Abstract
In this crossover study in 12 healthy volunteers, coadministration of amprenavir (1,200 mg; single dose) with grapefruit juice slightly reduced the maximum concentration of drug in serum (Cmax) compared to administration with water (7.11 versus 9.10 μg/ml), slightly increased the time to Cmax (1.13 versus 0.75 h), and did not affect the area under the concentration-time curve from 0 to 12 h (AUC0-12), the AUC0-∞, or the concentration at 12 h. Therefore, grapefruit juice does not clinically significantly affect amprenavir pharmacokinetics.
The potent human immunodeficiency virus type 1 (HIV-1) protease inhibitor amprenavir is a substrate and inhibitor of cytochrome P450 3A4. Some components of grapefruit juice, including flavonoids, naringin, and 6′,7′-dihydroxybergamottin, could potentially reduce first-pass metabolism of amprenavir since they also inhibit enterocyte cytochrome P450 3A4 (1). A previous study showed that coadministration of grapefruit juice with another HIV-1 protease inhibitor, saquinavir, increased bioavailability twofold (4). The primary objective of this study was to investigate whether there was a drug interaction between amprenavir and grapefruit juice. The secondary objective was to evaluate the safety of amprenavir in combination with grapefruit juice.
(This study was previously presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Ontario, Canada, 17 to 20 September 2000 [D. Demarles, C. Gillotin, S. Bonaventure-Paci, I. Vincent, S. Fosse, and A. M. Taburet, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1643, 2000].)
This was a phase I, open-label, randomized, single-dose, two-period, crossover study. The study protocol was reviewed and approved by the Ethical Review Committee of Poitou-Charentes and the study was done in accordance with the version of the Declaration of Helsinki applicable at the time. We estimated that using 12 volunteers would give 90% power to detect an increase of at least 25% in the area under the concentration-time curve (AUC) for amprenavir. The calculation was based on the intrasubject variability of the amprenavir AUC estimated at 17% in a previous study (5). Therefore, we recruited 12 healthy adult volunteers (6 males and 6 females). Key inclusion criteria were weight between 55 and 95 kg, body mass index from 20 to 28 kg/m2, HIV seronegativity, and a negative pregnancy test for females. We collected 17 blood samples over 24 h for each treatment period and monitored volunteers for adverse events. Standard hematological and clinical chemistry assessments were done. The volunteers received a single dose of 1,200 mg of amprenavir in a soft gelatin capsule (Agenerase; Glaxo SmithKline, Marly-le-Roi, France) with 200 ml of water or grapefruit juice (Minute Maid; Coca Cola, Paris, France) on two occasions separated by a 3-day washout period. Amprenavir concentrations in plasma were determined using a validated high-performance liquid chromatography assay (2). The limit of quantification was 40 ng/ml.
Pharmacokinetic parameters of amprenavir were calculated using noncompartmental methods with WinNonlin Pro Software (version 1.5; Scientific Consulting Inc., Cary, N.C.). The maximum concentration (Cmax), the time to Cmax (Tmax), the concentration at 12 h (C12), the last quantifiable concentration following dosing (Clast), and the time to Clast (Tlast) were taken from the raw data. The AUCs from 0 to 12 h (AUC0-12) and from 0 h to Tlast (AUClast) were calculated using the linear trapezoidal method. Individual estimates of the terminal elimination rate constant (lambda-z) for amprenavir were obtained by log-linear regression of the terminal portions of the plasma concentration-versus-time curves. The AUC from 0 h to infinity (AUC0-∞) was calculated as AUClast + Clast/lambda-z. Single-dose pharmacokinetics parameters except Tmax were log transformed prior to analysis using analysis of variance, including treatment, sequence of treatment, period, and subject within sequence effects. The effect of gender was initially included to test its significance. No significant gender effects were found, so it was removed from the final model. Estimates of treatment effect were based on the ratio of the geometric least-squares means and presented on the untransformed scale with the associated 90% confidence interval. The analysis of Tmax was based on nonparametric methods. Estimates of median differences between treatment with corresponding 90% confidence intervals were calculated based on the Koch method (3).
