Abstract
XMAP215/TOGp family members and KinI kinesins are conserved microtubule (MT)-regulatory proteins, and have been viewed as possessing prominent antagonistic stabilizing/destabilizing activities that must be balanced. Here, interdependencies between TOGp and the KinI kinesin MCAK were analyzed in human leukemia cells. A system was established that permits inducible overexpression in homogeneous cell populations that simultaneously synthesize interfering short hairpin RNAs. We present evidence that the functional interplay of TOGp and MCAK proteins is manifested as three distinct phenotypes during the cell cycle. The first involves a role for TOGp in protecting spindle MTs from MCAK activity at the centrosome, which appears essential to prevent the formation of disorganized multipolar spindles. The second phenotype involves TOGp-dependent counteraction of excessive MCAK activity during mitosis, which recapitulates the previously established plus-end specific counteractive activities in vitro. The third involves an unexpected destabilization of the interphase MTs by overexpressed TOGp, a phenotype that requires endogenous MCAK. We hypothesize that TOGp-dependent prevention of MCAK-mediated spindle disorganization, as evidenced by depletion experiments, reflects a primary physiological role for TOGp in human somatic cells.
Keywords: catastrophe, monoastral, pericentrin, RNA interference, XKCM1
Introduction
Microtubules (MTs) are polar dynamic polymers that are responsible for chromosome segregation during mitosis and a multitude of cell-specific functions during interphase (for a review, see Desai and Mitchison, 1997). The dynamics of MTs are dramatically changed at the transition from interphase to mitosis, which results in a reorganization of the MT system, culminating in mitotic spindle formation. Many conserved proteins that regulate MT dynamics have been identified (for a review, see Howard and Hyman, 2003). One of these, XMAP215/TOGp, is responsible for stimulating rapid assembly at MT plus-ends in Xenopus egg extracts (Gard and Kirschner, 1987) and for causing a dramatic increase in both the growth and shrinkage rates during in vitro assembly of purified tubulin (Vasquez et al, 1994). Subsequent studies in Xenopus egg extracts have identified XMAP215/TOGp as a major MT stabilizing factor. This protein opposes the destabilizing activity of the KinI kinesin member XKCM1/MCAK (hereafter referred to as MCAK because they are orthologs), which functions by promoting catastrophes, that is, transitions from growing to shrinking MTs (Tournebize et al, 2000). By using a system with purified components, it has been shown that the opposing activities of these two proteins can generate the rapid MT dynamics observed in intact cells (Kinoshita et al, 2001).
Based on the findings outlined above, a general model has been proposed in which cell cycle regulation of MT dynamics is explained by a reduced ability of MAP215/TOGp to antagonize catastrophes upon entry into mitosis (for a review, see Kinoshita et al, 2002), possibly as a result of CDK1-mediated phosphorylation (Vasquez et al, 1999). This would facilitate destabilization of MT plus-ends by constitutively active catastrophe promotors such as MCAK, which would explain the increase in MT dynamism observed during spindle formation (Salmon et al, 1984; Belmont et al, 1990). Given the degree of conservation from yeast to mammals (for a review, see Ohkura et al, 2001), it has been assumed that the prominent MT-stabilizing function of XMAP215/TOGp in embryonic Xenopus systems is general for all cell systems (Kinoshita et al, 2002). Here we have investigated whether the current model of antagonizing stabilizing/destabilizing activities of the human homolog of XMAP215, TOGp, and MCAK applies to human somatic cells. The experimental system involved analysis of the MT system during interphase and mitosis of human leukemia cells depleted of and/or overexpressing these two MT regulators. The data from overexpression experiments confirm the potential of TOGp to counteract destabilizing MCAK activity at MT plus-ends, but this mechanism was only evident during mitosis. Most importantly, however, analysis of cells depleted of these proteins has suggested a novel and unexpected essential role of TOGp to protect spindle MTs from MCAK activity at the centrosome. This protective activity of TOGp provides an explanation for the previously observed requirement for TOGp during the formation of bipolar spindles (Gergely et al, 2003).
Results
Phenotypes of human cells singly or co-depleted of TOGp and MCAK
To evaluate the interdependence of TOGp and MCAK in the human K562 leukemia cell line, expression levels were reduced by RNAi-mediated gene silencing. The approach relies on constitutive production of short hairpin RNAs (shRNA) by means of an RNA polymerase III promoter (Brummelkamp et al, 2002). To allow rapid selection of homogeneous transfected cell populations producing specific shRNA, we used a replicating shuttle vector system that confers hygromycin resistance. As shown in Figure 1A, shRNA targeting of CaMKIIγ, MCAK, or TOGp resulted in diminishing levels of the targeted proteins. It can also be seen that the present system allows simultaneous depletion of both MCAK and TOGp. Arbitrary quantification, obtained from serial dilutions of cell lysates, revealed a 10–20-fold specific decrease in TOGp or MCAK after 6 days of culture, and unaltered levels of tubulin. Cell counting showed that while CaMKIIγ or MCAK depletion had no effect or an intermediate effect, respectively, TOGp depletion blocked cell proliferation (Figure 1B). Surprisingly, the data also show that simultaneous depletion of MCAK restored cell proliferation of TOGp-depleted cells to the intermediate level, observed after depletion of MCAK alone.
Figure 1.

Protein expression and cell proliferation of K562 leukemia cell lines producing interfering shRNA. (A) Cells were transfected with a replicating shuttle vector that directs synthesis of shRNA designed to target CaMKIIγ, MCAK or TOGp. Nontransfected cells were counter-selected by culturing in the presence of hygromycin, which killed off most nontransfected cells within 3 days. Total cellular lysates were analyzed by immunoblots using the indicated antibodies for detection. (B) Viable cells grown in the presence of hygromycin were determined on the days indicated. The data plotted represent the mean of triplicate determinations. All data in this and subsequent figures have been reproduced in at least three independent transfection experiments.
Analysis of DNA content after 4 days of shRNA-mediated depletion revealed an essentially normal cell cycle distribution among cells producing shRNA-CaMKIIγ and shRNA-MCAK, while production of shRNA-TOGp caused a marked increase in the peak corresponding to G2/M DNA (Figure 2). This alteration in the DNA profiles is consistent with the observed increase in the mitotic index of shRNA-TOGp-producing cells (Figure 2, upper panels; see % mitotic cells). Most importantly, and consistent with the data obtained by cell counting, the data also show that the mitotic index of TOGp and MCAK co-depleted cells is essentially the same as for cells depleted of MCAK alone, which suggests suppression of the mitotic interference caused by TOGp depletion alone.
