Abstract
Amino acid alterations in or flanking conserved motifs making up the active binding sites of penicillin-binding proteins (PBPs) 1a, 2b, and 2x of pneumococci were correlated with changes in affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor for these PBPs. Four penicillin-susceptible (PSSP), eight penicillin-intermediate (PISP), and six penicillin-resistant (PRSP) pneumococci were studied by DNA sequencing of the penicillin-binding sites of the pbp1a, -2x, and -2b genes of strains and by determining 50% inhibitory concentrations of the seven agents for PBP1a, -2x, and -2b. Two PSSP strains had alterations in PBP2x (L546→V) (one strain) or PBP2b (T445→A) (one strain). All eight PISP strains had at least two alterations--T338→P or A or H394→Y in PBP2X and T445→A in BPB2b. All PRSP strains had the same changes seen in PISP strains, as well as T371→A or S substitutions in PBP1a. The two most resistant PRSP strains had a second change in PBP2x (M339→F) in a conserved motif. The affinities of penicillin and ampicillin for all three PBPs were decreased for PRSP and most PISP strains. The affinity of amoxicillin for PBP1a and -2x was decreased only for PRSP. Cefaclor and cefprozil showed decreased affinity of PRSP but not PISP for all three PBPs. Cefuroxime showed decreased affinity of PISP and PRSP for PBP1a and -2x but no change for PBP2b. Cefditoren showed no difference in PBP affinity based on penicillin or cefditoren MICs, indicating a different PBP target for this agent. Overall, the MICs for and PBP affinities of the strains correlated with the changes found in the PBP active binding sites.
The worldwide incidence of infections caused by Streptococcus pneumoniae isolates resistant to penicillin and other antimicrobial agents has increased at an alarming rate during the past 2 decades (2). In a recent U.S. study, penicillin-nonsusceptible pneumococci were found in 50.4% of 1,476 strains, and the prevalence of macrolide-resistant strains was 65.6% in penicillin-resistant strains (20). The higher the penicillin MIC, the more likely it is that the strain will be multidrug resistant. Multidrug-resistant (including resistance to fluoroquinolones) pneumococci have been reported in Hong Kong (19), Canada (7), and Spain (24), and the clonal spread of these strains from country to country and continent to continent is of concern. There is an urgent need for oral β-lactams that can be used for outpatient treatment of infections, such as pneumonia, bronchitis, sinusitis, and otitis media, caused by penicillin- and macrolide-nonsusceptible pneumococci. Amoxicillin has excellent in vitro antipneumococcal activity (1, 3, 18) and has been shown to select for resistant laboratory mutants less frequently than other compounds (27, 29). Previous studies have documented the potent in vitro antipneumococcal activity of cefditoren, with MICs at which 50 and 90% of isolates are inhibited of 0.5 and 1.0 μg/ml, respectively, against penicillin-resistant strains (35, 36). Cefditoren also selects for resistant laboratory mutants very rarely, comparably to amoxicillin (C. L. Clark, K. Nagai, B. E. Dewasse, G. A. Pankuch, L. M. Ednie, M. R. Jacobs, and P. C. Appelbaum, unpublished data).
Penicillin-binding proteins (PBPs) are cell wall transpeptidases that catalyze the assembly of cell wall peptidoglycan by transpeptidation of the peptide side chains of murein units. Six PBPs are found in S. pneumoniae: the five high-molecular-mass PBPs (PBP1a [79.7 kDa], PBP1b [89.6 kDa], PBP2x [82.3 kDa], PBP2a [80.8 kDa], and PBP2b [82.3 kDa]) and one low-molecular-mass PBP (PBP3 [45.2 kDa]) (10-12, 13-18). The high-molecular-mass PBPs are made up of an N-terminal hydrophobic region, a central penicillin-binding domain, and a C-terminal domain. The active site of transpeptidase activity is formed by three conserved amino acid motifs, SXXK, SXN, and KT(S)G. These motifs occur at amino acid positions 370 to 373, 428 to 430, and 557 to 559 in PBP1a, at positions 337 to 340, 395 to 397, and 547 to 549 in PBP2x, and at positions 385 to 388, 442 to 444, and 614 to 616 in PBP2b (17, 18). Changes in these motifs, or in the positions flanking these motifs, are associated with low-affinity variants of the PBPs. These changes are the results of point mutations in strains or recombination of PBP genes with PBP genes of other pneumococci or of viridans streptococci to form mosaic genes. Penicillin resistance in S. pneumoniae is mediated by stepwise alterations of PBPs (18). Specifically, decreased affinity of PBP1a, -2x, and -2b for β-lactams has been reported to play an important part in resistance (4-6, 18, 23, 32). Alterations in the conserved motifs in PBP2b are associated with resistance to penicillin (14), and alterations in PBP2x mediate low-level resistance to cephalosporins (4, 8, 12, 14). Additional alterations in PBP1a raise penicillin G MICs to ≥1 μg/ml and cefotaxime MICs to ≥0.5 μg/ml (4, 26, 33, 37). These three PBPs are considered to be the key targets for these agents and were therefore chosen for examination in this study.
