Abstract
Transforming growth factor-β (TGF-β), one of the most abundant cytokines in bone matrix, has positive and negative effects on bone formation, although the molecular mechanisms of these effects are not fully understood. Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, induce bone formation in vitro and in vivo. Here, we show that osteoblastic differentiation of mouse C2C12 cells was greatly enhanced by the TGF-β type I receptor kinase inhibitor SB431542. Endogenous TGF-β was found to be highly active, and induced expression of inhibitory Smads during the maturation phase of osteoblastic differentiation induced by BMP-4. SB431542 suppressed endogenous TGF-β signaling and repressed the expression of inhibitory Smads during this period, possibly leading to acceleration of BMP signaling. SB431542 also induced the production of alkaline phosphatase and bone sialoprotein, and matrix mineralization of human mesenchymal stem cells. Thus, signaling cross-talk between BMP and TGF-β pathways plays a crucial role in the regulation of osteoblastic differentiation, and TGF-β inhibitors may be invaluable for the treatment of various bone diseases by accelerating BMP-induced osteogenesis.
Keywords: BMP, kinase inhibitor, osteoblast, signal cross-talk, TGF-β
Introduction
Bone repair is one of the most important and urgent subjects for our aging society. Bone morphogenetic proteins (BMPs), members of the transforming growth factor-β (TGF-β) superfamily, are expected to be applied to the treatment of various orthopedic diseases, including bone fracture and spinal fusion (Boden et al, 2002; Govender et al, 2002). BMPs are able to promote osteogenesis, chondrogenesis, and adipogenesis, whereas they inhibit myogenesis of mesenchymal progenitor cells (Reddi, 1998). More than a dozen BMPs have been identified, including BMP-2, BMP-4, BMP-6, BMP-7, and growth/differentiation factor-5. When recombinant human BMP-2 is applied to a matrix carrier protein and implanted subcutaneously, mesenchymal cells infiltrate into the matrix from the surrounding tissues; then, the matrix is degraded and replaced by trabecular bone and cartilage (Wozney and Rosen, 1998). However, the bone formation by BMP-2 is a self-limiting process, suggesting the presence of endogenous inhibitors of bone formation (Valentin-Opran et al, 2002).
TGF-β, the prototype of the TGF-β superfamily, is stored abundantly in bone, and has potent effects on osteoblasts. Three different isoforms of TGF-β, that is, TGF-β1, -β2, and -β3, with essentially similar bioactivities, have been identified in mammals (Roberts and Sporn, 1990). TGF-β has been reported to exhibit both positive and negative effects on bone. Exogenously injected TGF-β induced bone formation on periosteum (Noda and Camilliere, 1989; Joyce et al, 1990), whereas transgenic mice overexpressing TGF-β2 in bone exhibited an osteoporotic phenotype characterized by increased activities of osteoblasts and osteoclasts and impaired matrix mineralization by osteoblasts (Erlebacher and Derynck, 1996). Alliston et al (2001) reported that TGF-β inhibits osteoblast differentiation through modulation of the expression and transcriptional activity of Runx2. However, the roles of endogenous TGF-β in bone formation have not been fully elucidated.
Members of the TGF-β superfamily transduce their signals through two types of serine/threonine kinase receptors, termed type I and type II (Heldin et al, 1997; Derynck and Zhang, 2003; Shi and Massague, 2003). The type II receptors are constitutively active kinases, which phosphorylate type I receptors upon ligand binding. Seven type I receptors termed activin receptor-like kinase (ALK)-1 through -7 have been identified in mammals. BMPs bind to ALK-2, ALK-3, and ALK-6, whereas TGF-βs and activins bind to ALK-5 and ALK-4, respectively. ALK-7 has been shown to transduce signals for Nodal (Reissmann et al, 2001). Upon activation by type II receptors, ALKs transduce signals to Smads in the cytoplasm. Eight different Smads have been identified in mammals and classified into three groups, that is, receptor-regulated Smads (R-Smads), common-partner Smads (Co-Smads) and inhibitory Smads (I-Smads). Smad1, Smad5, and Smad8 are R-Smads activated by BMPs, whereas Smad2 and Smad3 are activated by TGF-βs and activin (Miyazawa et al, 2002). Smad4 is the Co-Smad shared by signaling pathways for BMPs and those for TGF-β/activin. Smad6 and Smad7 are I-Smads in mammals. Smad6 preferentially suppresses BMP signaling, whereas Smad7 inhibits both BMP and TGF-β/activin signaling.
As the roles of endogenous TGF-β signaling in bone formation are not well understood, we examined whether TGF-β could inhibit the osteoblastic differentiation induced by BMPs. For this purpose, we used a novel synthetic compound, SB431542, which has been shown to inhibit ALK-4/5/7 kinase activity specifically, but not ALK-2/3/6 kinase activity (Callahan et al, 2002; Inman et al, 2002a). We found that SB431542 enhanced myoblastic differentiation as well as osteoblastic differentiation of mouse C2C12 cells. SB431542 induced osteoblast differentiation in the maturation phase, possibly through inhibition of the expression of I-Smads. Moreover, SB431542 acted on multipotent human mesenchymal stem cells (hMSC) and enhanced osteoblastic differentiation and promoted matrix mineralization.
Results
SB431542 enhances differentiation of C2C12 cells into myoblasts
We first confirmed the effect of SB431542 by a luciferase assay using a TGF-β-responsive promoter-reporter construct, p3TP-Lux. SB431542 at 1 and 3 μM suppressed transcriptional activities induced by a constitutively active ALK-5 (ALK-5-TD) to 40 and 28%, respectively, compared to the control mouse C2C12 cells treated with 0.01% dimethyl sulfoxide (DMSO). Immunoblot analysis using a phospho-Smad2 antiserum also revealed that SB431542 at 1 μM efficiently inhibited the phosphorylation of Smad2 induced by TGF-β (data not shown). Although cytotoxic effects on C2C12 cells were not observed even at 10 μM of SB431542, we used 1 μM of SB431542 in the following experiments in order to minimize unexpected effects of SB431542 other than inhibition of TGF-β signaling.
C2C12 cells differentiate into myoblasts and form myotubes when cultured in low (less than 5%) serum-containing media. As it has been reported that exogenously applied TGF-β inhibits myogenesis (Liu et al, 2001), we examined whether SB431542 affects differentiation of C2C12 cells through inhibition of endogenous TGF-β signaling. As shown in Figure 1A, SB431542 accelerated myotube formation of C2C12 cells not only in low (2.5 and 5%)-serum-containing media but also in high (10 and 20%)-serum-containing media.
Figure 1.