Figure 2.

Cell cycle phenotypes of K562 leukemia cell lines producing interfering shRNA. DNA content was determined by flow cytometry 4 days after transfection with either Vector-Co or the indicated shRNA-producing derivative(s). The mitotic index is given as a percentage of total cells in each panel. The middle panels show the distribution of the indicated types of mitotic figure and the data plotted represent the mean of duplicate determinations (n=300). The lower panels show representative images of mitotic figures double-stained for DNA and MTs. Bar: 10 μm.
Examination of mitotic figures revealed the expected low levels of abnormal spindles among Vector-Co- or shRNA-CaMKIIγ-transfected cells, while synthesis of either shRNA-MCAK or shRNA-TOGp resulted in various levels of increased mitotic abnormalities (Figure 2, lower panels). MCAK depletion, which only resulted in a minor increase in the mitotic index, was primarily associated with a modest increase in mitotic cells with monoastral spindles with many microtubules that extend out towards the cell periphery, which is consistent with the effect of MCAK-specific antibodies injected into mitotic PtK2 cells (Kline-Smith and Walczak, 2002). In agreement with a previous study (Gergely et al, 2003), the data also show that TOGp depletion causes the accumulation of highly characteristic multipolar spindles, termed type B in Figure 2. The bipolar and monoastral spindles still present in TOGp-depleted cells had no distinctive features and appeared similar to those observed in vector-Co cells. It is notable that type B multipolar spindles have a disorganized appearance, with multiple MT asters of various sizes in which most MTs appear to be attached to chromosomes in the vicinity of each aster. It can also be seen in Figure 2 that type B multipolar spindles are very rare among Vector-Co-, CaMKIIγ- or MCAK-depleted cells. In these cell populations, essentially all multipolar spindles are of type A (i.e., well organized from three, and in some cases four, poles; Figure 2, lower panels). Most importantly, the distribution of spindle abnormalities in TOGp/MCAK co-depleted cells appears to be remarkably similar to the distribution in cells depleted of MCAK alone. Thus, simultaneous depletion of MCAK suppresses the appearance of type B multipolar spindles in TOGp-depleted cells, which is consistent with the relieved proliferation block (Figure 1B) and the decreased accumulation of mitotic cells (Figure 2).
It may appear paradoxical that TOGp-depleted cells, which did not increase in cell number over several days of culture (Figure 1) and contain type B multipolar spindles, still contain a significant fraction of cells in G1 (Figure 2). However, previous observations of live TOGp-depleted human cells have revealed that the majority of cells with disorganized multipolar spindles eventually divide (Gergely et al, 2003). The resulting interphase cells exhibited multiple or satellite nuclei, which is likely to result in a G1 block. However, in the present study, we observed no significant drop in cell viability during a 6-day culture period (unpublished data), which probably reflects the relative resistance of K562 leukemia cells to DNA-damage-induced apoptosis (Shao et al, 1997).
Type A and type B mitotic spindles were characterized by dual labeling of tubulin and the centrosomal protein pericentrin. As expected, the rare type A multipolar spindles present among Vector-Co cells contained pericentrin at each of the multiple spindle poles (n=20, unpublished data). However, analysis of the type B multipolar spindles of TOGp-depleted cells showed that the majority contained centrosomes at only two of the multiple spindle poles (Figure 3A and B), which shows that the type B multipolar phenotype is not caused by nucleation from multiple centrosomes. Furthermore, dual labeling of tubulin and the NuMA marker for the minus end of microtubules revealed that each one of the spindle poles of multipolar TOGp-depleted cells stained intensively for NuMA (Figure 3C). Thus, the majority of disorganized multipolar type B spindles contain two centrosomally associated MT-asters and additional acentrosomal MT-asters. Moreover, both types of MT-asters are gathered at the MT minus-ends and have chromosomes attached at the MT plus-ends. These data are consistent with a model implying that type B spindles evolve gradually during entry into mitosis by the release of spindle MTs from the centrosomal MT-asters.
Figure 3.
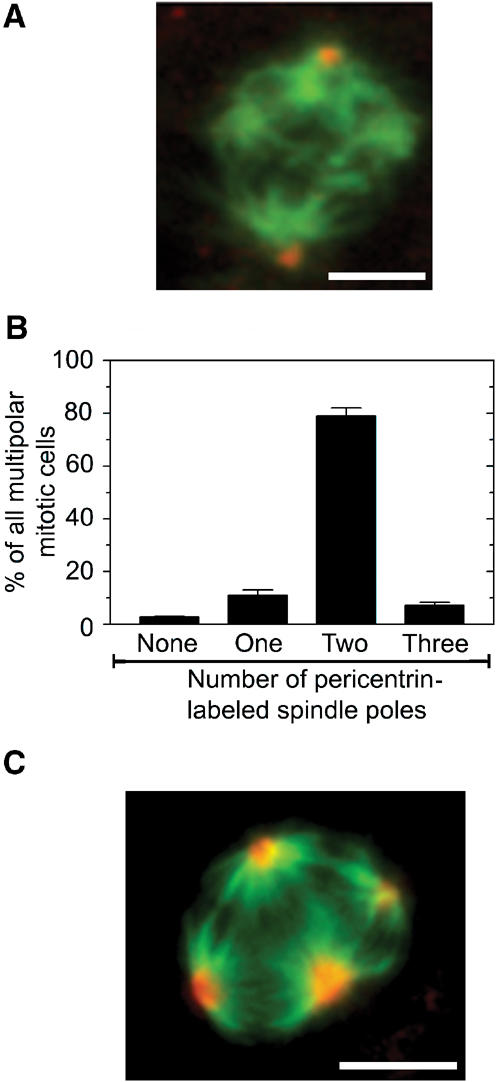
Only two of the poles of type B multipolar spindles of TOGp-depleted cells contain pericentrin, but all spindle pools contain NuMA. Transfected K562 cell lines producing shRNA-TOGp were generated as in Figure 1, and cultured for 4 days. (A) Representative epifluorescence image of a mitotic cell stained with anti-α-tubulin (green) and anti-pericentrin (red). (B) The distribution of the number of spindle poles in individual cells with type B multipolar spindles, as determined by pericentrin-labeled dots positioned at the spindle pole. The data are the average of the means of duplicate determinations (n=200 mitotic cells). (C) Representative epifluorescence image of a mitotic cell stained with anti-α-tubulin (green) and anti-NuMA (red). Inspection of individual mitotic TOGp-depleted cells revealed the association of NuMA with the center of each aster in all cells analyzed (n=100). Bar: 10 μm.