The purpose of this study was to determine the association between PBP affinity for various β-lactams and the gene sequences of the conserved motifs of selected PBPs, using a selection of penicillin-susceptible, -intermediate, and -resistant pneumococci. The β-lactams used, penicillin G, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor, were chosen to represent commonly used therapeutic agents or, in the case of cefditoren, a new potent oral cephalosporin.
MATERIALS AND METHODS
Strains.
Eighteen recent clinical pneumococcal strains (four penicillin-susceptible [PSSP] strains for which the penicillin MICs were ≤0.06 μg/ml, eight penicillin-intermediate [PISP] strains for which the penicillin MICs were 0.125 to 1.0 μg/ml, and six penicillin-resistant [PRSP] strains for which the penicillin MICs were ≥2.0 μg/ml) in our collection were used for this study. The strains were not clonally related and were all isolated from different geographic locations. NCCLS breakpoints were used for interpretation of penicillin G susceptibility categories (28).
MIC testing.
MIC testing was done by the agar dilution method using Mueller-Hinton agar with 5% defibrinated sheep blood as previously described (35, 36). Plates were incubated for 20 h in air after inoculation. Susceptibility testing was performed for penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor. Amoxicillin was obtained from SmithKline Beecham (King of Prussia, Pa.), cefditoren was obtained from Meiji Seika Kaisha (Yokohama, Japan), and the other compounds were obtained from their respective manufacturers.
Sequencing of pbp1a, -2x, and -2b genes by PCR.
Template DNA for PCR was prepared using the Prep-A-Gene kit (Bio-Rad, Hercules, Calif.) as recommended by the manufacturer. A 1.2-kb segment of pbp1a was amplified using the primers and cycling conditions described by Asahi and Ubukata (5). A 2-kb segment of pbp2x and a 1.4-kb segment of pbp2b were amplified using primers and cycling conditions described previously (23, 38). PCR products were purified from primers and excess nucleotides using a QIAquick PCR purification kit (Qiagen, Valencia, Calif.) as recommended by the manufacturer and were sequenced directly with an Applied Biosystems model 373A DNA sequencer using the following additional primers: for pbp1a, 5′-2377GCAAGTAGTGAAAAGATGGCT2400-3′, 5′-2545TGTCGGTCATCATATAGGC2527-3′, and 5′-2963GATTGTGAAGTTGAACTATCTG2944-3′; for pbp2x, 5′-991GGAACAGAACAAGTTTCCCAAC1112-3′, 5′-1354GATGCCACGATTCGAGATTGGG1375-3′, 5′-1645TTTACAGCTATTGCTATTGATGG1667-3′, 5′-1488CCAGGTAGCATCTCCCAT1471-3′, and 5′-2105AGAGAGTCTTTCATAGCTGAAGC2083-3′; for pbp2b, 5′-1196TTGCTGAAAAGTTATTTCAATTC1218-3′, 5′-1466ATTGTCTTCCAAGGTTCAGCT1486-3′, 5′-1775TTCCTTTGGGCAGTTTGATAAC1794-3′, and 5′-CAACAATACGAGGAGCCACA-3′.
The results of DNA sequencing were aligned using Vector NTI 6.0 (Infomax, Inc., Bethesda, Md.) and compared to DNA and amino acid sequences from the penicillin-susceptible S. pneumoniae R6 strain as previously reported (10, 15, 21, 22, 25, 31, 37, 39).
PBP binding assays.
PBP binding assays were performed as described previously (27). Five hundred microliters of early-log-phase culture was centrifuged for 3 min at 5,000 × g. The cells were resuspended in 0.1 M phosphate buffer containing unlabeled drug and incubated for 15 min at 37°C. [3H]benzylpenicillin (2 μCi; Amersham Life Sciences, Piscataway, N.J.) was added, and the mixture was incubated for 15 min at 37°C. the cells were lysed by the addition of 0.1 M phosphate buffer with 0.1% Triton X-100. A 10-fold excess of unlabeled benzylpenicillin was added, and the mixture was incubated for 15 min at 37°C. Samples were resuspended in 40 μl of lysis buffer (2% sodium dodecyl sulfate, 4% 2-mercaptoethanal, 10% glycerol, and 0.002% bromphenol blue in 1 M Tris [pH 6.8] buffer) and heated to 100°C for 3 min. Labeled PBPs were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 10% gel and visualized by fluorography using preflashed Hyperfilm MP (Amersham Pharmacia Biotech, Piscataway, N.J.). PBP bands were interpreted by determining 50% inhibitory concentrations (IC50s) for PBP1a, PBP2x, and PBP2b bands as follows. Fluorograms were converted to electronic format using a video camera, and the IC50s of each agent were determined for the PBP bands based on the intensity of each band using Molecular Analyst software (Bio-Rad, Inc.). IC50s were defined as the concentration of each agent that resulted in a 50% decrease in intensity of PBP bands on fluorograms compared to controls without the addition of unlabeled competitive compounds (30, 31). Saturation of PBPs by radiolabeled benzylpenicillin in competition experiments was addressed as described previously (30, 31).