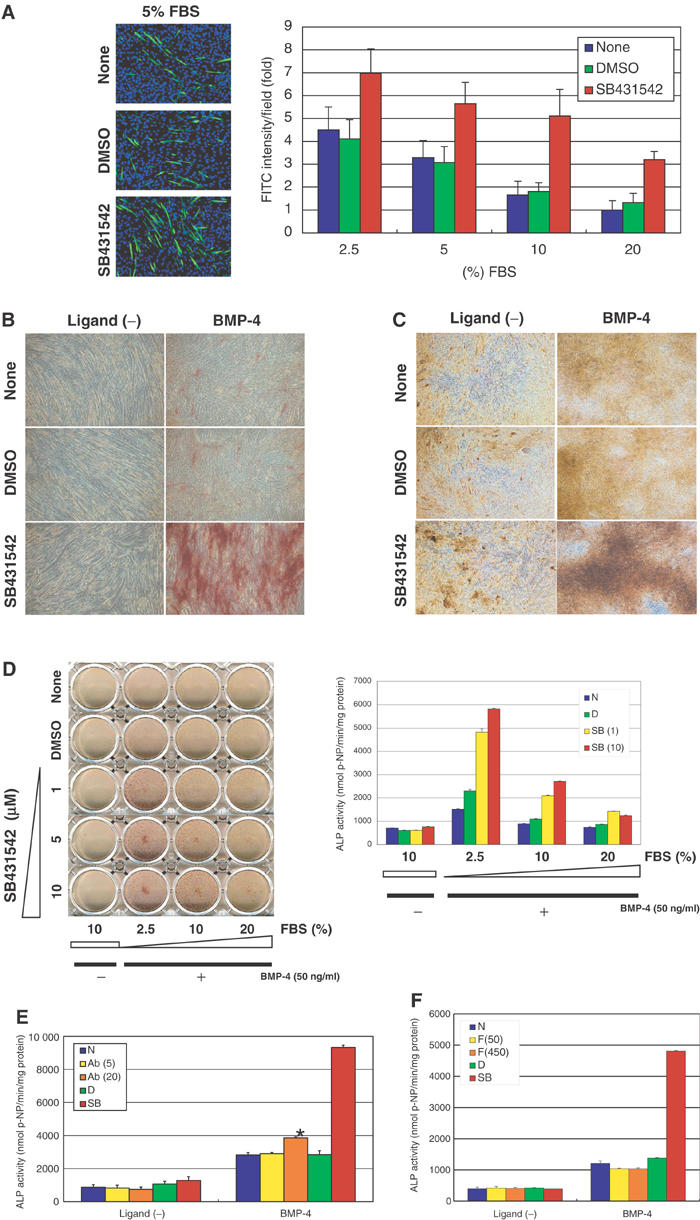
Effects of SB431542 on myoblastic and osteoblastic differentiation of C2C12 cells. (A) Myogenic differentiation of C2C12 cells in the presence of various concentrations of FBS (between 2.5 and 20%), with or without DMSO (0.01%) or SB431542 (1 μM). Myogenic differentiation was monitored by immunocytochemistry using antimyosin heavy-chain antibody (green), 4 days after stimulation. Nuclei were counterstained by DAPI (blue) (left panel). Intensities of the staining by antimyosin heavy-chain antibody were measured, and shown as fold induction compared to the value of the cells cultured in 20% FBS without DMSO (right panel). (B, C) Osteogenic differentiation of C2C12 cells in the presence of 5% FBS, induced by BMP-4 (50 ng/ml) with or without DMSO (0.01%) or SB431542 (1 μM). ALP staining was performed to monitor osteoblastic differentiation at day 9 (B). In vitro bone nodule formation was determined at day 15 by Van Gieson staining, which gives a pink color for collagen and a brown-yellow color for unmineralizaed nodules (C). (D) The effect of SB431542 on BMP-4 (50 ng/ml)-induced osteoblastic differentiation was assessed in the presence of various concentrations of FBS (between 2.5 and 20%) in C2C12 cells. The left panel shows ALP staining, and the right panel demonstrates ALP activity of parallel samples at day 9. N, none; D, DMSO; SB, SB431542. (E, F) Effects of anti-TGF-β1/2/3 neutralizing antibody (5 and 20 μg/ml) (E) or follistatin (50 and 450 ng/ml) (F) on BMP-4 (50 ng/ml)-induced osteoblastic differentiation of C2C12 cells. ALP activity was measured 9 days after induction. Ab, antibody; F, follistatin. In panel E, ALP activity was significantly increased by 20 μg/ml of anti-TGF-β antibody in the presence of BMP-4 (*), compared to that treated only with BMP-4 (P<0.05).
SB431542 promotes osteoblastic differentiation of C2C12 cells induced by BMP-4
We next examined the effects of SB431542 on osteoblastic differentiation induced by BMP-4. BMPs inhibit myogenic differentiation of C2C12 cells, and induce their osteoblastic differentiation in low-serum-containing media (Katagiri et al, 1994). However, high doses of BMP-2/4 (300 ng/ml) or BMP-7 (1000 ng/ml) were used to induce osteoblastic differentiation of C2C12 cells (Fujii et al, 1999; Lee et al, 2000). We also found that although 50 ng/ml BMP-4 efficiently inhibited myotube formation of C2C12 cells in low-serum-containing media, it induced only weak osteoblastic differentiation when assessed by production of the early osteoblast differentiation marker alkaline phosphatase (ALP) (Figure 1B). Interestingly, when SB431542 was added, C2C12 cells exhibited strikingly enhanced osteoblast differentiation by 50 ng/ml BMP-4 (Figure 1B). The effects of BMP-4 and SB431542 were further confirmed by the formation of bone nodules in vitro. BMP-4 was able to induce bone nodule formation of C2C12 cells, and SB431542 greatly enhanced the effect of BMP-4 (Figure 1C). These findings suggested that inhibition of endogenous TGF-β signaling may accelerate osteoblastic differentiation.
Although ALP production was significantly increased by BMP-4 in the presence of SB431532 in low-serum-containing media, the effects of BMP-4 and SB431542 were not remarkable in high-serum-containing media (Figure 1D). When SB431542 was added at high concentrations (up to 10 μM), it could only partially induce osteoblastic differentiation of C2C12 cells in the presence of 10 or 20% fetal bovine serum (FBS). These findings suggest that molecules contained in serum, including TGF-β as well as other factor(s), antagonized osteoblastic differentiation by BMP-4.
We also found that anti-TGF-β neutralizing antibody (20 μg/ml) enhanced BMP-4-induced ALP induction (Figure 1E), although it was less potent in this respect than SB431542. As endogenously produced TGF-β may act in an autocrine fashion, SB431542 acting in the cytoplasm may block TGF-β signaling more efficiently than TGF-β antibody. It is also possible that other TGF-β superfamily ligands, including activins, whose activities are blocked by SB431542 (Inman et al, 2002a), may be involved in the regulation of osteoblastic differentiation of C2C12 cells. However, follistatin, a natural antagonist for activins, failed to enhance ALP synthesis induced by BMP-4 (Figure 1F), excluding the possibility that endogenous activins play a major role in this process.
Regulation of osteoblast markers by BMP-4 and SB431542
In order to elucidate how SB431542 accelerates the osoteoblastic differentiation of C2C12 cells induced by BMP-4, we analyzed the expression profiles of osteoblast markers by quantitative real-time reverse transcription polymerase chain reaction (RT–PCR). BMPs have been reported to induce the osteogenic transcription factors Runx2/Cbfa1 (Ducy et al, 1997; Komori et al, 1997) and Osterix (Nakashima et al, 2002), which are responsible for osteoblast commitment. Runx2 mRNA level was gradually increased by BMP-4 to a peak at 48 h and gradually decreased thereafter (Figure 2A). SB431542 suppressed the Runx2 expression after 96 h. Osterix (Osx) was transiently and strongly induced by BMP-4; it increased after 2 h, reached a peak level at 24 h, and decreased thereafter (Figure 2B). SB431542 suppressed the transient increase in Osx by about 30%. Thus, inhibition of endogenous TGF-β signaling did not significantly accelerate the effects of BMP-4 in the commitment phase of osteoblastic differentiation of C2C12 cells.
Figure 2.