TOGp has an essential role to protect spindle MTs at the centrosome from MCAK activity
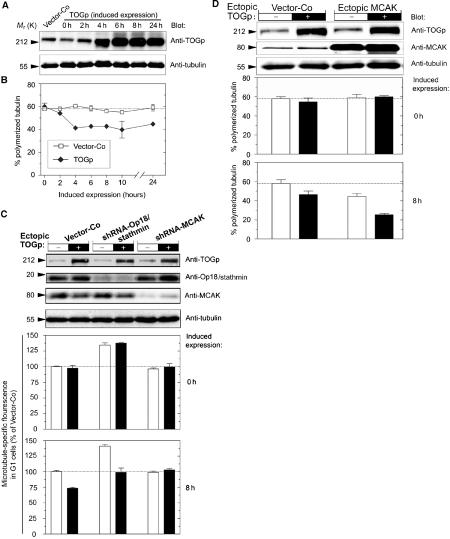
A more stringent evaluation of the apparent requirement of the MCAK protein for manifestation of the characteristic multipolar phenotype of TOGp-depleted cells requires a system that allows inducible expression of MCAK in cells co-depleted of TOGp and MCAK. This was achieved by cotransfection of the episomally replicating shRNA-producing vectors with an episomally replicating pMEP vector, which allows tightly regulated expression of transfected genes from the hMTIIa promotor. Accordingly, cells were transfected with a mixture of vectors that direct constitutive synthesis of shRNA-TOGp and shRNA-MCAK, and inducible expression of the Xenopus MCAK ortholog, which is not targeted by human-specific interfering RNA. To select homogeneous cell populations, transfected cells were counterselected for 4 days with hygromycin. Immunoblotting showed that this system allows co-depletion of TOGp and MCAK and that 24 h of Cd2+ induction increases the level of MCAK protein to close to normal levels (Figure 4). It is also shown in Figure 4 that prior to induced Xenopus MCAK expression (i.e., 0 h), TOGp- and MCAK-depleted cells have a distribution of spindle abnormalities that is consistent with data on doubly depleted cells in Figure 2. Importantly, 24 h of induced Xenopus MCAK expression caused a major increase in type B multipolar spindles, which are characteristic of cells depleted of TOGp alone. Thus, the severe type B multipolar phenotype of TOGp-depleted cells depends on the MCAK protein.
Figure 4.

MCAK expression is required for generation of the type B multipolar spindles in TOGp-depleted cells. Cells were transfected either with pMEP vector alone or with a mix of replicating shuttle vectors that direct constitutive synthesis of shRNA-TOGp and shRNA-MCAK, and inducible expression of MCAK, as described under Materials and methods. After 4 days of culture, Cd2+ was added for 24 h to specifically induce ectopic MCAK from the hMTIIa promoter of the pMEP vector. Upper panels show immunoblots of total cellular lysates using the indicated antibody for detection prior to or after induced MCAK expression. Lower panels show the distribution of the indicated types of mitotic figure after 0 and 24 h of induced MCAK expression. The data plotted represent the means of duplicate determinations (n=250).
Given the present demonstration that MCAK is essential for generating type B multipolar spindles in TOGp-depleted cells, we hypothesize that TOGp, from its previously demonstrated centrosomal localization (Charrasse et al, 1998; Gergely et al, 2003), is essential to protect spindle MTs from MCAK activity at the centrosome. The model, outlined as mechanism 1 in Scheme 1, implies that MCAK-dependent release of spindle MTs from the centrosomes is the cause of the observed acentrosomal asters of MTs. This model would explain the phenotypic observation that TOGp depletion causes the characteristic type B spindles, while co-depletion of TOGp and MCAK has the same comparably modest spindle phenotype as MCAK depletion alone.
Figure 8.

Model of TOGp and MCAK interdependence during interphase and mitosis. (1) Depiction of how TOGp from its centrosomal (pink oval) location is postulated to be essential for protection of spindle MTs against the destabilizing activity of MCAK. The predominant centrosomal location of TOGp is consistent with the observation that TOGp protects spindle MTs from MCAK activity at the centrosome. Moreover, a postulated loss of plus-end protection by TOGp depletion would predict accumulation of type I spindles, which are indicative of excess catastrophe promotion at the plus-end (Segerman et al, 2003), and not the observed disorganized type B multipolar spindles in TOGp-depleted cells. This protective function of TOGp at the centrosome is only evident during mitosis, which is consistent with an indirect attachment of minus-ends of spindle MTs to centrosomes, while interphase MTs are embedded in the centrosome and thereby protected from MCAK activity. This level of TOGp function is suggested by depletion experiments. (2) Depiction of how TOGp expressed at elevated levels protects free spindle MT plus-ends from excess MCAK-dependent destabilization. This mechanism of TOGp function is consistent with analysis of overexpressed phenotypes during mitosis. (3) Depiction of how TOGp may mediate MCAK-dependent destabilization of interphase MTs. This level of TOGp function is suggested by the fact that destabilization of interphase MTs by overexpressed TOGp is dependent on the endogenous MCAK protein. We hypothesize that mechanism 1, which has support from depletion experiments, probably reflects the most physiologically important role of TOGp in human somatic cells, while the other two mechanisms may only be relevant in human K563 leukemia cells under conditions of overexpression.
Overexpression phenotypes of TOGp and MCAK during mitosis
To further evaluate the functional interdependencies of TOGp and MCAK, cell cycle profiles and mitotic figures were analyzed in cells overexpressing these proteins alone, or in combination. This approach involved a previously described strategy for inducible coexpression of genes carried on pMEP vectors (Gradin et al, 1998). Immunoblot analysis (Figure 5) showed that both TOGp and MCAK are extensively overexpressed, both alone and in combination. To determine the consequences of excess TOGp and MCAK activities during spindle formation, DNA profiles and mitotic figures were analyzed after 20 h of induced expression (Figure 5). The data show that ectopic TOGp causes no significant interference during mitosis, while, as expected from a previous study, ectopic MCAK causes accumulation of mitotic cells (Kline-Smith and Walczak, 2002). It was also shown that the MCAK-mediated mitotic block was mainly associated with an increased frequency of the type I abnormal spindles, which have features indicative of excess catastrophe promotion (Segerman et al, 2003), namely that most kinetochore MTs were absent and MTs appeared as two dense star-like asters (Figure 5, lower panel). It should be noted that MCAK overexpression caused only a minor increase in the frequency of multipolar spindles, which were all of type A and not the disorganized type B that is characteristic of TOGp-depleted cells (see Figure 2). Most importantly, the MCAK-mediated mitotic block is suppressed by coexpression of TOGp, as evidenced by both a relieved mitotic block and the disappearance of type I abnormal spindles. The observed accumulation of mitotic cells after 20 h in the presence of paclitaxel shows that transfected cells are not blocked at any stage of the interphase of the cell cycle (see inserted DNA profiles in Figure 5). It follows that ectopic TOGp suppresses the mitotic overexpression phenotype of MCAK by a mechanism that allows overexpressing cells to divide.
