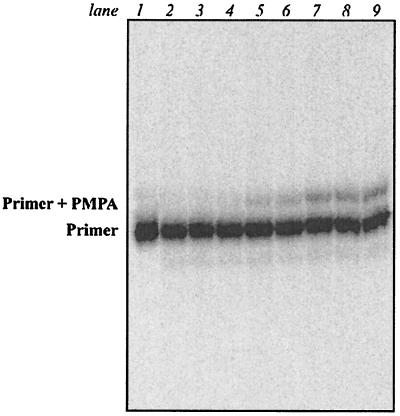
FIG. 2.
Kinetics of PMPA incorporation catalyzed by DNA pol δ. Reaction mixtures containing pol δ (0.6 U/ml) and PMPApp at various concentrations (left to right, lanes 2 to 9: 1, 5, 25, 125, 250, 500, 750, and 2,500 μM) were incubated in the presence of the template-primer DNA40/18 for 18 min at 37°C. Activity of pol δ-associated 3′-5′-exonuclease is detectable at all concentrations of PMPApp. Lane 1: primer only.
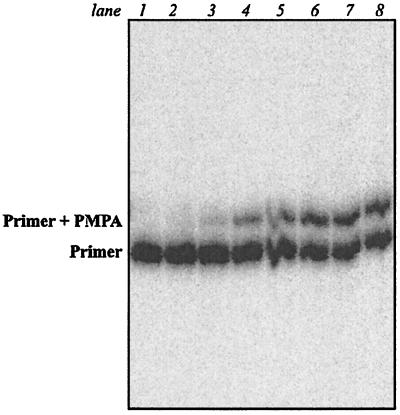
FIG. 3.
Kinetics of PMPA incorporation catalyzed by DNA pol α. Reaction mixtures containing pol α (4 U/ml) and PMPApp at various concentrations (left to right, lanes 2 to 8: 5, 25, 125, 250, 500, 750, and 1,500 μM) were incubated in the presence of the template-primer DNA40/18 for 32 min at 37°C. Lane 1: primer only.
TABLE 1.
Incorporation of PMPApp into template-primer DNA40/18 catalyzed by DNA pol α, δ, and ɛ∗a
| DNA pol | dATPKm (μM) | dATPVmax (pmol min−1 μg−1) | PMPAppKm (μM) | PMPAppVmax (pmol min−1 μg−1) | % fincb |
|---|---|---|---|---|---|
| pol α | 0.69 ± 0.28 | 8.5 ± 0.9 | 244 ± 87 | 3.2 ± 0.6 | 0.106 |
| pol δ + PCNA | 0.29 ± 0.05 | 0.58 ± 0.05 | 625 ± 73 | 0.34 ± 0.05 | 0.027 |
| pol ɛ∗ | 5.1 ± 1.1 | 1.3 ± 0.2 | 955 ± 203 | 0.20 ± 0.01 | 0.08 |
Reaction procedures were performed as described in the text. Kinetic constants are given as the means ± standard deviations from at least three independent experiments.
finc (%) = 100 × (PMPAppVmax × dATPKm)/(dATPVmax × PMPAppKm) (8).
TABLE 2.
Comparisons of ANP cytotoxicities to their interactions with DNA pol α, δ, and ɛ∗
| ANP |
ANPppKi/dNTPKmh
|
% fince, i
|
CC50f
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pol α | pol δ | pol ɛ*g | pol α | pol δ | pol ɛ*g | MT4j (μM) | CEMk (μM) | F-PBMCk (μM) | hRPTECl (μM) | HELm (μg/ml) | |
| PMEGa | 0.03 | ND | 0.004r | 6.4 | 40.2 | 5.9h | 11 | ≥0.8 | 0.23 | ND | 2.5 |
| PMEDAPb | 0.3 | 0.1 | 1.3r | 2.6 | 20.5 | 4.2h | 18 | ND | ND | ND | 40 |
| (S)-HPMPAc | 2.3 | 0.3 | 0.1r | 7.6 | 8.7 | 18.4r | 52; 26 | ND | ND | ND | 20 |
| PMEAd | 1.2 | 0.6 | 0.9r | 0.7 | 4.4 | 2.0r | 67 | 64 | 24 | 500 | 100 |
| (R)-PMPA | 10.2 | 10.2 | 15.6r | 0.11 | 0.03 | 0.08r | >300 | ≥500 | 165 | >2,000 | ND |
PMEG, 9-[(2-phosphonomethoxy)ethyl]guanine.