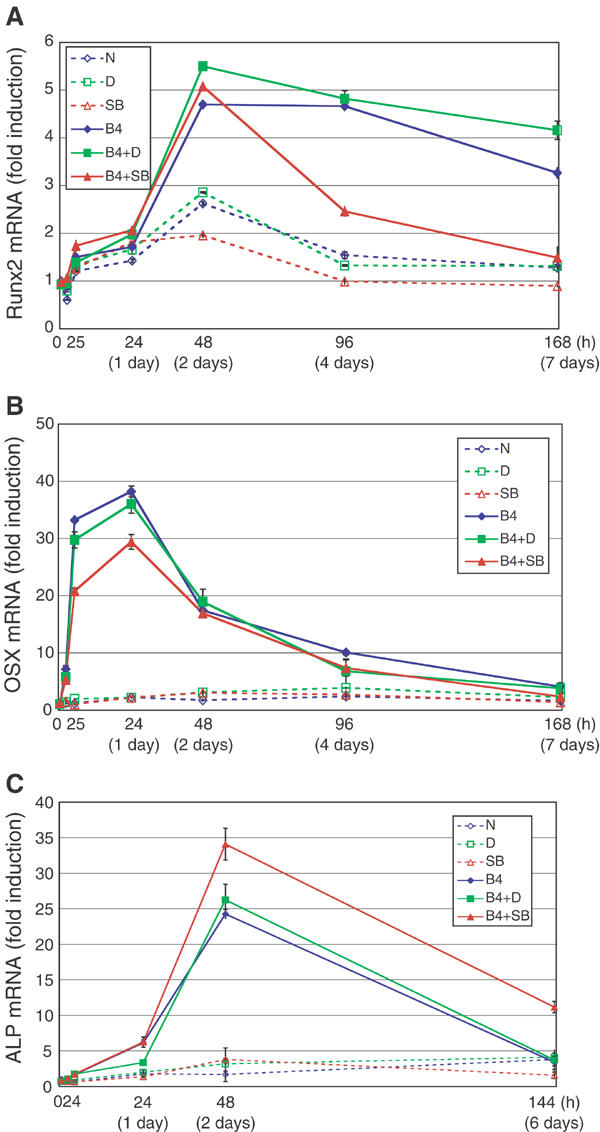
Expression profiles of Runx2, Osterix, and ALP mRNAs during myoblastic or osteoblastic differentiation of C2C12 cells treated with or without SB431542. C2C12 cells were treated or not with DMSO (0.01%) or SB431542 (1 μM) for 30 min in 5% FBS before stimulation. Then, cells were stimulated with or without BMP-4 (50 ng/ml) in combinations with DMSO or SB431542. Cells were harvested after stimulation at indicated time points, followed by quantitative RT–PCR. Quantitated mRNA values were normalized by the amounts of GAPDH mRNA, and results are given as fold induction. Runx2 gene (Runx2) (A), Osterix gene (Osx) (B), and liver/bone/kidney-type ALP gene (Akp2) (C) were determined. Solid lines reflect osteoblastic differentiation (treated with BMP-4), and broken lines reflect myoblastic differentiation (treated without BMP-4). N, none; D, DMSO; SB, SB431542; B4, BMP-4.
The commitment phase of osteoblastic differentiation occurs within the first 48 h upon BMP stimulation, and maturation of osteoblasts occurs thereafter. BMP-4 induced the expression of mRNA for liver/bone/kidney-type ALP gene (Akp2) until 48 h, and decreased thereafter (Figure 2C). Interestingly, SB431542 further induced the expression of Akp2 mRNA at 48 h upon addition of BMP-4 and this effect continued until day 6, suggesting that SB431542 accelerates osteoblastic differentiation at the later phase of osteoblastic differentiation.
Expression of TGF-βs and their receptors during osteoblastic differentiation
We examined whether endogenous TGF-β ligands and receptors were upregulated during osteoblastic differentiation, and whether SB431542 could affect their expression. Real-time RT–PCR analyses for TGF-β1 (Tgfb1), TGF-β2 (Tgfb2), TGF-β3 (Tgfb3), and TGF-β type I receptor ALK-5 (Tgfbr1) revealed that they were upregulated continuously during the osteoblastic differentiation phase of C2C12 cells (Figure 3A–D). Expression of TGF-β type II receptor (TβP-II; Tgfbr2) was increased from 48 h with or without BMP-4 stimulation (Figure 3E). TGF-β1 expression was upregulated three-fold by BMP-4, and SB431542 suppressed the expression of TGF-β1 after 48 h. Expression of TGF-β2 and -β3 was greatly enhanced by BMP-4; although expression of TGF-β2 was enhanced by SB431542, that of TGF-β3 was not significantly affected. ALK-5 was induced by BMP-4, and SB431542 did not significantly affect its expression. Expression of TβR-II was suppressed by SB431542 at day 7 in the BMP-4-treated cells. Thus, expression of most of the TGF-β ligands and their receptors was induced in the maturation phase of osteoblastic differentiation, although the effects of SB431542 on these molecules appeared to be variable.
Figure 3.
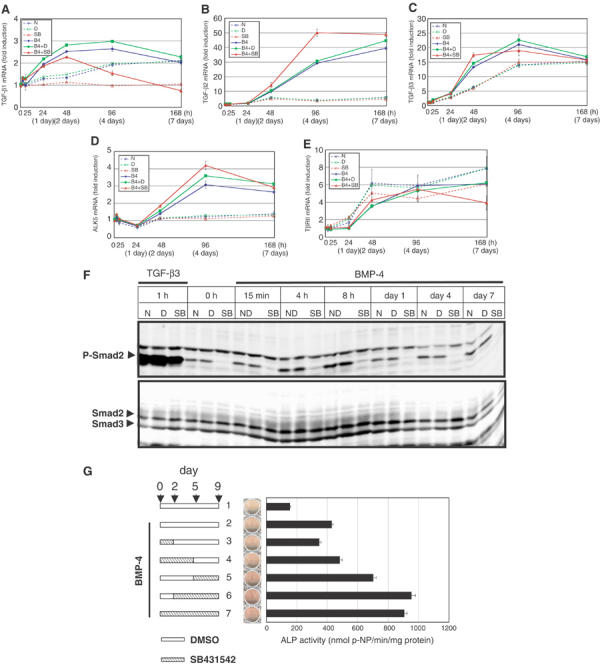
Endogenous TGF-β signaling is upregulated during the maturation phase. (A–E) mRNA expression patterns of ligands and receptors for TGF-β were determined during myoblastic or osteoblastic differentiation of C2C12 cells. C2C12 cells were treated as in Figure 2 and real-time RT–PCR analyses were performed. TGF-β1 gene (Tgfb1) (A), TGF-β2 gene (Tgfb2) (B), TGF-β3 gene (Tgfb3) (C), ALK5 gene (Tgfbr1) (D), and TGF-β type II receptor gene (Tgfbr2) (E) were determined. N, none; D, DMSO; SB, SB431542; B4, BMP-4. (F) C2C12 cells were pretreated or not with DMSO (0.01%) or SB431542 (1 μM) for 30 min in 5% FBS before stimulation. Cells were treated with BMP-4 (50 ng/ml) in combinations with or without DMSO or SB431542. TGF-β3 (1 ng/ml) was used as a control for phosphorylation of Smad2 and inhibition by SB431542. Whole-cell extracts were prepared at indicated time points, followed by immunoblotting. Activated Smad2 was detected by anti-phospho-Smad2 antibody. Smad2 and Smad3 were demonstrated by anti-Smad2/3 antibody. (G) Effects of BMP-4 and SB431542 were examined on different periods of osteoblastic differentiation of C2C12 cells. SB431542 (1 μM) was added to BMP-4 (50 ng/ml)-induced C2C12 cells at different periods as shown in the left panel. The middle panel demonstrates ALP staining. The right panel shows ALP activity.
When phosphorylation of Smad2 was examined in C2C12 cells, we found that phosphorylated Smad2 was detected up to day 7 after BMP-4 stimulation (Figure 3F); this may be induced by an increase in the expression of TGF-β ligands and their receptors during the osteoblastic differentiation. Although SB431542 exhibited variable effects on the expression of TGF-β ligands and receptors, the phosphorylation of Smad2 was efficiently abolished throughout this period, since SB431542 strongly suppressed the kinase activity of ALK-5.