Figure 5.

Overexpressed TOGp counteracts spindle disruption by overexpressed MCAK. K562 cells were transfected with pMEP-MCAK or Vector-Co and either additional Vector-Co (−) or pMEP-TOGp (+). The effect of ectopic TOGp and MCAK, either expressed alone or in combination, during spindle formation was analyzed after 20 h of Cd2+ induction. The upper panels show immunoblots of cell lysates using the indicated antibody for detection. The distribution of DNA content is shown in the absence or presence (inserts) of the mitosis-blocking drug paclitaxel (1 μM). The mitotic index is given as a percentage of total cells in each panel. Cells were categorized with respect to frequencies of bipolar, monoastral, multipolar and Type I spindles by inspection of cells double-stained for DNA and MTs. Examples of type I abnormal spindles spanning the different degrees of severity observed are shown at the bottom (bar: 10 μm). Data represent the means of duplicate determinations (n=400 mitotic cells).
Immunofluorescence analysis of endogenous TOGp confirmed the spindle localization with concentration at the spindle pole as previously observed (Charrasse et al, 1998; Gergely et al, 2003) (unpublished data). Under conditions of overexpression, the localization was similar, but TOGp staining of spindles was more intense and uniform (unpublished data). Given the higher levels of TOGp bound to spindle poles, it seems remarkable that TOGp overexpressed alone did not cause a clear mitotic phenotype. Taken together, the present data indicate that overexpressed TOGp does not need to be balanced in a reciprocal fashion by MCAK for the formation of a functional spindle to take place in somatic human cells.
The mitotic TOGp-depletion phenotype, which is suppressed by simultaneous MCAK depletion, is characterized by accumulation of disorganized type B multipolar spindles (Figure 2), while abnormal bipolar type I spindles are not observed (unpublished data). On the other hand, the mitotic overexpression phenotype of MCAK, which is suppressed by overexpressed TOGp, is primarily characterized by abnormal bipolar type I spindles (Figure 5). A common theme from these depletion and overexpression experiments appears to be that TOGp allows protection of the mitotic spindle from MCAK activity. Given the distinct features of multipolar type B and bipolar type I spindles, the protective effect of TOGp must occur at a different level and/or by a different mechanism. TOGp-mediated suppression of the mitotic MCAK overexpression phenotype, namely formation of abnormal bipolar type I spindles, is consistent with a mechanism that involves suppression of MCAK-mediated catastrophe promotion at the MT plus-ends. Given that XMAP215/TOGp has been shown to counteract MCAK by such a mechanism in Xenopus egg extracts and in vitro (Tournebize et al, 2000; Kinoshita et al, 2001), we propose that this mechanism of TOGp action would explain the counteraction of MCAK observed under overexpression conditions (outlined as mechanism 2 in Scheme 1).
Evaluation of the functional interplay of TOGp and MCAK during the interphase of the cell cycle
TOGp and MCAK have been shown to possess opposing MT regulatory activities in both interphase and mitotic extracts of Xenopus (Tournebize et al, 2000). To evaluate the importance of the interplay of MCAK and TOGp for the regulation of the interphase MT array in human cells, shRNA-transfected cells were analyzed over time. Given that shRNA-producing cells showed differences in cell cycle profiles (Figure 2), we determined the MT content specifically in cells with G1 content of DNA, by using a previously described strategy (Holmfeldt et al, 2003). Surprisingly, the data showed that 90–95% depletion (see Figure 1) of MCAK or TOGp alone, or in combination, had no detectable effects on the interphase MT polymer levels over a 6-day period (Figure 6A). We were also unable to detect any alteration in the interphase MT array in TOGp- or MCAK-depleted cells by immunofluorescence analysis (unpublished data). In the case of TOGp-depleted cells, these negative data are consistent with a previous report using the human HeLa cell line (Gergely et al, 2003).
Figure 6.

TOGp and MCAK do not exert antagonizing activities in human interphase cells. (A) Transfected K562 cell lines producing the indicated shRNA derivative were generated as in Figure 1. MT content among G1 interphase cells was analyzed on the indicated days by multi-parameter flow cytometry analysis. (B) The fraction of polymerized tubulin was analyzed as in panel (A) after 2 h, in the presence of graded concentrations of the microtubule-destabilizing drug nocodazole. Open bars represent Vector-Co cells and filled bars represent cells after 5 days of TOGp depletion. (C) Cells were cotransfected as indicated, with either Vector-Co or a replicating shuttle vector directing constitutive synthesis of the TOGp-specific interfering shRNA combined with either Vector-Co (−) or pMEP-MCAK (+) (see Materials and methods). After 5 days of culture, Cd2+ was added for 8 h to specifically induce ectopic TOGp from the hMTIIa promoter of the pMEP vector. Upper panels show immunoblots of total cellular lysates using the indicated antibody for detection. Lower panels show MT content among G1 interphase cells after 0 and 8 h of induced expression. It should be noted that the transfection protocol was designed for higher MCAK expression, as compared to the experiments shown in Figures 3 and 5 (see Materials and methods). All data plotted represent the means of duplicate determinations.
The negative data outlined above are put into perspective by the clear effect of Op18/stathmin depletion. The Op18/stathmin MT-destabilizing protein was only partially depleted by shRNA synthesis (approx. 70% at day 5; unpublished data), but MT polymer levels were still substantially elevated (Figure 6). A major MT-destabilizing role of Op18/stathmin is consistent with a previous report in which neutralizing antibodies or anti-sense RNA were used to inactivate or deplete Op18/stathmin in newt lung cells (Howell et al, 1999).