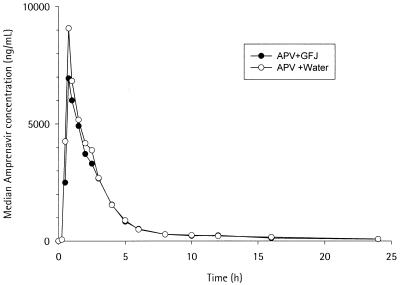
All 12 volunteers completed the study. The mean age was 36.5 years (standard deviation, 10.2) and the mean weight was 63.8 kg (standard deviation, 8.3). The pharmacokinetic parameters after dosing are summarized in Table 1. The addition of grapefruit juice resulted in a 22% decrease in the geometric least-squares mean Cmax and in an increase in the median Tmax (from 0.75 to 1.13 h). The median plasma concentration-time profiles for amprenavir taken with water and with grapefruit juice are presented in Fig. 1. The curves of each combination are superimposable. There were no adverse events, no clinically significant hematologic changes, and no clinical chemistry abnormalities observed in the study.
TABLE 1.
Pharmacokinetic parameters for healthy volunteers after administration of a single 1,200-mg dose of amprenavir with water or in combination with grapefruit juicea
| Parameter | APV plus GFJ | APV plus water | APV plus GFJ/APV plus water ratio | 90% Confidence interval | P value |
|---|---|---|---|---|---|
| Cmaxb (μg/ml) | 7.11 | 9.10 | 0.78 | 0.62-0.99 | 0.09 |
| Tmaxc (h) | 1.13 | 0.75 | 0.19 | 0.00-0.75 | 0.13 |
| C12b (μg/ml) | 0.21 | 0.19 | 1.13 | 0.95-1.35 | 0.22 |
| AUC0-12b (μg · h/ml) | 17.93 | 19.25 | 0.93 | 0.81-1.07 | 0.38 |
| AUC0-∞b (μg · h/ml) | 21.23 | 23.72 | 0.89 | 0.79-1.02 | 0.15 |
APV, amprenavir; GFJ, grapefruit juice.
Geometric least squares mean.
Hodges-Lehmann estimates of median and median difference.
FIG. 1.
Median plasma amprenavir (APV) concentration-time profile after administration of a single 1,200-mg dose of amprenavir with water and in combination with grapefruit juice (GFJ).
In this study we found that the coadministration of grapefruit juice with a single 1,200-mg dose of amprenavir caused only a slight change in the absorption rate of amprenavir: Cmax was reduced and Tmax was increased when amprenavir was taken with grapefruit juice. However, AUC0-12, AUC0-∞, and C12 (which is equivalent to the minimum concentration in serum for the standard 1,200-mg dose of amprenavir) were unaffected. As these latter parameters are more closely correlated with the antiviral response (5), we do not believe that the effects of grapefruit juice on amprenavir pharmacokinetics are clinically significant. The results of this study indicate that gut metabolism of amprenavir is low. Moreover, Sadler et al. showed that administration of a single dose of 600 mg of amprenavir taken with food by HIV-infected patients led to a 14% decrease in AUC0-∞, a delay in Tmax (from 1 to 1.75 h), and a 33% decrease in Cmax (6). Therefore, the change in amprenavir absorption parameters (Cmax and Tmax) by grapefruit juice observed in our study may be comparable to a slight food effect.
In conclusion, we found that coadministration of a single dose of 1,200 mg of amprenavir with grapefruit juice led to small changes in some pharmacokinetic parameters (Cmax and Tmax) but that these changes were not clinically significant. No adverse events were recorded in this study.
Acknowledgments
Funding for this study (PRO10021) was provided by Glaxo SmithKline Research and Development.
We thank Justin Cook for writing and editing assistance during preparation of the manuscript.
REFERENCES
- 1.Bailey, D., J. Malcolm, O. Arnold, and D. Spence. 1998. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 46:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayewardene, A., F. Zhu, F. Awweka, and J. Gambertoglio. 2001. Simple high performance liquid chromatography determination of the protease inhibitor indinavir in human plasma. J. Chromatogr. 707:203-211. [DOI] [PubMed] [Google Scholar]
- 3.Koch, G. 1972. The use of nonparametric methods in the statistical analysis of the two period change-over design. Biometrics 28:577-588. [PubMed] [Google Scholar]
- 4.Kupferschmidt, H., K. Fattinger, H. Reim Ha, F. Follath, and S. Krahenbuhl. 1998. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br. J. Clin. Pharmacol. 45:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler, B., C. Gillotin, Y. Lou, and D. Stein. 2001. In vivo effect of α1-acid glycoprotein on pharmacokinetics of amprenavir, a human immunodeficiency virus protease inhibitor. Antimicrob. Agents Chemother. 45:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadler, B., C. Hanson, G. Chittick, W. Symonds, and N. Roskell. 1999. Safety and pharmacokinetics of amprenavir (141W94), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob. Agents Chemother. 43:1686-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]