Statistical analysis.
Geometric mean MICs and correlation coefficients were determined using the statistical functions of Microsoft Excel 2000 (Microsoft Corporation, Redmond, Wash.).
RESULTS
Susceptibility of pneumococcal strains.
The origins of 18 clinical pneumococcal isolates are shown in Table 1. Clinical isolates from various countries and patients were chosen for this study. The MICs of the pneumococcal strains tested are shown in Table 2. The geometric mean values of MICs (and MIC ranges) in micrograms per milliliter for four PSSP strains were as follows: penicillin, 0.04 (0.03 to 0.06); amoxicillin, 0.03 (0.015 to 0.03); ampicillin, 0.08 (0.06 to 0.25); cefditoren, 0.03 (0.015 to 0.06); cefuroxime, 0.15 (0.06 to 0.25); cefprozil, 0.15 (0.06 to 0.5); and cefaclor, 0.6 (0.25 to 1). Those for eight PISP strains were as follows: penicillin, 0.25 (0.125 to 1); amoxicillin, 0.2 (0.06 to 1); ampicillin, 0.25 (0.06 to 1); cefditoren, 0.1 (0.03 to 0.25); cefuroxime, 1 (0.25 to 4); cefprozil, 0.8 (0.25 to 2); and cefaclor, 1.4 (0.5 to 8). Those for six PRSP strains were as follows: penicillin, 2.8 (2 to 4); amoxicillin, 2.5 (1 to 8); ampicillin, 6.3 (2 to 16); cefditoren, 1.3 (0.5 to 4); cefuroxime, 10.1 (4 to 32); cefprozil, 20.2 (16 to 32); cefaclor, 160 (64 to >256). The mean MICs for PISP were generally 2- to 6-fold higher than those for PSSP, whereas the geometric mean MICs for PRSP were generally 40- to 80-fold higher than those for PSSP and 10- to 25-fold higher than for PISP. However, there were some exceptions to these findings, with the mean MICs of cefprozil and cefaclor for PRSP being over 100-fold higher than the mean MICs for PSSP and those of cefaclor for PRSP being over 100-fold higher than the mean MICs for PISP.
TABLE 1.
Origins of 18 pneumococcal isolates tested in this study
| Strain | Sample no. | Age of patient (yr) | Site of isolation | Country |
|---|---|---|---|---|
| 1 | 49 | Unknown | Unknown | United States |
| 2 | 768 | 2 | Maxillary sinus | Mexico |
| 3 | 875 | 3 | Eye | United States |
| 4 | 891 | 78 | Sputum | United States |
| 5 | 731 | <1 | Ear | United States |
| 6 | 737 | 4 | Maxillary sinus | United States |
| 7 | 914 | <1 | Ear | United States |
| 8 | 714 | 12 | Blood | United States |
| 9 | 420 | Unknown | Nasopharynx | Bulgaria |
| 10 | 850 | 41 | Sputum | United States |
| 11 | 755 | 87 | Eye | United States |
| 12 | 866 | <1 | Eye | United States |
| 13 | 997 | Unknown | Blood | Canada |
| 14 | 703 | 3 | Ear | Mexico |
| 15 | 500 | Unknown | Blood | Korea |
| 16 | 799 | 3 | Nasopharynx | United States |
| 17 | 810 | 56 | Sputum | United States |
| 18 | 775 | 22 | Nasopharynx | United States |
TABLE 2.
MICs of seven agents against 18 study strains
| Strain | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| Penicillin G | Amoxicillin | Ampicillin | Cefditoren | Cefuroxime | Cefprozil | Cefaclor | |
| PSSP | |||||||
| 1 | 0.03 | 0.03 | 0.06 | 0.015 | 0.06 | 0.125 | 1 |
| 2 | 0.06 | 0.015 | 0.06 | 0.015 | 0.125 | 0.06 | 0.25 |
| 3 | 0.06 | 0.03 | 0.25 | 0.06 | 0.25 | 0.5 | 1 |
| 4 | 0.03 | 0.03 | 0.06 | 0.06 | 0.25 | 0.125 | 0.5 |
| PISP | |||||||
| 5 | 0.125 | 0.06 | 0.06 | 0.03 | 0.5 | 0.5 | 0.5 |
| 6 | 0.125 | 0.125 | 0.125 | 0.03 | 0.25 | 0.25 | 0.5 |
| 7 | 0.5 | 0.125 | 0.125 | 0.25 | 1 | 0.5 | 0.5 |
| 8 | 1 | 0.125 | 0.25 | 0.25 | 4 | 1 | 4 |
| 9 | 0.25 | 0.25 | 0.5 | 0.06 | 0.5 | 1 | 1 |
| 10 | 0.125 | 0.125 | 0.125 | 0.06 | 2 | 2 | 8 |
| 11 | 0.25 | 1 | 1 | 0.25 | 2 | 2 | 2 |
| 12 | 0.25 | 0.5 | 1 | 0.125 | 1 | 1 | 2 |
| PRSP | |||||||
| 13 | 2 | 1 | 8 | 1 | 8 | 16 | 128 |
| 14 | 4 | 1 | 2 | 1 | 8 | 16 | 64 |
| 15 | 2 | 2 | 8 | 1 | 8 | 16 | 128 |
| 16 | 2 | 2 | 2 | 0.5 | 4 | 16 | 64 |
| 17 | 4 | 8 | 16 | 2 | 6 | 32 | >256 |
| 18 | 4 | 8 | 16 | 4 | 32 | 32 | >256 |
DNA sequencing and amino acid alterations in PBP1a, -2x, and -2b.