To further evaluate the potential role of TOGp levels in the stability of interphase MT polymers, we incubated control and TOGp-depleted cells for 2 h in the presence of the microtubule-destabilizing drug nocodazole. The result shown in Figure 6B did not reveal any significant effect of TOGp depletion. Given that XMAP215/TOGp is critical for opposing the destabilizing activity of XKCM1/MCAK in interphase Xenopus egg extract (Tournebize et al, 2000), it was of particular interest to determine whether TOGp depletion modulates MCAK-mediated MT destabilization in human interphase cells. The immunoblots in Figure 6C show that ectopic MCAK expression is efficiently induced in both Vector-Co- and shRNA-TOGp-transfected cells. Moreover, analysis of transfected cells at 0 and 8 h of induction demonstrated that ectopic MCAK exerts an equal degree of MT-destabilizing activity in both control and TOGp-depleted cells. Thus, contrary to initial expectations, endogenous TOGp does not counterbalance MT destabilization by MCAK in human interphase cells.
It should be noted that 90–95% depletion was achieved in the present study (Figure 1A), and that less efficient depletion in embryonic amphibian systems has been shown to result in dramatic alterations in interphase MT content (Tournebize et al, 2000). Given that severe depletion of MCAK and TOGp alone or in combination does not cause significant alterations of MT polymer content and that these MT regulators do not exert opposing activities during interphase, the present experiments reveal major differences between embryonic amphibian extracts and intact mammalian cells.
Overexpression of TOGp causes MCAK-dependent destabilization of interphase MTs
The potential effect of overexpressed TOGp on interphase MTs was evaluated by Cd2+ induction of pMEP-TOGp-transfected cells, and protein expression levels and MT content were followed over time. As shown in Figure 7A, a four- to six-fold increase in total TOGp protein levels was observed within 4 h, which stayed constant up to 24 h. Interestingly, and contrary to the prevalent view of TOGp as a general stabilizer of MTs (Kinoshita et al, 2002), overexpression of TOGp resulted in a substantial decrease in interphase MT-polymer levels (Figure 7B).
Figure 7.
Overexpressed TOGp destabilizes interphase MTs by an MCAK-dependent mechanism. (A) Cell lysates from Vector-Co- or pMEP-TOGp-transfected K562 cells were analyzed by immunoblots, using the indicated antibodies for detection, after the indicated time of Cd2+-induced expression. Arbitrary quantification was obtained from serial dilutions of cell lysates, which revealed four- to six-fold increased expression of TOGp (24 h) and nonsignificant alterations in endogenous tubulin levels (lower panel). (B) The fraction of polymerized tubulin was analyzed at the indicated times after Cd2+ induction of Vector-Co- and pMEP-TOGp-transfected cells. (C) Cells were cotransfected as indicated with either Vector-Co or a replicating shuttle vector directing constitutive synthesis of the indicated interfering shRNAs combined with either Vector-Co (−) or pMEP-TOGp (+) (see Materials and methods). After 4 days of culture, Cd2+ was added for 8 h to specifically induce ectopic TOGp from the hMTIIa promoter of the pMEP vector. The upper panels show immunoblots of total cellular lysates using the indicated antibody for detection. Lower panels show MT content among G1 interphase cells after 0 and 8 h of induced expression. (D) Cells were transfected with the indicated pMEP4 derivative as described in Figure 5, and the upper panels show immunoblots of cell lysates using the indicated antibody for detection. The effect of ectopic TOGp and MCAK, either expressed alone or in combination, on MT-polymer content was analyzed after 8 h of Cd2+ induction. All data plotted represent the means of duplicate determinations.
To explore the dependence of the MT-destabilizing activity of overexpressed TOGp on other MT-regulatory proteins, we cotransfected with a replicating vector that directs inducible TOGp expression and with vectors directing the constitutive production of specific shRNAs. As shown by immunoblots in Figure 7C, this system allows high-level TOGp expression in Op18/stathmin or MCAK-depleted cells. Moreover, analysis of cells prior to induction (i.e., at 0 h) revealed the expected increase in MT content after Op18/stathmin depletion, while MCAK depletion had no effect. It is also evident that the hMTIIa promoter is tightly regulated since all TOGp-transfected cells had an MT content similar to that of their cognate controls at 0 h. Analysis after 8 h of induced expression showed the expected TOGp-dependent destabilization in Vector-Co cells, and also showed that the abnormally high MT content in Op18/stathmin depleted cells decreased to the control level. Importantly, however, overexpressed TOGp did not decrease the MT content in MCAK-depleted cells. Moreover, analysis of mitotic cells after 20 h of induced expression showed that ectopic TOGp does not cause detectable modulation of spindle assembly in MCAK-depleted cells (unpublished data). Thus, the destabilizing activity of singly overexpressed TOGp, which is evident exclusively in interphase cells (see Figure 5 above), requires the presence of the endogenous MCAK protein.
Given that overexpression of either TOGp or MCAK causes MT destabilization in interphase cells, it seemed likely that co-overexpression of these proteins would have an additive destabilizing effect. This was evaluated in a cotransfection experiment in which the DNA concentrations were adjusted such that overexpression of MCAK alone caused a similar degree of partial MT destabilization to overexpression of TOGp (Figure 7D). It is evident from the data shown in Figure 7D that co-overexpression of TOGp and MCAK caused the predicted additive destabilizing effect.
The combined data in Figures 6 and 7 demonstrate that TOGp and MCAK do not exert opposing activities in mammalian interphase cells, which contrasts with the previously demonstrated antagonistic interplay during mitosis. Our finding that the MT-destabilizing activity of overexpressed TOGp requires endogenous MCAK is still consistent with a co-dependence between these regulators. However, the data outlined in Figure 7 can clearly not be reconciled with the two distinct levels of TOGp function proposed above to explain TOGp-dependent protection of the mitotic spindle against endogenous or ectopic MCAK activity. Hence, this has prompted our proposal of a third possible mechanistic level of TOGp function during interphase, as depicted in Scheme 1.
Discussion
XMAP215/TOGp and MCAK are currently viewed as being the predominant regulators of MT dynamics, which exert reciprocal counteractive activities during both interphase and mitosis. As shown here, however, single depletion and co-depletion of TOGp and MCAK has no apparent effect on the MT content or stability during interphase. Thus, these proteins do not exert the counterbalancing activities in mammalian interphase cells that have been demonstrated in interphasic Xenopus egg extracts and in vitro (Tournebize et al, 2000; Kinoshita et al, 2001). Given 90–95% depletion levels and pronounced phenotypes during mitosis (Figures 2 and 6), our data suggest that TOGp and MCAK have no role in maintaining the interphase MT array. However, it cannot be excluded that low residual amounts of TOGp and/or MCAK in depleted cells are sufficient for essential functions during interphase but not during mitosis.