SB431542 is crucial for acceleration of the osteoblastic differentiation in the maturation phase
We next treated the BMP-4-induced C2C12 cells with SB431542 at various periods (Figure 3G). BMP-4 alone yielded a weak increase in ALP activity of C2C12 cells, and SB431542 had little effect when added between days 0 and 2 (Figure 3G, lanes 2 and 3). Treatment with SB431542 throughout 9 days resulted in maximum increase in ALP production. Intriguingly, removal of SB431542 for the first 2 days did not affect ALP production (Figure 3G, lanes 6 and 7). Moreover, treatment with SB431542 in the later phase of osteoblastic differentiation (days 5–9) resulted in higher ALP production than that in the earlier phase (days 0–5) (Figure 3G, lanes 4 and 5). These findings are consistent with the findings that endogenous TGF-β signaling is active in the maturation phase. Thus, SB431542 is more important for enhancement of osteoblastic differentiation in the maturation phase than in the commitment phase.
Enhanced phosphorylation of BMP-specific R-Smads by SB431542 at the later phase
Phosphorylation of BMP-specific R-Smads by BMP-4 was analyzed by immunobloting using phospho-Smad1/5-specific antibody with or without SB431542 treatment (Figure 4A). Phosphorylation of Smad1/5 was observed from 10 min after ligand stimulation, reached maximal level at 1 h, and declined thereafter. SB431542 did not exhibit significant effects at early time points; however, Smad1/5 phosphorylation was more strongly observed in the presence than in the absence of SB431542 after day 1.
Figure 4.
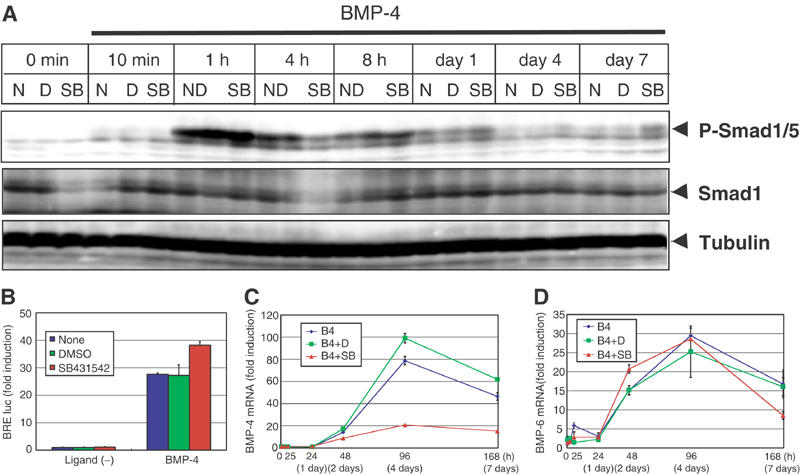
Effects of SB431542 on BMP-4-induced Smad signaling in C2C12 cells. (A) C2C12 cells were pretreated or not with DMSO (0.01%) or SB431542 (1 μM) for 30 min in 5% FBS before stimulation. Cells were treated with BMP-4 (50 ng/ml) in combinations with or without DMSO or SB431542. Whole-cell extracts were prepared at indicated time points, followed by immunoblotting. Activated BMP-specific R-Smads were detected by anti-phospho-Smad1/5 antibody. Smad1 was demonstrated by anti-Smad1 antibody. The bands for tubulin are shown as a loading control. (B) BRE-luc reporter was transfected into C2C12 cells 3 h before stimulation. Cells were stimulated or not with BMP-4 (50 ng/ml) with DMSO or SB431542, and harvested 36 h after stimulation. BRE-luc activities were normalized by TK-renilla luc activities. (C, D) mRNA expression patterns of BMP-4 and BMP-6 were determined during differentiation of C2C12 cells. C2C12 cells were treated as in Figure 2 and real-time RT–PCR analyses were performed. BMP-4 gene (Bmp4) (C) and BMP-6 gene (Bmp6) (D) were determined. N, none; D, DMSO; SB, SB431542; B4, BMP-4.
Analysis by a BMP-responsive promoter reporter construct BRE-luc containing the Id-1 promoter (Korchynskyi and ten Dijke, 2002) revealed that SB431542 enhanced the transcriptional activity induced by BMP-4 (Figure 4B). Thus, although SB431542 accelerates the osteoblastic differentiation of C2C12 cells by BMP-4 mainly acting during the maturation phase, it also enhances BMP signaling in the earlier phase by enhancing the phosphorylation of BMP-specific Smads.
Expression of BMP ligands was examined by real-time PCR analyses. As shown in Figure 4C and D, expression of BMP-4 and BMP-6 was strongly induced at the maturation phase of osteoblastic differentiation. Interestingly, expression levels of BMP-4, but not those of BMP-6, were suppressed by SB431542, suggesting that TGF-β signaling plays an important role in the induction of endogenous BMP-4. However, since Smad1/5 was more strongly phosphorylated in the presence than in the absence of SB431542 at days 4 and 7 (Figure 4A), exogenously added BMP-4 may have sufficiently compensated the decrease of endogenous BMP-4 during this period.
Suppression of I-Smad expression by SB431542
In order to elucidate how SB431542 accelerates oteoblastic differentiation in the osteoblast maturation phase, we analyzed the expression of various molecules that regulate the effects of TGF-β and BMPs. Among these, we found that the expression profiles of I-Smads are regulated by SB431542. Expression of the Smad7 gene (Madh7) was rapidly induced at 2 h, and decreased at 5 h. Thereafter, a mild increase of Smad7 was observed after 48 h (Figure 5A). When treated with SB431542, the induction of Smad7 after 48 h was suppressed. Expression of the Smad6 gene (Madh6) was also regulated by BMP-4; a rapid increase of Smad6 was observed in a manner similar to that of Smad7, and an increase after 24 h was more prominent for Smad6 than for Smad7 (Figure 5B). SB431542 did not significantly alter the expression of Smad6 in the early phase, but suppressed the increase of Smad6 in the maturation phase.
Figure 5.