PMEDAP, 9-[(2-phosphomethoxy)ethyl]-2,6-diaminopurine.
(S)-HPMPA, (S)-9-[(3-hydroxy-2-phosphonomethoxy)propyl]adenine.
PMEA, 9-[(2-phosphonomethoxy)ethyl]adenine.
Percent finc = 100 × (ANPppVmax × dNTPKm)/(dNTPVmax × ANPppKm) (see reference 8).
CC50, 50% cytotoxic concentration; ND, not determined.
h, human DNA polymerases; r, rat DNA polymerases.
See reference 21.
See reference 1.
See reference 11.
See reference 2.
In addition to being recognized as a substrate, PMPApp may also act as an inhibitor of DNA pol. To analyze its inhibitory activity toward pol α, δ, and ɛ*, we used poly(dT)-oligo(dA)12-18 as a template-primer. Kinetic analysis of the experiments showed that PMPApp was a very weak competitive (for pol δ and pol ɛ*) or uncompetitive (for pol α) inhibitor, with Ki values more than 10-fold higher than Km values for dATP (Table 3). On the other hand, HIV RT is relatively strongly inhibited by PMPApp, with a PMPAppKi/dATPKm ratio equal to 0.34 (9). Our data correlate with the observation that PMPApp exhibits little inhibitory effect on the in vitro model of eukaryotic DNA replication at concentrations as high as 1 mM (27). When compared to the results of a previous study (performed under identical conditions), PMPApp seems to be an at least 10-fold-weaker inhibitor of DNA pol than PMEApp, the active form of another antiretroviral drug, adefovir (21). Moreover, it has been shown that PMPApp is also a poor inhibitor and substrate of DNA pol β as well as of mitochondrial DNA pol γ (9, 13). This feature distinguishes PMPApp from several nucleoside analogs presently used in anti-HIV therapies, e.g., didanosine, zalcitabine, and stavudine (4, 9, 13, 24).
TABLE 3.
Inhibition of DNA pol α, δ, and ɛ∗ with PMPApp on template-primer poly(dT)-oligo(dA)12-18a
| DNA pol | Kinetic constant valued
|
PMPAppKi/dATPKm | |
|---|---|---|---|
| dATPKm | PMPAppKi | ||
| pol α | 10.0 ± 1.1 | 102.0 ± 9.5c | 10.2 |
| pol δ + PCNA | 0.71 ± 0.08 | 7.2 ± 1.3b | 10.2 |
| pol ɛ∗ | 6.1 ± 2.3 | 95.3 ± 13.5b | 15.6 |
Reaction procedures were performed as described in the text. Kinetic constants are given as the means ± standard deviations from three independent experiments.
Competitive inhibition.
Uncompetitive inhibition.
Micromolar.
Taken together, PMPApp was found to be an exceptionally poor substrate and a weak inhibitor for all three replicative DNA pol. These observations indicate that PMPApp may minimally interfere with nuclear DNA synthesis and at least partially explain the low toxicity of tenofovir in a number of cell culture models (1, 10, 11, 29) as well as the favorable safety profile in treatment of HIV-infected patients with its oral prodrug, tenofovir disoproxil fumarate (see reference 3 and R. Schooley, R. Myers, P. Ruane, G. Beall, H. Lampiris, M. Miller, R. Mills, and I. McGowan, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 692, 2000).
Acknowledgments
We thank M. Otmar for the synthesis of PMPA diphosphate, L. Tovcigrecko for excellent assistance, and T. Cihlar from Gilead Sciences for comments and review of the manuscript.