We have also shown that cell division is not completely blocked among cells depleted of MCAK alone or co-depleted of MCAK and TOGp, which contrasts with the dramatic and complete effect of TOGp depletion alone (Figures 1 and 2). Moreover, while overexpression of TOGp was found to counteract spindle disruption by excess MCAK activity, ectopic TOGp by itself did not interfere significantly with spindle formation (Figure 5). Thus, MCAK and TOGp activities do not necessarily need to be balanced for the formation of functional spindles. Taken together, our data show that the prevalent view of XMAP215/TOGp as the master regulator of MT dynamicity, which must be reciprocally balanced by MCAK for the formation of a functional spindle, does not apply to somatic human cells.
The importance of TOGp and MCAK during spindle formation has also been studied recently by mutant analysis of the TOGp and MCAK homologs in fission yeast (Garcia et al, 2002). This study provided unexpected results, since the two TOGp homologs of fission yeast (Alp14 and Dis1) share an essential, rather than opposing, function with the two KinI homologs (Klp5 and Klp6) during spindle formation. It is presently unclear to what extent the yeast system is comparable to human somatic cells, but it is still notable that, similar to the present study, the data from fission yeast also refute a general reciprocal antagonistic relationship between TOGp and MCAK.
In this study, phenotypic evidence is presented that TOGp has the potential to act via three different mechanisms involving differential interdependence between the MT-directed activities of TOGp and MCAK during the cell cycle. Mechanism 1, as depicted in Scheme 1, implies that TOGp, from its centrosomal location, is important for protection of the free MT minus-ends from the otherwise deleterious activity of MCAK. In a recent study, the multipolar depletion phenotype of TOGp-depleted cells was interpreted to suggest that centrosomally located TOGp is required for the formation of bipolar spindles (Gergely et al, 2003). The present study demonstrates that this is not the case, since the characteristic type B multipolar phenotype is only observed in the presence of the endogenous MCAK protein (Figures 2 and 3). Moreover, we also show here that cells co-depleted of TOGp and MCAK form bipolar spindles with appreciable efficiency and proliferate at the same rate as cells depleted of MCAK alone (Figures 1 and 2). The model that emerges from our data implies that at least one essential function of TOGp during bipolar spindle formation involves protection from the otherwise deleterious MCAK activity. Given that TOGp has a centrosomal location during mitosis (Charrasse et al, 1998; Gergely et al, 2003), we hypothesize that lack of TOGp-dependent protection at the centrosome results in MCAK-dependent release of spindle MTs and a consequent disorganized multipolar spindle, as outlined under Scheme 1.
As depicted in Scheme 1, differential modes of MT attachment to the centrosome during mitosis and interphase provide an explanation for the apparent requirement for protection of spindle MTs at the centrosome during mitosis, but not during interphase. Thus, while the minus-ends of spindle MTs are only indirectly attached to the centrosome at the spindle pole, which may expose the minus-end to destabilizing MCAK activity, essentially all interphase MTs have their minus-ends embedded in the centrosome (Bornens, 2002).
Our model of the genesis of type B multipolar spindles outlined in Scheme 1 predicts a gradual evolution of the TOGp phenotype during entry into mitosis. This is indeed consistent with recent time-lapse studies, which show that TOGp-depleted human cells enter a prolonged prometaphase period in which some of the chromosomes repeatedly align on a metaphase plate-like structure and then revert to a more disorganized state (Gergely et al, 2003). A gradual evolution during entry into mitosis is also consistent with the observation that type B multipolar spindles contain only two centrosomally associated MT-asters and that additional asters are acentrosomal (Figure 3).
Cells depleted of either MCAK alone, or in combination with TOGp, show a comparably modest mitotic phenotype but frequent errors are still evident during spindle formation. These data are consistent with the finding that MCAK is essential for a high fidelity of spindle formation (Kline-Smith and Walczak, 2002). By comparing cell proliferation and gross spindle morphologies of cells depleted of MCAK alone with MCAK/TOGp co-depleted cells, we did not obtain evidence to suggest MCAK-independent functions of TOGp in somatic human cells. However, more detailed future analysis of co-depleted cells may still reveal TOGp-mediated activities in intact cells, which are independent of MCAK.
In Scheme 1, we hypothesize that mechanism 1 of TOGp function, which is based on depletion experiments, is exerted at the minus-ends, while mechanism 2 of TOGp function, which is based on overexpression experiments, is exerted at the plus-end. The MT plus-end mechanism 2 is based on previous demonstrations of MT plus-end specific counteraction of the catastrophe-promoting activity of MCAK in Xenopus egg extracts (Tournebize et al, 2000) and in vitro (Kinoshita et al, 2001). Assuming that mechanisms 1 and 2 of TOGp function are exerted at different MT ends, it follows that the mechanisms involved are not identical, which is consistent with the observed differences in functional interplay with MCAK as outlined in Scheme 1.
The postulated TOGp-dependent protection of spindle MTs at the centrosome (i.e. mechanism 1 of TOGp function) may either be exerted as a reciprocally counteractive activity that can be out-competed by overexpressed MCAK or, alternatively, as a robust protection of spindle MTs at the centrosome at sufficient stoichiometry to resist even excessive MCAK activity. The present data support the latter alternative since overexpression of MCAK results in abnormal bipolar type I spindles, and not appearance of the disorganized type B multipolar spindles that are the hallmark of TOGp-depleted cells (Figures 2 and 5). Given that the MT plus-end specific activities of MCAK and TOGp have been shown to be reciprocally counteractive in vitro and in embryonic amphibian systems (Tournebize et al, 2000; Kinoshita et al, 2001), the present results are consistent with the idea that TOGp exerts distinct types of activities at different MT ends, as proposed in Scheme 1. It is notable that differential functions of XMAP215/TOGp at minus- and plus-ends are also consistent with in vitro experiments, which have shown that MT polymerization/depolymerization rates are only increased at MT plus-ends (Vasquez et al, 1994). Interestingly, unpublished observations indicate that XMAP215/TOGp actually stabilizes MTs at the minus-ends similarly to a conventional MAP, which would be consistent with mechanism 1 of TOGp function (Lynne Cassimeris, Lehigh University, personal communication).