The amino acid sequences of the three conserved PBP motifs of the three PBPs studied are shown in Table 3. There were no changes in the conserved motifs of PBP1a for PSSP or PISP, while all PRSP had T371→A or S substitutions in the STMK motif and no changes in the other two PBP1a motifs.
TABLE 3.
Sequences of the three conserved amino acid motifs of PBPs 1a, 2x, and 2b that form the active penicillin-binding site cavities of the PBPs in S. pneumoniae R6 and the 18 study strains (sequences flanking these motifs are also shown where relevant)
| Strain | Changes in amino acids of conserved PBP sites making up active penicillin-binding site of PBP:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1a
|
2x
|
2b
|
|||||||
| 370-373a | 428-430 | 557-559 | 337-340 | 394-397 | 546-549 | 385-388 | 442-445 | 614-616 | |
| R6 | STMKb | SRN | KTG | STMK | HSSN | LKSG | SVVK | SSNT | KTG |
| 1 | -c | - | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | VKSG | - | - | - |
| 3 | - | - | - | - | - | - | - | SSNA | - |
| 4 | - | - | - | - | - | - | - | - | - |
| 5 | - | - | - | - | YSSN | - | - | SSNA | - |
| 6 | - | - | - | SPMK | - | - | - | SSNA | - |
| 7 | - | - | - | - | YSSN | - | - | SSNA | - |
| 8 | - | - | - | SPMK | - | - | - | SSNA | - |
| 9 | - | - | - | SAMK | - | - | - | SSNA | - |
| 10 | - | - | - | SAMK | - | - | - | SSNA | - |
| 11 | - | - | - | SAMK | - | VKSG | - | SSNA | - |
| 12 | - | - | - | SAMK | - | VKSG | - | SSNA | - |
| 13 | SAMKd | - | - | SAMK | - | VKSG | - | SSNA | - |
| 14 | SAMK | - | - | SAMK | - | VKSG | - | SSNA | - |
| 15 | SAMK | - | - | SAMK | - | VKSG | - | SSNA | - |
| 16 | SAMK | - | - | SAMK | - | VKSG | - | SSNA | - |
| 17 | SSMK | - | - | SAFK | - | VKSG | - | SSNA | - |
| 18 | SSMK | - | - | SAFK | - | VKSG | - | SSNA | - |
Amino acid position numbers of amino acids shown in column below.
For S. pneumoniae R6, conserved amino acid motifs are shown in boldface and flanking amino acids (where applicable) are shown in regular typeface.
-, no change in amino acid motifs of test strains from those of S. pneumoniae R6.
Changes in amino acid motifs of test strains from those of S. pneumoniae R6 are shown in boldface for study strains.
The STMK motif of PBP2x showed no changes in PSSP strains, while T338→A or P substitutions were found in six of eight PISP and in all PRSP strains. Additionally, the two most resistant PRSP strains (strains 17 and 18) showed M339→F substitutions. Changes in the amino acid before the other two motifs of PBP2x were found in two PISP strains for the SSN motif (H394→Y) and for one PSSP, two PISP, and all PRSP strains for the KSG motif (L546→V).
The only change found in the motifs of PBP2b were a T444→A substitution in the position following the central SSN motif. This change was found in one PSSP and all PISP and PRSP strains.
Other amino acid changes noted were a Y insertion in PBP2b between positions 429 and 430 in PISP strain 10. There were many other amino acid changes at other positions not known to be associated with the active binding sites of these enzymes.
Affinities of agents tested for PBP1a, -2x, and -2b.