This work was supported by the program of key projects no. S4055109 of the Academy of Sciences of the Czech Republic.
REFERENCES
- 1.Balzarini, J., T. Vahlenkamp, H. Eqberink, K. Hartmann, M. Witvrouw, C. Pannecouque, P. Casara, J. F. Nave, and E. De Clercq. 1997. Antiretroviral activities of acyclic nucleoside phosphonates [9-(2-phosphonylmethoxyethyl)-adenine, 9-(2-phosphonylmethoxyethyl)guanine, (R)-9-(2-phosphonylmethoxy-propyl)adenine, and MDL 74,968] in cell cultures and murine sarcoma virus-infected newborn NMRI mice. Antimicrob. Agents Chemother. 41:611-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Holý, J. Jindřich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barditch-Crovo, P., S. G. Deeks, A. C. Collier, S. Safrin, D. Coakley, M. D. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus type 1-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkuš, G., J. M. Hitchcock, and T. Cihlar. 2002. Mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkuš, G., I. Votruba, B. Otová, M. Otmar, and A. Holý. 1999. HPMPApp as a substrate toward replicative DNA polymerases α, δ and ɛ, p. 289-293. In A. Holý and M. Hocek (ed.), Collection symposium series, vol. 2. Institute of Organic Chemistry and Biochemistry, Academy of Sciences of Czech Republic, Prague. [Google Scholar]
- 6.Birkuš, G., I. Votruba, A. Holý, and B. Otová. 1999. 9-[2-(Phosphonomethoxy)ethyl]adenine (PMEApp) as a substrate toward replicative DNA polymerases α, δ, ɛ and ɛ*. Biochem. Pharmacol. 58:487-492. [DOI] [PubMed] [Google Scholar]
- 7.Birkuš, G., P. Kramata, I. Votruba, B. Otová, M. Otmar, and A. Holý. 1998. Nonproteolyzed form of DNA polymerase ɛ from T-cell spontaneous lymphoma of Sprague-Dawley inbred rat: isolation and characterization. Collect. Czech. Chem. Commun. 63:723-731. [Google Scholar]
- 8.Boosalis, M. S., J. Petruska, and M. F. Goodman. 1987. DNA polymerase insertion fidelity. Gel assay for site-specific kinetics. J. Biol. Chem. 262:14689-14696. [PubMed] [Google Scholar]
- 9.Cherrington, J. M., S. J. W. Allen, N. Bischofberger, and M. S. Chen. 1995. Kinetic interaction of the diphosphates of 9-(2-phosphonylmethoxyethyl)adenine and other anti-HIV active purine congeners with HIV reverse transcriptase and human DNA polymerases α, β, and γ. Antivir. Chem. Chemother. 6:217-221. [Google Scholar]
- 10.Cihlar, T., G. Birkuš, D. E. Greenwalt, and M. J. M. Hitchcock. Tenofovir exhibits low cytotoxicity in various human cell types—comparison with other nucleoside reverse transcriptase inhibitors. Antivir. Res., in press. [DOI] [PubMed]
- 11.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 12.Cihlar, T., and N. Bischofberger. 1998. PMEA and PMPA: acyclic nucleoside phosphonates with potent anti-HIV activity, p. 105-116. In H. Van der Goot (ed.), Trends in drug research II. Elsevier, Amsterdam, The Netherlands.