While overexpressed TOGp exerted the expected counteraction of MCAK during spindle formation (Figure 5), analysis of interphase cells revealed that ectopic TOGp exerted an unexpected destabilization of MTs (Figure 7). It is presently unclear how TOGp could function differently during interphase and mitosis, but this may be explained by cell cycle-regulated phosphorylation of TOGp by CDK1 (Vasquez et al, 1999). The observed destabilization of interphase MTs by ectopic TOGp is strictly dependent on the presence of endogenous MCAK (Figure 7), which provides an additional example of functional interplay between these two proteins. However, destabilization of interphase MTs by overexpressed MCAK was unaltered by TOGp depletion (Figure 6C), which indicates a nonreciprocal dependency of these two proteins under overexpression conditions during interphase. The observed TOGp-dependent destabilization of interphase MTs contrasts with the prevailing view of this protein as an important MT stabilizer. However, a plus-end specific MT-destabilizing activity of purified XMAP215/TOGp and the budding yeast homolog Stu2p has recently been reported (van Breugel et al, 2003; Shirasu-Hiza et al, 2003). A model was proposed in which the XMAP215/TOGp protein destabilized the pause state of MTs and thereby resulted in increased dynamism (Shirasu-Hiza et al, 2003). However, given that TOGp-mediated MT destabilization in human interphase cells was shown to require MCAK (Figure 7), the relevance of this model in explaining the present observations of destabilization of interphase MTs by overexpressed TOGp alone remains unclear.
Depletion of either TOGp or MCAK, alone or in combination, has no detectable effect on interphase MT stability (Figure 6), and it is notable that all evidence for mechanisms 2 and 3 of TOGp function has been obtained under conditions of overexpression. While overexpression experiments can be mechanistically informative, their physiological relevance is more questionable, since nonphysiological levels of proteins may affect additional targets that do not normally come into play. Therefore, we propose that mechanism 1, which has support from depletion experiments, probably reflects the most physiologically important role of TOGp in human somatic cells. However, given the strong evidence for the importance of mechanism 2 in TOGp function derived from studies of Xenopus egg extracts and MT assembly in vitro (Tournebize et al, 2000; Kinoshita et al, 2001), both mechanisms 2 and 3 of TOGp function may still be physiologically relevant during some developmental stages and/or in some tissue types.
Materials and methods
DNA constructs, transfection, and cell culture
A pMEP4 shuttle vector derivative expressing full-length TOGp was constructed by ligation of a SacII to KpnI fragment of pBS-TOGp (Spittle et al, 2000) between the corresponding sites of a pMEP4 plasmid with a modified multiple cloning site cassette. The pMEP4 shuttle vector derivatives expressing the Xenopus ortholog of MCAK, originally termed XKCM1 (Walczak et al, 1996), have been described previously (Holmfeldt et al, 2002). For simplicity, this derivative is referred to as MCAK in the present study.
Replicating EBV-based shuttle vectors for expression of short hairpin RNA (shRNA) were constructed by replacing the 945-bp XbaI to BamHI fragment of pMEP4, which contains the hMTIIa promotor, with a PCR-generated fragment containing the polymerase III H1-RNA gene promotor (Baer et al, 1990) with specific gene-targeting sequences located downstream. The sequence of the H1 RNA promotor and the design of the gene-targeting sequence was as described previously (Brummelkamp et al, 2002). In brief, a 19-nt sequence from the target transcript was separated by the sequence TTC AAG AGA from the reverse complement of the same sequence, and the sequence TTT TT that serves as a termination signal for RNA polymerase III. The targeting sequences (AA(19 nt)TT), chosen as described by (Elbashir et al, 2001), included the following 19-nt sequences: TOGp (accession: X92474): TGT CTT ACT GGC CTG GCT G; MCAK (accession: NM_006845): GAT CCA ACG CAG TAA TGG T; CaMKIIγ (accession: L07044): CAC CAC CAC AGA AGA TGA G; Op18/stathmin (accession: NM_005563): GTC CCA TGA AGC TGA GGT C. A BLAST search of the NCBI database ensured specific targeting of the cognate mRNA. The coding sequences of PCR-generated fragments were confirmed by nucleotide sequence analysis using the ABI PRISM dye terminator cycle sequencing kit from Perkin-Elmer.
Single transfection and cotransfection using pMEP4 derivatives, and subsequent selection of hygromycin-resistant cell lines over 4–6 days, were performed as described (Gradin et al, 1998), using a total of 18 μg DNA. For co-expression of TOGp and MCAK derivatives, we used equal amounts of DNA in a mix with a total of 18 μg pMEP-derivative DNA. Conditional expression/co-expression was induced from the hMTIIa promoter, which can be suppressed by cultivation in a specifically formulated medium and subsequently induced by 0.5 μM Cd2+ (Gradin et al, 1998). Transfection of replicating shuttle vectors that direct constitutive synthesis of specific interfering shRNA was performed according to the same basic protocol as described for pMEP vectors, with 2.5 μg of each of the shRNA-producing vectors mixed with empty pMEP vector up to a total quantity of 18 μg DNA. For inducible ectopic expression in shRNA-synthesizing cells, we used two distinct protocols. Firstly, in experiments in which TOGp was overexpressed in cells depleted of MCAK, or vice versa, 2.5 μg of the shRNA derivative was mixed with 15.5 μg of the pMEP derivative directing inducible ectopic expression. Secondly, in experiments in which MCAK was expressed in cells co-depleted of both TOGp and MCAK, 2.5 μg of each of the shRNA-producing vectors was mixed with 2.0 μg of pMEP-MCAK up to a total amount of 18 μg DNA. In the latter protocol, the amount of pMEP-MCAK DNA, which has a coding sequence that is not targeted by human-specific shRNA-TOGp, was adjusted to result in induced expression to a level comparable with the endogenous MCAK level. The data from transfection cell lines shown in this report have been reproduced in at least three independent transfection experiments.