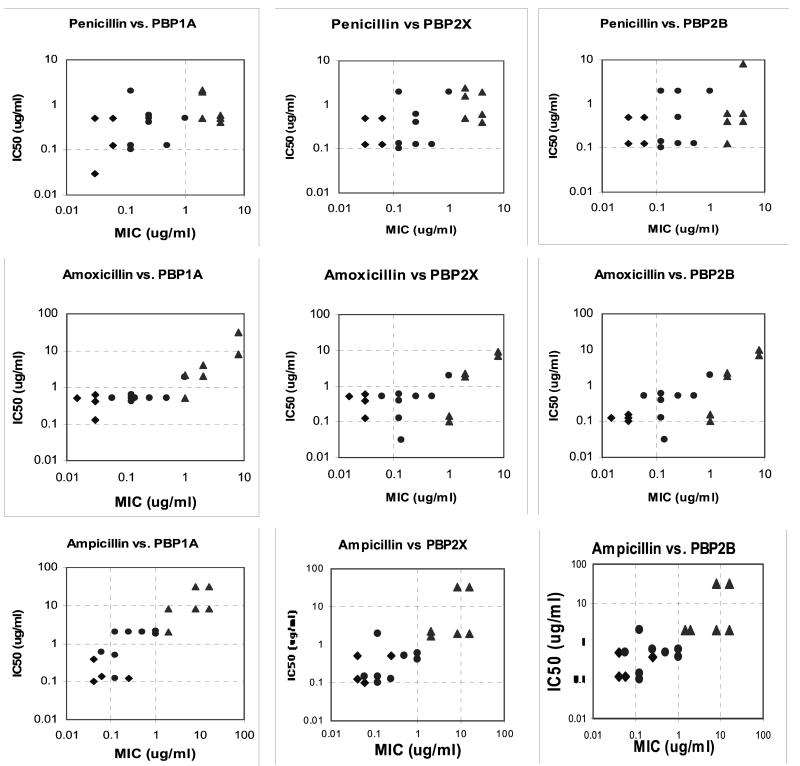
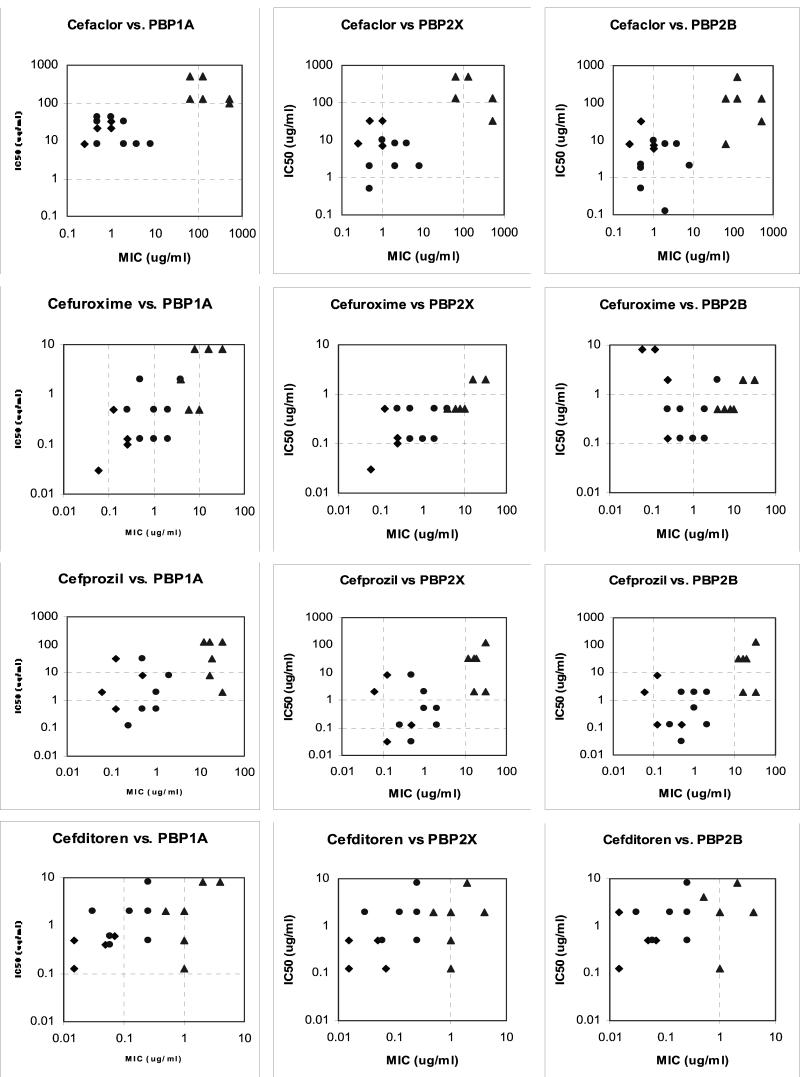
Graphs of MICs versus IC50s for PBP1a, -2x, and -2b of the antimicrobial agents for the 18 pneumococcal strains tested are shown in Fig. 1. Overall, the affinity of penicillin for each PBP generally decreased as the MICs increased, but overall agreement was poor (R2 = 0.23 for PBP1a, 0.27 for PBP2x, and 0.12 for PBP2b). Ampicillin showed a more striking correlation between PBP affinity and MICs for all three PBPs, with a more linear relationship for all three PBPs tested than was found with the other agents tested in this study (R2 = 0.76 for PBP1a, 0.65 for PBP2x, and 0.62 for PBP2b). Amoxicillin showed a different affinity pattern, with a bimodal pattern for all three PBPs separating most of the strains for which the amoxicillin MICs were <1 μg/ml from those for which the amoxicillin MICs were ≥1 μg/ml. The amoxicillin MICs of PRSP strains 14 and 15 (both 1 μg/ml) did not appear to be related to affinity for PBP2x or PBP2b (IC50s, 0.1 to 0.15 μg/ml). Cefaclor showed a clear bimodal distribution separating PSSP-PISP strains from PRSP strains. Cefprozil showed a bimodal distribution of PBP affinities similar to that of cefaclor, but the decrease in PBP affinities was disproportionate to the rise in MICs, suggesting another target for this agent. Cefuroxime showed a pattern different from that seen with the other agents studied, with linear correlation between affinities and MICs for PBP1a (R2 = 0.54) and -2x (R2 = 0.52) but no relationship for PBP2b. In fact, the affinities of PISP and PRSP strains for PBP2b were higher than those of some PSSP strains. Cefditoren showed no clear association between PBP affinities and MICs, and increases in MICs did not appear related to affinities for the three PBPs tested.