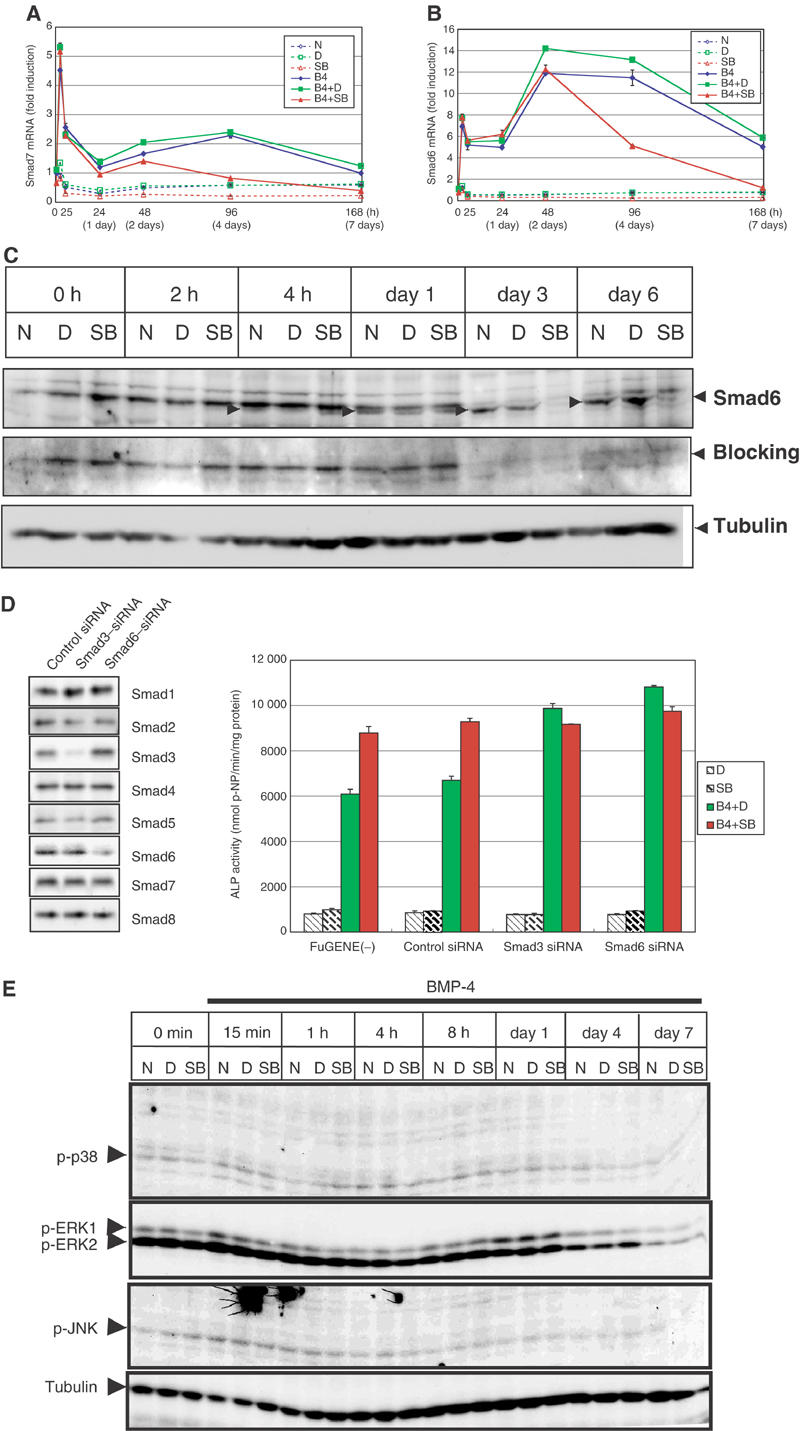
Effects of SB431542 on expression of I-Smads. (A, B) Quantitative RT–PCR for Smad7 gene (Madh7) (A) and Smad6 gene (Madh6) (B) was performed as in Figure 2. (C) Immunoblot analysis for endogenous Smad6. C2C12 cells were pretreated or not with DMSO (0.01%) or SB431542 (1 μM) for 30 min in 5% FBS before stimulation. Cells were stimulated with BMP-4 (50 ng/ml) in combinations with or without DMSO or SB431542. Whole-cell extracts were prepared at indicated time points. Smad6 was detected by anti-Smad6 antiserum (RPR). Bands specifically detected by the antiserum are indicated by arrowheads. Specificity of the bands was confirmed by reblotting of the membrane with blocking of the antiserum with the peptide used for immunization. The bands for tubulin are shown as a loading control. (D) Effects of the Smad3 and Smaf6 siRNA on BMP-4-induced osteoblastic differentiation of C2C12 cells. As the effects of siRNA were transient, C2C12 cells were treated with 150 ng/ml BMP-4 and ALP activity was determined at day 6. Expression levels of each Smad protein in the presence of control, Smad3, or Smad6 siRNA were determined by immunoblotting using transfected C2C12 cells (left panel). (E) Phosphorylation of MAPKs during osteoblastic differentiation. C2C12 cells were treated as in Figure 4A, and subjected to immunoblotting using anti-phospho-p38, anti-phospho-ERK1/2, or anti-phospho-JNK antibodies. The bands for tubulin are shown as a loading control.
To confirm repression of Smad6 mRNA expression by SB431542 in the maturation phase, immunoblot analysis using anti-Smad6 serum was performed (Figure 5C). We detected 70-kDa bands, which were specifically detected by anti-Smad6 antibody from 4 h after BMP-4 stimulation. The bands increased in intensity after 4 h, but SB431542 abolished expression of the bands in the maturation phase, consistent with the expression profiles of Smad6 mRNA obtained by real-time RT–PCR.
As expression of Smad6 is more strongly induced than Smad7 in C2C12 cells during the maturation phase, we next knocked down the expression of Smad6 mRNA by the Smad6 siRNA. The effects of siRNA were transient; therefore, C2C12 cells were treated with 150 ng/ml BMP-4 and ALP activity was determined at day 6. Under this condition, silencing of the Smad6 gene resulted in enhancement of the synthesis of ALP induced by BMP-4, whereas the control siRNA did not exhibit significant effects (Figure 5D). In the presence of SB431542, however, silencing of the Smad6 gene did not lead to further increase of ALP in the BMP-4-treated cells. Thus, Smad6 may play a major role in enhancement of osteoblastic differentiation induced by SB431542 in the BMP-4-treated C2C12 cells. In addition, we knocked down the expression of Smad3 mRNA, and found that the Smad3 siRNA exhibited effects very similar to the Smad6 siRNA. These findings suggest that TGF-β/Smad3 signaling plays an important role in the induction of Smad6.
R-Smads are not the only mediators of BMP signaling. Mitogen-activated protein kinases (MAPKs), including p38 MAPK, have been suggested to play roles in BMP-induced ALP induction (Lai and Cheng, 2002; Nohe et al, 2002). Moreover, Smad7 has been shown to activate p38 and JNK MAPKs in certain types of cells (Mazars et al, 2001; Edlund et al, 2003). In the BMP-4-treated C2C12 cells, however, immunoblot analysis using antibodies specific to each phosphorylated MAPK revealed no significant effects of SB431542 on ERK1/2, JNK, or p38 (Figure 5E).
SB431542 promotes osteoblastic differentiation of hMSCs
Finally, we examined the effects of SB431542 on hMSCs. hMSCs differentiate into osteoblasts and, in contrast to C2C12 cells, they undergo matrix mineralization by a medium containing dexamethasone, ascorbic acid, and β-glycerophosphate (termed osteogenesis induction medium; OS) (Pittenger et al, 1999). When SB431542 was applied to hMSCs, it greatly induced the production of ALP (Figure 6A, left panel). Moreover, the matrix mineralization induced by the OS medium was strikingly enhanced by SB431542 (Figure 6A, right panel).
Figure 6.
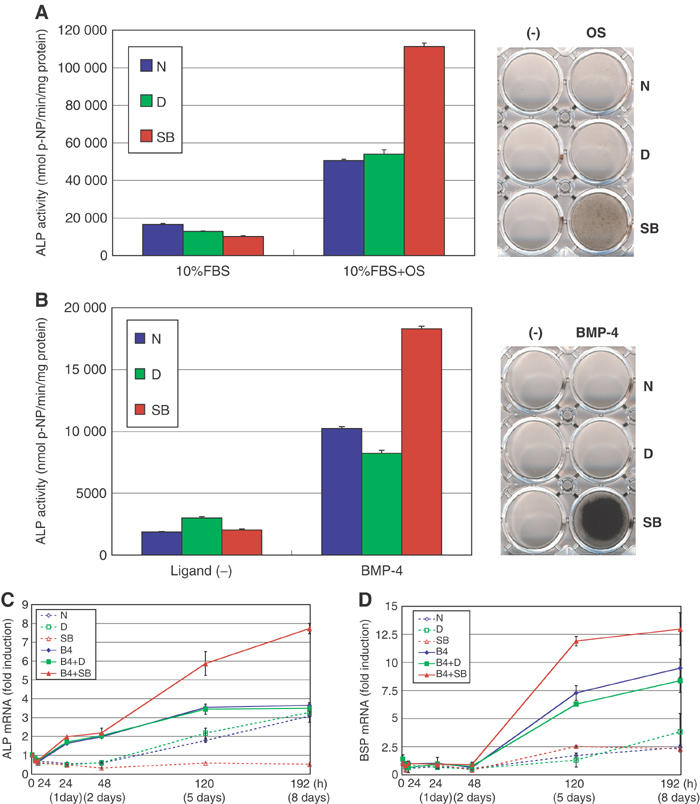
Effects of SB431542 on osteoblastic differentiation of hMSCs. (A) hMSCs were differentiated into osteoblasts by OS medium in combination with or without DMSO or SB431542. The left panel shows ALP activity at day 7, and the right panel demonstrates calcium deposition on matrix as determined by von Kossa staining at day 14 after induction. (B) hMSCs were induced to differentiate into osteoblasts by BMP-4 (50 ng/ml) in serum-free media containing ITS supplements, 0.05 mM ascorbic acid-2-phosphate, and 10 mM β-glycerophosphate in combination with or without DMSO or SB431542. ALP activity was measured on day 7 (left panel) and von Kossa staining was performed on day 14 after induction (right panel). (C, D) Quantitative RT–PCR for human ALP gene (ALPL) (C) and BSP gene (IBSP) (D) was performed as described in Figure 2. hMSCs were treated with or without BMP-4 (50 ng/ml) in serum-free media containing ITS supplements with or without DMSO or SB431542 as described in panel B.