- 13.Cihlar, T., and M. S. Chen. 1997. Incorporation of selected nucleoside phosphonates and anti-human immunodeficiency virus nucleotide analogues into DNA by human DNA polymerases α, β and γ. Antivir. Chem. Chemother. 8:87-195. [Google Scholar]
- 14.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holy. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 15.Fien, K., and B. Stillman. 1992. Identification of replication factor C from Saccharomyces cerevisiae: a component of the leading-strand DNA replication complex. Mol. Cell. Biol. 12:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holý, A., and M. Masojídková. 1995. Synthesis of enantiomeric N-(2-phosphonomethoxypropyl) derivatives of purine and pyrimidine bases. 1. The stepwise approach. Collect. Czech. Chem. Commun. 60:1196-1212. [Google Scholar]
- 17.Holý, A., and I. Rosenberg. 1987. Synthesis of 9-(2-phosphonylmethoxyethyl)-adenine and related compounds. Collect. Czech. Chem. Commun. 52:2801-2809. [Google Scholar]
- 18.James, J. S. 2001. Tenofovir approved: broad indication. AIDS Treat. News 373:2-3. [PubMed] [Google Scholar]
- 19.Kramata, P., and K. M. Downey. 1999. 9-(2-Phosphonylmethoxyethyl) derivatives of purine nucleotide analogs: a comparison of their metabolism and interaction with cellular DNA synthesis. Mol. Pharmacol. 56:1262-1270. [DOI] [PubMed] [Google Scholar]
- 20.Kramata, P., K. M. Downey, and L. R. Paborsky. 1998. Incorporation and excision of 9-(2-phosphonylmethoxyethyl)guanine (PMEG) by DNA polymerase δ and ɛ in vitro. J. Biol. Chem. 273:21966-21971. [DOI] [PubMed] [Google Scholar]
- 21.Kramata, P., I. Votruba, B. Otová, and A. Holý. 1996. Different inhibitory potencies of acyclic phosphonomethoxyalkyl nucleotide analogs toward DNA polymerases α, δ and ɛ. Mol. Pharmacol. 49:1005-1011. [PubMed] [Google Scholar]
- 22.Kramata, P., J. erný, G. Birkuš, I. Votruba, B. Otová, and A. Holý. 1995. DNA polymerases α, δ and ɛ from T-cell spontaneous lymphoblastic leukemia of Sprague-Dawley inbred rat: isolation and characterization. Collect. Czech. Chem. Commun. 60:1555-1572. [Google Scholar]
- 23.Krejèová, R., K. Horská, I. Votruba, and A. Holý. 2000. Phosphorylation of purine (phosphonomethoxy)alkyl derivatives by mitochondrial AMP kinase (AK2 type) from L1210 cells. Collect. Czech. Chem. Commun. 65:1653-1668. [Google Scholar]
- 24.Martin, J. L., C. E. Brown, N. Matthews-Davis, and J. E. Reardon. 1994. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob. Agents Chemother. 38:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otová, B., A. Holý, I. Votruba, M. Sladká, V. Bílá, B. Mejsnarová, and V. Lešková. 1997. Genotoxicity of purine acyclic nucleotide analogs. Folia Biol. (Prague) 43:225-229. [PubMed] [Google Scholar]
- 26.Pauwels, R., J. Balzarini, D. Schols, M. Baba, J. Desmyter, L. Rosenberg, A. Holy, and E. De Clercq 1988. Phosphonylmethoxyethyl purine derivatives, a new class of anti-human immunodeficiency virus agents. Antimicrob. Agents Chemother. 32:1025-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisarev, V. M., S. H. Lee, M. C. Connelly, and A. Fridland. 1997. Intracellular metabolism and action of acyclic nucleoside phosphonates on DNA replication. Mol. Pharmacol. 52:63-68. [DOI] [PubMed] [Google Scholar]
- 28.Robbins, B. L., J. Greenhaw, M. C. Connelly, and A. Fridland. 1995. Metabolic pathways for activation of the antiviral agent 9-(2-phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob. Agents Chemother. 39:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivas, R. V., and A. Fridland. 1998. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob. Agents Chemother. 42:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suo, Z., and K. A. Johnson. 1998. Selective inhibition of HIV-1 reverse transcriptase by an antiviral inhibitor, (R)-9-(2-phosphonylmethoxypropyl)adenine. J. Biol. Chem. 273:27250-27258. [DOI] [PubMed] [Google Scholar]
- 31.Veselý, J., A. Merta, I. Votruba, I. Rosenberg, and A. Holý. 1990. The cytostatic effects and mechanism of action of antiviral acyclic adenine nucleotide analogs in L1210 mouse leukemia cells. Neoplasma 37:105-110. [PubMed] [Google Scholar]