FIG. 1.
Correlation between MICs and IC50s for PBPs 1a, 2x, and 2b of PSSP (⧫), PISP (•), and PRSP (▵) for the agents tested.
Correlation of MICs, PBP mutations, and PBP affinities.
The associations shown in this study between MIC changes and PBP affinity changes show which agents bind to the PBPs studied and whether the PBP affinities of these agents change as the MICs rise. However, correlation of these changes with changes in the conserved motifs of the PBP binding sites was more complex. Nevertheless, it appears that the single changes detected either in the STMK motif or flanking the SSN motifs of PBP2x are associated with decreased susceptibility and affinity of PISP strains for penicillin, and addition of a change in the STMK motif of PBP1a is associated with decreased susceptibility and affinity of PRSP strains. No changes in any of the three motifs in PBP1a were detected in PSSP or PISP strains. The changes found in the amino acids preceding the SSN motif of PBP2x (H394→Y) and following the SSN motif of PBP2b (T445→A) show no clear associations with the penicillin MIC or affinity changes but could contribute to resistance when present with changes in other key motifs of the PBPs.
DISCUSSION
PBP1a, -2x, and -2b are generally recognized as the major PBPs associated with the activities of penicillins and some cephalosporins (e.g., cefaclor and cefprozil), and PBP1a and -2x are generally recognized as the major PBPs associated with those of other cephalosporins (e.g., cefotaxime and cefuroxime) (15, 17). However, other PBPs and other mechanisms, such as altered stem peptide (mur) genes, altered histidine protein kinase CiaH, and altered glycosyltransferase CpoA, can also affect β-lactam activity (16, 21, 34). The low-affinity variants of PBPs are the results of recombination of the genes coding for these proteins with genes of other species, such as viridans streptococci. Much work has been done on laboratory mutants with defined point mutations in PBPs, but these relationships are more difficult to determine in clinical isolates. Laboratory transformation experiments have shown that low-affinity forms of PBP2b and -2x confer low-level penicillin resistance and are prerequisites for high-level resistance (12, 14, 17, 22). High-level penicillin resistance requires the presence of a low-affinity form of PBP1a as well as either low-affinity PBP2x or low-affinity PBP2x and -2b (17, 33). Our findings were in general agreement with these reports, with the affinities of isolated PBPs, as well as the PBP active binding site genes, confirming these relationships.
The changes we found in the STMK motif of PBP2x (T338→A or P substitution) are identical to those previously reported (4, 8). However, the two most resistant strains we studied, strains 17 and 18, had an additional M339→F substitution, which has been reported in clinical isolates of cefotaxime-resistant strains in Japan (cefotaxime MICs, 2 to 8 μg/ml) and the United States (cefotaxime MICs, 8 to 32 μg/ml) (4, 8). These two strains were also remarkable for having higher amoxicillin and ampicillin MICs than penicillin MICs and very high cefaclor MICs (>256 μg/ml). These two amino acid substitutions in the STMK motif of PBP2x have been shown to increase cefotaxime MICs from 0.016 to 1 μg/ml in pneumococcal transformants (4). Additional strains need to be examined to determine the significance of this finding. Hakenbeck and coworkers also reported PBP2x T550→A and Q552→E substitutions, which are close to the K547SG motif, to be associated with cephalosporin resistance in a laboratory mutant of pneumococci (17). We found no T550 substitutions and could not confirm this association. However, we did find Q552→E substitutions in two PSSP and three PISP without significant cephalosporin resistance in these strains, suggesting that the reported association may not be valid in clinical isolates.
The PBP2b T445→A substitution found in all PISP and PRSP and one PSSP has been found in all low-level β-lactam-resistant pneumococci examined, as well as in Streptococcus mutans (11, 14). A PBP1a with a T371→A or S substitution has also been associated with decreased β-lactam affinity of this PBP and high-level penicillin resistance, as we found in this study (4, 15, 33). A 4-amino-acid substitution in PBP1a, T574SQF→NTGY, has also been described as being important in PBP1a with respect to the interaction with penicillin and the development of resistance (33). We found this change in strains 7, 9, 10, and 12 to 18 in this study, but we are unsure of its significance, as this change was not near one of the PBP penicillin-binding sites and showed no clear correlation with susceptibility results.