We next examined the effects of SB431542 on hMSCs in the presence of BMP-4. Using serum-free/ITS media containing ascorbic acid and β-glycerophosphate, we found that BMP-4 induced the production of ALP in hMSCs, and that this effect was greatly enhanced by SB431542 (Figure 6B, left panel). Notably, hMSCs produced over 20 times higher ALP levels than C2C12 cells. Moreover, SB431542 and BMP-4 exhibited strong synergism in matrix mineralization. Calcium was deposited on the dish in the presence of BMP-4 and SB431542, and the deposition of calcium was stronger than in OS medium in the presence of SB431542 but without BMP-4 (Figure 6B, right panel).
Expression of ALP in hMSC in the serum-free/ITS media was examined by real-time PCR. As shown in Figure 6C, BMP-4 induced the expression of ALP, which was further enhanced by SB431542 at days 5 and 8. Moreover, we found that expression of bone sialoprotein (BSP) was induced by BMP-4, and SB431542 induced further increase of its expression at days 5 and 8 (Figure 6D).
Discussion
Both TGF-β and BMP-4 belong to the TGF-β superfamily. They bind to corresponding type II and type I receptors, and transmit intracellular signals through Smad proteins (Heldin et al, 1997; Derynck and Zhang, 2003; Shi and Massague, 2003). Although cross-talk between the TGF-β superfamily and other signaling pathways has been reported (Derynck et al, 2001), cross-talk between TGF-β and BMP signaling pathways has not been fully elucidated. TGF-β blocks both osteogenic and myogenic differentiation of C2C12 cells, whereas BMPs block myogenesis but promote osteogenesis. Thus, it is of great interest to elucidate how the myogenic and osteogenic differentiation processes are regulated by TGF-β and BMPs.
TGF-β has been shown to inhibit muscle differentiation of C2C12 cells through functional repression of myogenic transcription factors, including MyoD, by Smad3 (Liu et al, 2001). Endogenous TGF-β signaling may play an important role in inhibition of myoblastic differentiation of C2C12 cells. Thus, SB431542, which specifically inhibits ALK-4/5/7 kinase, efficiently induced myogenesis of C2C12 cells. Although BMPs also inhibit differentiation of C2C12 cells into myoblasts, the molecular mechanisms for this process have not been fully elucidated. Inhibition of myoblastic differentiation was achieved with 50 ng/ml of BMP-4, whereas induction of osteoblastic differentiation required higher concentrations of BMP-4. Thus, BMP-4 may inhibit the myoblastic differentiation of C2C12 cells independently of induction of differentiation into osteoblasts.
The present study suggested the presence of cross-talk between these two signaling pathways in the commitment phase as well as the maturation phase. Phosphorylation of BMP-specific R-Smads was detected as early as 10 min upon ligand stimulation, reached a maximal level at 1 h, and declined thereafter (Figure 4A). It has been suggested that phosphorylation levels of R-Smads may reflect the status of activation of type I receptors, since R-Smads are continuously shuttling between the nucleus and cytoplasm, and they may be phosphorylated as long as their receptors are activated (Inman et al, 2002b). Interestingly, phosphorylation of BMP-specific R-Smads by BMP-4 was more strongly observed in the presence than in the absence of SB431542 from days 1 to 7. Although inhibition of TGF-β signaling did not dramatically affect osteoblastic differentiation in the commitment phase (Figure 3G), transcription of a BMP-specific target gene, Id-1, was enhanced by SB431542 (Figure 4B), suggesting that suppression of TGF-β signaling may lead to enhancement of BMP signaling in the early phase. As expression of I-Smads is not significantly affected by SB431542 until day 2, mechanism(s) other than induction of I-Smads play a role in the enhancement of BMP signaling by SB431542 during this period. As Smad4 is shared by the TGF-β and BMP pathways, inhibition of TGF-β signaling by SB431542 may result in an increase in the pool of Smad4 available for BMP signaling (Candia et al, 1997; see Supplementary Figure S1).
In the maturation phase, expression of TGF-βs and their receptors was induced, suggesting that endogenous TGF-β signaling is activated during this period (Figure 7). I-Smads have been reported to be induced by TGF-β and BMPs, and to form negative feedback loops for regulation of TGF-β superfamily signaling (Heldin et al, 1997). Consistent with upregulation of endogenous TGF-β signaling, expression levels of Smad6 and Smad7 were increased after 48 h, possibly leading to inhibition of BMP as well as TGF-β signaling. As SB431542 inhibits endogenous TGF-β signaling, it represses the expression of Smad6 and Smad7 in the maturation phase, resulting in enhancement of BMP signaling. Silencing of the Smad6 gene resulted in an increase of ALP synthesis even in the absence of SB431542 (Figure 5D). Moreover, blockade of TGF-β signaling by the Smad3 siRNA exhibited effects similar to those induced by the Smad6 siRNA. Thus, inhibition of I-Smad expression during the maturation phase may be one of the most critical mechanisms in the acceleration of osteoblastic differentiation induced by SB431542.
Figure 7.
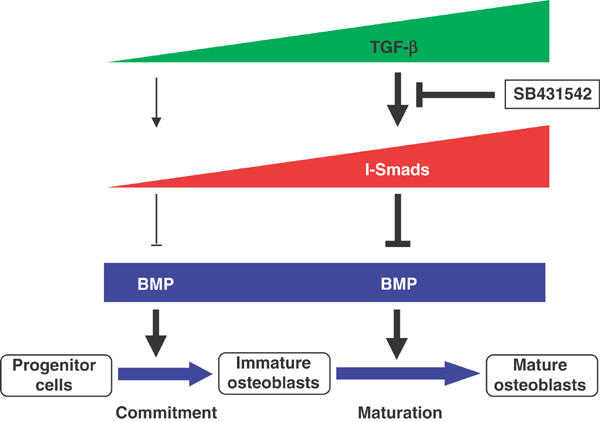
Schematic model of cross-talk between BMP and TGF-β signaling during osteoblastic differentiation. The process of osteoblastic differentiation of mesenchymal progenitor cells induced by BMP-4 is shown. BMP-4 acts on mesenchymal progenitor cells, and induces osteoblastic differentiation through the early commitment phase and the late maturation phase. The continuous presence of exogenous BMP-4 is essential for induction of osteoblastic differentiation (data not shown). Production of TGF-β is strongly induced in the maturation phase, and inhibitory Smads, Smad6 and Smad7, are induced during this period, possibly through TGF-β. The TGF-β receptor kinase inhibitor SB431542 inhibits endogenous TGF-β signaling, and suppresses the expression of I-Smads. The decrease in I-Smads may result in acceleration of BMP signaling and enhancement of osteoblastic differentiation.
We have found that BMP-4 and BMP-6 mRNAs were also induced at the maturation phase, suggesting that I-Smads may also be induced by endogenous BMPs. Moreover, expression of BMP-4 mRNA, but not that of BMP-6 mRNA, was suppressed by SB431542. However, phosphorylation of Smad1/5 was upregulated by SB431542 in the presence of exogenous BMP-4 (Figure 4A), suggesting that exogenously added BMP-4 sufficiently compensated the downregulation of BMP-4 mRNA by SB431542. It is also possible that other BMP family ligands are differentially regulated by SB431542. So far, most studies have examined the effects of TGF-β and BMP on Smad6/7 expression only for short periods (up to 48 h), and their effects at later phases have not been extensively examined. It is possible that different transcription factors may cooperate with TGF-β-specific or BMP-specific R-Smads for regulation of transcription of Smad6/7 during this period, and this should be determined in the future.