Immunoblotting and immunofluorescence
Immunoblotting and subsequent detection using the ECL detection system (Amersham Pharmacia Biotech) were performed using anti-α-tubulin (B-5-1-2, Sigma), affinity-purified rabbit anti-MCAK (Kline-Smith and Walczak, 2002), goat anti-CaMKIIγ (SC-1541, Santa Cruz Biotechnology), rabbit anti-Op18 (Gradin et al, 1998), and rabbit anti-TOGp C-terminus (Cassimeris et al, 2001). Centrosomes were immunolocalized by pericentrin staining, as described previously (Holmfeldt et al, 2003). The same general strategy, combined with an anti-NuMA antibody (clone A73-B/D12, www.neomarkers.com), was used to localize the NuMA protein. Analysis of cellular MT content by flow cytometry (>90% of all cells were included in the acquisition gate and >150 000 cells were collected) was performed using a FACS Calibur instrument (Becton Dickinson) as described previously (Holmfeldt et al, 2001), with modifications allowing determination of MT content at distinct phases of the cell cycle, detailed in Holmfeldt et al (2003). For characterization of spindles by immunofluorescence analysis, cells were permeabilized with saponin (0.2%) in MT-stabilizing buffer and subsequently fixed in 4% paraformaldehyde/0.5% glutaraldehyde, followed by quenching with NaBH4 (Holmfeldt et al, 2001). MTs and DNA were co-stained using Alexa Fluor 488-conjugated anti-α-tubulin and propidium iodide, and analyzed by epifluorescence. Confocal microscopy was performed using a Leica SP2 confocal imager system.
Supplementary Material
Supplementary Material
Acknowledgments
We thank Lynne Cassimeris for supplying anti-TOGp antibodies, unpublished data and helpful comments. We are grateful to Claire Walczak for providing MCAK cDNA, anti-MCAK antibodies, unpublished data and helpful comments, Christian Larroque for TOGp cDNA, Sidney Altman for the H1 RNA promotor, Sofia Edin and Thomas Grundström for the shRNA-CaMKIIγ derivative, and Victoria Shingler for discussions. Alistair Kidd's editing of this manuscript is also appreciated. This work was supported by the Swedish Research Council.
References
- Baer M, Nilsen TW, Costigan C, Altman S (1990) Structure and transcription of a human gene for H1 RNA, the RNA component of human RNase P. Nucleic Acids Res 18: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ (1990) Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 62: 579–589 [DOI] [PubMed] [Google Scholar]
- Bornens M (2002) Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol 14: 25–34 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Gard D, Tran PT, Erickson HP (2001) XMAP215 is a long thin molecule that does not increase microtubule stiffness. J Cell Sci 114: 3025–3033 [DOI] [PubMed] [Google Scholar]
- Charrasse S, Schroeder M, Gauthier-Rouviere C, Ango F, Cassimeris L, Gard DL, Larroque C (1998) The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. J Cell Sci 111: 1371–1383 [DOI] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13: 83–117 [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498 [DOI] [PubMed] [Google Scholar]
- Garcia MA, Koonrugsa N, Toda T (2002) Spindle-kinetochore attachment requires the combined action of Kin I-like Klp5/6 and Alp14/Dis1-MAPs in fission yeast. EMBO J 21: 6015–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL, Kirschner MW (1987) A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol 105: 2203–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely F, Draviam VM, Raff JW (2003) The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev 17: 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin HM, Larsson N, Marklund U, Gullberg M (1998) Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol 140: 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt P, Brattsand G, Gullberg M (2002) MAP4 counteracts microtubule catastrophe promotion but not tubulin-sequestering activity in intact cells. Curr Biol 12: 1034–1039 [DOI] [PubMed] [Google Scholar]
- Holmfeldt P, Brattsand G, Gullberg M (2003) Interphase and monoastral-mitotic phenotypes of overexpressed MAP4 are modulated by free tubulin concentrations. J Cell Sci 116: 3701–3711 [DOI] [PubMed] [Google Scholar]
- Holmfeldt P, Larsson N, Segerman B, Howell B, Morabito J, Cassimeris L, Gullberg M (2001) The catastrophe-promoting activity of ectopic Op18/stathmin is required for disruption of mitotic spindles but not interphase microtubules. Mol Biol Cell 12: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Hyman AA (2003) Dynamics and mechanics of the microtubule plus end. Nature 422: 753–758 [DOI] [PubMed] [Google Scholar]
- Howell B, Deacon H, Cassimeris L (1999) Decreasing oncoprotein 18/stathmin levels reduces microtubule catastrophes and increases microtubule polymer in vivo. J Cell Sci 112: 3713–3722 [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Arnal I, Desai A, Drechsel DN, Hyman AA (2001) Reconstitution of physiological microtubule dynamics using purified components. Science 294: 1340–1343 [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Habermann B, Hyman AA (2002) XMAP215: a key component of the dynamic microtubule cytoskeleton. Trends Cell Biol 12: 267–273 [DOI] [PubMed] [Google Scholar]
- Kline-Smith SL, Walczak CE (2002) The microtubule-destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell 13: 2718–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H, Garcia MA, Toda T (2001) Dis1/TOG universal microtubule adaptors—one MAP for all? J Cell Sci 114: 3805–3812 [DOI] [PubMed] [Google Scholar]
- Salmon ED, Leslie RJ, Saxton WM, Karow ML, McIntosh JR (1984) Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol 99: 2165–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerman B, Holmfeldt P, Morabito J, Cassimeris L, Gullberg M (2003) Autonomous and phosphorylation-responsive microtubule-regulating activities of the N-terminus of Op18/stathmin. J Cell Sci 116: 197–205 [DOI] [PubMed] [Google Scholar]
- Shao RG, Shimizu T, Pommier Y (1997) 7-Hydroxystaurosporine (UCN-01) induces apoptosis in human colon carcinoma and leukemia cells independently of p53. Exp Cell Res 234: 388–397 [DOI] [PubMed] [Google Scholar]
- Shirasu-Hiza M, Coughlin P, Mitchison T (2003) Identification of XMAP215 as a microtubule-destabilizing factor in Xenopus egg extract by biochemical purification. J Cell Biol 161: 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittle C, Charrasse S, Larroque C, Cassimeris L (2000) The interaction of TOGp with microtubules and tubulin. J Biol Chem 275: 20748–20753 [DOI] [PubMed] [Google Scholar]
- Tournebize R, Popov A, Kinoshita K, Ashford AJ, Rybina S, Pozniakovsky A, Mayer TU, Walczak CE, Karsenti E, Hyman AA (2000) Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat Cell Biol 2: 13–19 [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A (1996) XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84: 37–47 [DOI] [PubMed] [Google Scholar]
- van Breugel M, Drechsel D, Hyman A (2003) Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer. J Cell Biol 161: 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RJ, Gard DL, Cassimeris L (1994) XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J Cell Biol 127: 985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RJ, Gard DL, Cassimeris L (1999) Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil Cytoskeleton 43: 310–321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material