Our study has extended our knowledge of the complex interrelationships among MICs, PBP affinities, and PBP binding site changes, utilizing clinical isolates. Single-amino-acid substitutions in or flanking the STMK motif of PBP2x and the SSN motif of PBP2b appear to be the predominant changes in PISP strains. A substitution in the STMK motif of PBP1a, in addition to the changes associated with PISP strains, appears to correlate with PRSP strains. An intriguing finding of our study is a second substitution in the STMK motif of PBP2x, associated with the most resistant strains and strains with higher aminopenicillin than penicillin MICs. The associations demonstrated among MICs, PBP affinities, and PBP structures are useful in illustrating the mechanisms underlying the MIC variations of the β-lactams studied. In particular, these associations may explain the improved activity of amoxicillin compared to penicillin against many PISP strains and the higher MICs of cefuroxime, cefprozil, and cefaclor against PRSP strains. However, we have no explanation for the lack of correlation between PBP affinities and MICs for cefditoren. The affinity of amoxicillin for PBP2b decreased for PISP and PRSP strains, but the affinity of this agent for PBP1a and -2x did not decrease for most PISP strains, in agreement with the amoxicillin MICs (0.125 μg/ml) for some PISP, such as strains 7 and 8, being lower than the penicillin MICs (0.5 to 1 μg/ml).
Substitutions outside the specific areas examined might also contribute to resistance, either by providing compensatory structural alterations stabilizing the primary active-site mutation or by alterations further affecting the active-site structure and function directly via a distance effect. In addition, other mechanisms are involved in the activities of β-lactams in pneumococci. Filipe and Tomasz (13) have postulated that more distant mutations, such as murM and murN genes, which code for stem peptides that cross-link peptidoglycan chains, might play a role in the expression of penicillin resistance in pneumococci. These aspects were not explored in the present study. Additionally, it is recognized that the methodological limitations inherent in IC50 determinations might have limited our ability to correlate these results with MICs. Also, what is actually measured in competition assays is complex and includes acylation, deacylation, and binding reactions. Additional experiments, such as transformation of modified PBPs from selected strains with raised penicillin MICs into susceptible strains to see if the PBP changes are responsible for the level of resistance found in clinical isolates may also yield valuable information.
We should not forget that these relationships are not the only determinants of the clinical relevance of these agents, which is also determined by the pharmacokinetic parameters achieved with the dosing regimen chosen and the pharmacodynamic properties of the agent (9).
Acknowledgments
This study was supported in part by grants from Glaxo SmithKline Pharmaceuticals (King of Prussia, Pa.) and TAP Pharmaceutical Products, Inc. (Lake Forest, Ill.).
We thank Kimiko Ubukata for her critical comments on the manuscript.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 2000. Microbiological and pharmacodynamic considerations in the treatment of infection due to antimicrobial-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 31(Suppl. 2):S29-S34. [DOI] [PubMed] [Google Scholar]
- 4.Asahi, Y., Y. Takeuchi, and K. Ubukata. 1999. Diversity of substitutions within or adjacent to conserved amino acid motifs of penicillin-binding protein 2X in cephalosporin-resistant Streptococcus pneumoniae isolates. Antimicrob. Agents Chemother. 43:1252-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahi, Y., and K. Ubukata. 1998. Association of a Thr-371 substitution in a conserved amino acid motif of penicillin-binding protein 1A with penicillin resistance of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2267-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcus, V. A., K. Ghanekar, M. Yeo, T. J. Coffey, and C. G. Dowson. 1995. Genetics of high level penicillin resistance in clinical isolates of Streptococcus pneumoniae. FEMS Microbiol. Lett. 126:299-303. [DOI] [PubMed] [Google Scholar]
- 7.Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 8.Coffey, T. J., M. Daniels, L. K. McDougal, C. G. Dowson, F. C. Tenover, and B. G. Spratt. 1995. Genetic analysis of clinical isolates of Streptococcus pneumoniae with high-level resistance to expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 39:1306-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Dowson, C. G., A. Hutchison, and B. G. Spratt. 1989. Nucleotide sequence of the penicillin-binding protein 2B gene of Streptococcus pneumoniae strain R6. Nucleic Acids Res. 17:7518.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowson, C. G., T. J. Coffey, C. Kell, and R. A. Whiley. 1993. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol. Microbiol. 9:635-643. [DOI] [PubMed] [Google Scholar]
- 12.Dowson, C. G., A. P. Johnson, E. Cercenado, and R. C. George. 1994. Genetics of oxacillin resistance in clinical isolates of Streptococcus pneumoniae that are oxacillin resistant and penicillin susceptible. Antimicrob. Agents Chemother. 38:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filipe, S. R., and A. Tomasz. 2000. Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc. Natl. Acad Sci. 97:4891-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grebe, T., and R. Hakenbeck. 1996. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of β-lactam antibiotics. Antimicrob. Agents Chemother. 40:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakenbeck, R., A. König, I. Kern, M. van der Linden, W. Keck, D. Billot-Klein, R. Legrand, B. Schoot, and L. Gutmann. 1998. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level β-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J. Bacteriol. 180:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakenbeck, R., T. Grebe, D. Zähner, and J. B. Stock. 1999. β-Lactam resistance in Streptococcus pneumoniae: penicillin-binding proteins and non-penicillin-binding proteins. Mol. Microbiol. 33:673-678. [DOI] [PubMed] [Google Scholar]