We have also examined the expression of antagonists for BMPs, including noggin, chordin, cerberus, Dan, c-Ski, SnoN, and I-Smads, by DNA microarray analysis using Affymetrix GenChip (S Maeda et al, unpublished data). Only Smad6 was significantly induced at 72 h upon BMP-4 stimulation, and suppressed by SB431542. Smad7 was not significantly induced by BMP-4 at 72 h, but its expression was suppressed by SB431542. These findings are consistent with the data obtained by real-time PCR analysis shown in Figure 5A and B, and support the notion that I-Smads play important roles in regulation of the osteoblastic differentiation at the maturation phase.
As osteoblasts mineralize the bone matrix after maturation, I-Smad-mediated blockade of BMP signaling in the maturation phase may suppress overmineralization and maintain normal bone mineral density. It has been reported that when TGF-β2 was overexpressed in bone in transgenic mice, bone mineralization was defective and bone was osteoporotic, although osteocytes were increased in number (Erlebacher and Derynck, 1996). SB431542 may interfere with this negative feedback regulation to support BMP-induced bone formation.
hMSCs can differentiate into a variety of mesenchymal lineages, including adipocytes, chondrocytes, and osteoblasts. Importantly, hMSCs can undergo matrix mineralization in vitro (Pittenger et al, 1999) and ex vivo (Kale et al, 2000). SB431542 enhanced ALP production in hMSCs, and promoted matrix mineralization in OS medium as well as in serum-free/ITS medium. Although BMP was not exogenously added to the OS medium, we observed that endogenous BMP-4 and BMP-6 mRNA was induced under this condition (see Supplementary Figure S2A and B). Moreover, expression of BMP-4 was enhanced by SB431542 under this condition. In conclusion, our findings strongly suggest that inhibition of endogenous TGF-β signaling with activated BMP signaling directs differentiation of hMSCs to osteoblasts. TGF-β receptor inhibitors may thus be invaluable as novel bone-inducing agents, particularly in BMP-induced bone formation.
Materials and methods
Cell cultures and myoblastic and osteoblastic differentiation
C2C12 cells were obtained from American Type Culture Collection. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 20% FBS and 100 U/ml penicillin G and 100 μg/ml streptomycin. Myogenic differentiation was induced by lowering the concentration of FBS to 5% unless specifically described. Osteoblastic differentiation of C2C12 cells was induced by 50 ng/ml recombinant human BMP-4 (Genzyme/Techne) in 2.5 or 5% FBS-containing medium unless specifically described. SB431542 was generated as described (Callahan et al, 2002), and dissolved at a concentration of 10 mM in DMSO as a 10 000× stock solution. As SB431542 was dissolved in DMSO, 0.01% DMSO was used as a control for SB431542 in each experiment. Neutralizing monoclonal antibody against TGF-β1, -β2, and -β3 (1D11) was obtained from R & D Systems. Follistain was kindly provided by Dr H Sugino. hMSCs were purchased from Poietics. hMSCs were maintained in DMEM containing 10% FBS and 100 U/ml penicillin G and 100 μg/ml streptomycin. Osteoblastic differentiation of hMSCs was induced by adding 0.1 μM dexamethasone, 0.05 mM ascorbic acid-2-phosphate, and 10 mM β-glycerophosphate to medium (OS, osteogenesis induction medium), or by BMP-4 (50 ng/ml) in serum-free media containing ITS supplements (Sigma), 0.05 mM ascorbic acid-2-phosphate, and 10 mM β-glycerophosphate.
Immunocytochemistry
C2C12 cells were fixed in 4% formaldehyde in PBS containing 0.2% Triton X-100, followed by PBS washing. Cells were blocked with 1% goat serum in PBS for 20 min. Myosin heavy chain was detected by incubating with monoclonal antibody MF20 (Developmental Studies Hybridoma Bank, provided by Donald A Fishman, MD) at 1:100 dilution in PBS containing 1% goat serum for 1 h. Cells were washed with PBS and incubated with biotinylated anti-mouse IgG (Vector Laboratories) for 1 h, and washed with PBS, followed by incubation with FITC-labeled avidin DCS (Vector laboratories) for 1 h. The nuclei were stained by 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes). Fluorescence was examined using an Olympus IX71 microscope. Intensities of the staining by antimyosin heavy-chain antibody were measured by Integrated Intensity Program of MetaVue (Universal Imaging Corporation).
ALP assays, Van Gieson staining, and von Kossa staining
Quantitative analysis of ALP activity was performed as previously described (Fujii et al, 1999), using Sigma Fast p-nitrophenyl phosphate tablet sets (Sigma). The protein concentration in each extract was measured by DC protein assay (Bio-Rad) using bovine serum albumin as a standard. Histochemical analysis of ALP activity was performed using the ALP staining kit (#85L-3R; Sigma) according to the manufacturer's protocol. Bone nodule formation was examined by Van Gieson staining as described (Harris et al, 1994; Feng et al, 2002). Calcium deposition was visualized by the von Kossa method. Cells were fixed in 3% glutaraldehyde in PBS, washed with PBS, and rinsed with distilled water. Fixed cells were incubated with 2.5% silver nitrate during exposure to light for 60 min, and then washed and developed with 0.5% hydroquinone for 2 min. Excess silver was washed out with 5% sodium thiosulfate for 2 min.
Quantitative real-time RT–PCR
Total RNA was extracted from C2C12 cells or hMSC using Trizol reagent (Invitrogen). cDNA was synthesized by the Thermoscript RT–PCR system (Invitrogen). Real-time PCR was performed using SYBR Green PCR master mix (Applied Biosystems) and the ABI Prism 7000 Sequence detection system (Applied Biosystems). All samples were run in duplicate in each experiment. Primers used are listed in ‘Supplementary information'. The relative quantities were determined using a standard curve generated by serial dilution (1:1, 1:5, 1:25, 1:125, 1:625) of a mixture of cDNAs from C2C12 cells or hMSC treated or not with BMP-4. The samples to be measured were diluted to 1:25. The specificity of detected signals was confirmed by a dissociation curve consisting of a single peak. Values were normalized by GAPDH.
Luciferase assay
C2C12 cells were seeded in duplicate in 12-well plates, followed by transient transfection with 0.15 μg of BRE-luc (Korchynskyi and ten Dijke, 2002) and 0.005 μg of TK-renilla reporter per well. At 3 h after transfection, cells were stimulated or not with BMP-4 (50 ng/ml) in combination with or without DMSO (0.01%) or SB431542 (1 μM). Cells were harvested 36 h after stimulation. Luciferase activity was measured by an AutoLumat LB953 (Berthold Technologies). BRE-luc activities were normalized by TK-renilla activity.
Immunoblotting
C2C12 cells were harvested with RIPA buffer containing 150 mM NaCl, 50 mM Tris–HCl, pH 8.0, 1% NP-40, 0.1% SDS, 0.5% deoxycholate, 1% aprotinin, and 1 mM phenylmethylsulfonyl fluoride. The lysates were sonicated and centrifuged. The supernatants were measured for protein concentrations, and equal amounts (150 μg) of lysates were applied in SDS sample buffer (100 mM Tris–HCl, pH 8.8, 0.01% bromophenol blue, 36% glycerol, 4% SDS, 10 mM DTT), followed by SDS–PAGE in 5–12% gradient gel. Separated proteins were electrotransferred to Pall FluoroTrans W PVDF membrane (Pall) and immunoblotted with anti-phospho-Smad2 antibody (Upstate), anti-Smad2/3 antibody (BD Transduction Laboratories), anti-phospho-Smad1/5 antibody (Cell Signaling), anti-Smad1 antibody (A-4) (Santa Cruz), anti-Smad6 antiserum (RPR) (provided by Dr C-H Heldin), anti-phospho-ERK-1/2, anti-phospho-JNK, anti-phospho-p38 (Cell Signaling), or anti-α-tubulin antibody (DM 1A) (Sigma). The peptide used in generating anti-Smad6 antiserum (RPR) was obtained from Dr C-H Heldin. Signals were detected using luminol sodium salt HG (Wako).