- 17.Hakenbeck, R., K. Kaminski, A. König, M. van der Linden, and J. R. Paik. 1999. Penicillin-binding proteins in beta-lactam-resistant Streptococcus pneumoniae. Microb. Drug Resist. 5:91-99. [DOI] [PubMed] [Google Scholar]
- 18.Hakenbeck, R. 1999. β-Lactam-resistant Streptococcus pneumoniae: epidemiology and evolutionary mechanism. Chemotherapy 45:83-94. [DOI] [PubMed] [Google Scholar]
- 19.Ho P.-K., T.-L. Que, N.-C. Tsang, et al. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs, M. R., S. Bajaksouzian, A. Zilles, G. Lin, et al. 1999. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. Surveillance study. Antimicrob. Agents Chemother. 43:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauss, J., and R. Hakenbeck. 1997. A mutation in the d,d-carboxypeptidase penicillin-binding protein 3 of Streptococcus pneumoniae contributes to cefotaxime resistance of the laboratory mutant C604. Antimicrob. Agents Chemother. 41:936-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laible, G., R. Hakenbeck, M. A. Sicard, B. Joris, and J. M. Ghuysen. 1989. Nucleotide sequences of the pbp2X genes encoding the penicillin-binding proteins 2X from Streptococcus pneumoniae R6 and a cefotaxime-resistant mutant, C506. Mol. Microbiol. 3:1337-1348. [DOI] [PubMed] [Google Scholar]
- 23.Laible, G., B. G. Spratt, and R. Hakenbeck. 1991. Interspecies recombinational events during the evolution of altered PBP2X genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5:1993-2002. [DOI] [PubMed] [Google Scholar]
- 24.Liñares, J., A. G. de la Campa, and R. Pállares. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. N. Engl. J. Med. 341:1546-1547. [DOI] [PubMed] [Google Scholar]
- 25.Martin, C., B. Thomas, and R. Hakenbeck. 1992. Nucleotide sequences of genes encoding penicillin-binding proteins from Streptococcus pneumoniae and Streptococcus oralis with high homology to Escherichia coli penicillin-binding proteins 1A and 1B. J. Bacteriol. 174:4517-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz, R., C. G. Dowson, M. Daniels, T. J. Coffey, C. Martin, R. Hakenbeck, and B. G. Spratt. 1992. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 6:2461-2465. [DOI] [PubMed] [Google Scholar]
- 27.Nagai, K., T. A. Davies, B. E. Dewasse, G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro development of resistance to ceftriaxone, cefprozil and azithromycin in Streptococcus pneumoniae. J. Antimicrob. Chemother. 46:909-915. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 29.Pankuch, G. A., S. A. Jueneman, T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 1998. In vitro selection of resistance to four β-lactams and azithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2914-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sifaoui, F., M. D. Kitzis, and L. Gutmann. 1996. In vitro selection of one-step mutants of Streptococcus pneumoniae resistant to different oral β-lactam antibiotics is associated with alteration of PBP2X. Antimicrob. Agents Chemother. 40:152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sifaoui, F., E. Varon, M. D. Kitzis, and L. Gutmann. 1998. In vitro activity of sanfetrinem and affinity for the penicillin-binding proteins of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:173-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, A. M., and K. P. Klugman. 1995. Alterations in penicillin-binding protein 2B from penicillin-resistant wild-type strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 39:859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, A. M., and K. P. Klugman. 1998. Alterations in PBP1A essential for high-level penicillin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, A. M., and K. P. Klugman. 2001. Alterations in MurM, a cell wall muropeptide branching enzyme, increase high-level penicillin and cephalosporin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2393-2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spangler, S. K., M. R. Jacobs, and P. C. Appelbaum. 1996. Activities of RPR 106972 (a new oral streptogramin), cefditoren (a new oral cephalosporin), two new oxazolidiones (U-100592 and U-100766), and other oral and parenteral agents against 203 penicillin-susceptible and -resistant pneumococci. Antimicrob. Agents Chemother. 40:481-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spangler, S. K., M. R. Jacobs, and P. C. Appelbaum. 1997. Time-kill studies on susceptibility of nine penicillin-susceptible and -resistant pneumococci to cefditoren compared with nine other β-lactams. J. Antimicrob. Chemother. 41:1033-1036. [DOI] [PubMed] [Google Scholar]
- 37.Ubukata, K., T. Muraki, A. Igarashi, Y. Asahi, and M. Konno. 1997. Identification of penicillin and other beta-lactam resistance in Streptococcus pneumoniae by polymerase chain reaction. J. Infect. Chemother. 3:190-197. [DOI] [PubMed] [Google Scholar]
- 38.Yamane, A., H. Nakano, Y. Asahi, K. Ubukata, and M. Konno. 1996. Directly repeated insertion of 9-nucleotide sequence detected in penicillin-binding protein 2B gene of penicillin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:1257-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao, G., T. I. Meier, J. Hoskins, and K. A. McAllister. 2000. Identification and characterization of the penicillin-binding protein 2a of Streptococcus pneumoniae and its possible role in resistance to β-lactam antibiotics. Antimicrob. Agents Chemother. 44:1745-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]