RNA interference and oligonucleotides
RNA interference was performed as described by Brummelkamp et al (2002). Briefly, C2C12 cells were transfected with pSUPER vectors using FuGENE6 transfection reagent (Roche Applied Science). To generate pSUPER constructs, oligonucleotides corresponding to Smad3-pSUPER (forward: 5′-gatccccGGCCATCACCACGCAGAACttcaagagaGTTCT GCGTGGTGATGGCCtttttggaaa-3′; reverse: 5′-agcttttccaaaaaGGCCATCACCACGCAGAACtctcttg aaGTTCTGCGTGGTGATGGCCggg-3′), Smad6-pSUPER (forward: 5′-gatccccCCCCTACCACTTCAGCCGGttcaagagaCCGGC TGAAGTGGTAGGGGtttttggaaa-3′; reverse: 5′-agcttttccaaaaaCCCCTACCACTTCAGCCGGtctcttg aaCCGGCTGAAGTGGTAGGGGggg-3′), and NC1-pSUPER (negative control) (forward: 5′-gatccccAAGCGCGCAAAGTAGGATTGCttcaagagaGCA ATCCTACTTTGCGCGCTTtttttggaaa-3′; reverse: 5′-agcttttccaaaaaAAGCGCGCAAAGTAGGATTGCtctct tgaaGTTCTGCGTGGTGATGGCCggg-3′) were annealed, followed by ligation into the pSUPER vector, which was digested with BglII/HindIII. Silencing of the genes was confirmed by inhibition of the expression of corresponding mRNAs using real-time RT–PCR (data not shown). To confirm the knockdown of corresponding Smad proteins, C2C12 cells were transfected with Smad cDNAs (Myc or FLAG-tagged constructs; 0.3 or 0.6 μg per well) and siRNA pSUPER constructs (NC1, Smad3, Smad6; 1.0 μg per well). Expression levels of proteins were determined by immunoblotting using Myc (9E10) or FLAG (M2) antibodies.
Supplementary Material
Supplementary Figure S1
Inhibition of endogenous TGF-β signals induces slightly increased interaction between Samd5 and Smad4. C2C12 cells were treated with BMP-4 in the presence (SB) or absence (D) of SB431542 for indicated periods, and cells were subjected to anti-Smad5 immunoprecipitation followed by anti-Smad4 immunoblotting (top panel). Cell lysates were precipitated in the absence of serum (S(−)) as a control. Expression levels of Smad5 and Smad4 were determined (lower two panels). The bands indicated by * (middle panel) are nonspecific bands detected also in the absence of serum.
Supplementary Figure S2A and B
Expression of human BMP-4 and BMP-6 in hMSC during osteoblastic differentiation. Quantitative RT-PCR for human BMP-4 gene (BMP4) (A) and BMP-6 gene (BMP6) (B) was performed using hMSC in OS medium in combination with or without DMSO or SB431542, as described in Figure 6A.
Supplementary information: Sequences of oligonucleotides used for RT-PCR.
Acknowledgments
We thank Y Inada, K Shirakawa, and M Takahata for their help in generating siRNA constructs. This research was supported by Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and grants from the Public Trust Haraguchi Memorial Cancer Research Fund and the Vehicle Racing Commemorative Foundation of Japan.
References
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R (2001) TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J 20: 2254–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden SD, Kang J, Sandhu H, Heller JG (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 27: 2662–2673 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J, Laping NJ (2002) Identification of novel inhibitors of the transforming growth factor β1 (TGF-β1) type 1 receptor (ALK5). J Med Chem 45: 999–1001 [DOI] [PubMed] [Google Scholar]
- Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW (1997) Cellular interpretation of multiple TGF-β signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124: 4467–4480 [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A (2001) TGF-β signaling in tumor suppression and cancer progression. Nat Genet 29: 117–129 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584 [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89: 747–754 [DOI] [PubMed] [Google Scholar]
- Edlund S, Bu S, Schuster N, Aspenstrom P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landstrom M (2003) Transforming growth factor-β1 (TGF-β)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-β-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell 14: 529–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A, Derynck R (1996) Increased expression of TGF-β2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol 132: 195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M (2002) Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res 17: 1822–1831 [DOI] [PubMed] [Google Scholar]
- Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K (1999) Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell 10: 3801–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84A: 2123–2134 [DOI] [PubMed] [Google Scholar]
- Harris SE, Sabatini M, Harris MA, Feng JQ, Wozney J, Mundy GR (1994) Expression of bone morphogenetic protein messenger RNA in prolonged cultures of fetal rat calvarial cells. J Bone Miner Res 9: 389–394 [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Miyazono K, ten Dijke P (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471 [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS (2002a) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62: 65–74 [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Hill CS (2002b) Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-β receptor activity. Mol Cell 10: 283–294 [DOI] [PubMed] [Google Scholar]
- Joyce ME, Roberts AB, Sporn MB, Bolander ME (1990) Transforming growth factor-β and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 110: 2195–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale S, Biermann S, Edwards C, Tarnowski C, Morris M, Long MW (2000) Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol 18: 954–958 [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127: 1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764 [DOI] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P (2002) Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 277: 4883–4891 [DOI] [PubMed] [Google Scholar]
- Lai CF, Cheng SL (2002) Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-β in normal human osteoblastic cells. J Biol Chem 277: 15514–15522 [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC (2000) Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20: 8783–8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Black BL, Derynck R (2001) TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15: 2950–296611711431 [Google Scholar]
- Mazars A, Lallemand F, Prunier C, Marais J, Ferrand N, Pessah M, Cherqui G, Atfi A (2001) Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem 276: 36797–36803 [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K (2002) Two major Smad pathways in TGF-β superfamily signalling. Genes Cells 7: 1191–1204 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29 [DOI] [PubMed] [Google Scholar]
- Noda M, Camilliere JJ (1989) In vivo stimulation of bone formation by transforming growth factor-β. Endocrinology 124: 2991–2994 [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P (2002) The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 277: 5330–5338 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147 [DOI] [PubMed] [Google Scholar]
- Reddi AH (1998) Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol 16: 247–252 [DOI] [PubMed] [Google Scholar]
- Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH (2001) The orphan receptor ALK7 and the activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev 15: 2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB (1990) The transforming growth factor-βs. In Peptide Growth Factors and Their Receptors, part I, Sporn MB , Roberts AB (eds) , pp 419–472. Berlin, Germany: Springer-Verlag [Google Scholar]
- Shi Y, Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700 [DOI] [PubMed] [Google Scholar]
- Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel GE (2002) Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop 395: 110–120 [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V (1998) Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop 346: 26–37 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Inhibition of endogenous TGF-β signals induces slightly increased interaction between Samd5 and Smad4. C2C12 cells were treated with BMP-4 in the presence (SB) or absence (D) of SB431542 for indicated periods, and cells were subjected to anti-Smad5 immunoprecipitation followed by anti-Smad4 immunoblotting (top panel). Cell lysates were precipitated in the absence of serum (S(−)) as a control. Expression levels of Smad5 and Smad4 were determined (lower two panels). The bands indicated by * (middle panel) are nonspecific bands detected also in the absence of serum.
Supplementary Figure S2A and B
Expression of human BMP-4 and BMP-6 in hMSC during osteoblastic differentiation. Quantitative RT-PCR for human BMP-4 gene (BMP4) (A) and BMP-6 gene (BMP6) (B) was performed using hMSC in OS medium in combination with or without DMSO or SB431542, as described in Figure 6A.
Supplementary information: Sequences of oligonucleotides used for RT-PCR.


