Abstract
A new delivery system, Ionic Amphiphilic Biovector (ABV), comprised of anionic lipids (dipalmitoyl phosphatidyl glycerol) included in a cationic cross-linked polysaccharide matrix was used as a reservoir for amphotericin B (AmB). Two ABV formulations exhibited an in vitro and in vivo efficacy similar to commercial AmBisome against Leishmania donovani-infected mice. The higher stability of these ABV formulations indicates their potential for further development and applications.
Drugs available for the treatment of visceral leishmaniasis remain unsatisfactory. Considerable interest has developed in the use of amphotericin B (AmB) for the chemotherapy of visceral leishmaniasis since the worldwide spread of resistance to the first-line pentavalent antimonials (2). However, the use of AmB is limited by its toxic side effects, in particular cardiotoxicity and nephrotoxicity. The use of lipidic formulations (AmBisome, Amphocil, and Abelcet) improve the therapeutic index by decreasing the side effects of AmB as well as by enhancing its activity against the parasite (3). Nevertheless, the use of these formulations is limited by their price and the lack of stability in aqueous suspensions, resulting in complicated clinical protocols.
We report here on the design and antileishmanial evaluation of new stable ionic amphiphilic formulations of AmB. The new formulations were assessed in vitro on AmB-resistant intramacrophagic amastigotes and compared to Fungizone and AmBisome since previous studies have shown that new micellar formulations enhanced AmB activity in the case of an AmB-resistant Leishmania donovani line (4).
The Ionic Amphiphile Biovector (ABV) is a new type of drug carrier having both ionic hydrophilic and hydrophobic properties. The ionic amphiphilic properties are obtained by the inclusion of an ionic lipid phase in an oppositely charged polymer hydrogel network. The resulting polymer-lipid matrix loads easily and can be used to stabilize a large spectrum of insoluble hydrophobic or polar amphiphilic drugs. Charge sign, charge density, and size (micro or nanoparticles) are predefined by the conditions used for the chemical synthesis of the ionic hydrogel matrix. Ionicity and hydrophobicity can be easily tailored after completion of the synthesis by the quantity and type of lipid phase incorporated into the polymeric network (5).
The possibility of obtaining a particle size in the range of 100 nm enables the use of ABV for the intravenous (i.v.) administration of insoluble drugs.
In this work, we report on an evaluation of the capacity of cationic ABV with differing DPPG (dipalmitoyl phosphatidyl glycerol) contents to include and stabilize AmB in a submicroscopic particle. The efficacy of two selected ABV/AmB formulations differing in DPPG content was the evaluated with a L. donovani-infected mice model after i.v. administration, and the results were compared to the commercially available formulation AmBisome.
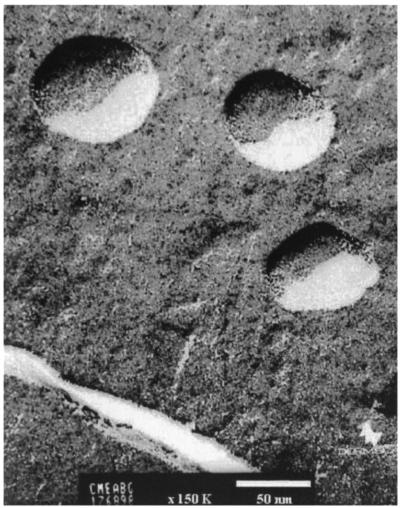
Cationic particles were obtained by the cross-linking of maltodextrins (Roquette Freres, Lestrem, France) with epichlorohydrin (Fluka) and further modification with glycidyl trimethyl ammonium (Fluka) as described elsewhere (1). Particles with a 60-nm diameter were obtained by high-pressure homogenization and were purified by ultrafiltration and microfiltration. Figure 1 shows an electron micrograph of the cationic polysaccharide particles (PSP). The cationic charge density (quaternary ammonium content) was 1.2 mEq/g.
FIG. 1.
Cryofracture transmission electron microscopy of cationic polysaccharide nanoparticles (PSP). Samples were frozen at −200°C and cryofracture was performed at −150°C on a Reicher-Jung Cryofract. Transmission electron microscopy was performed on a JEOL 1200 EX. Magnification, ×135,000.
ABV were prepared by mixing an ethanol solution of DPPG (16 g/liter) with the particle suspension (5 g/liter) at different ratios, from 10 to 40% (wt/wt), and a temperature above the melting temperature (Tm) of DPPG (>45°C). The final ethanol content was around 25% and was eliminated by diafiltration. AmB loading was performed by adding a dimethyl sulfoxide solution of the drug (50 g/liter) to an aqueous suspension of ABV (10 g/liter) by using the same ratios (10 to 40% [wt/wt]). The maximum dimethyl sulfoxide concentration, around 10%, was eliminated by diafiltration.
For all studies, the ABV concentration was kept constant at 10 mg/ml. The AmB concentration was in the range of 1 to 4 mg/ml. The solubility of free AmB in these conditions was lower than 0.1 mg/ml.
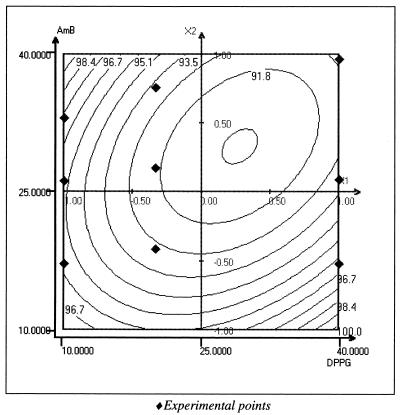
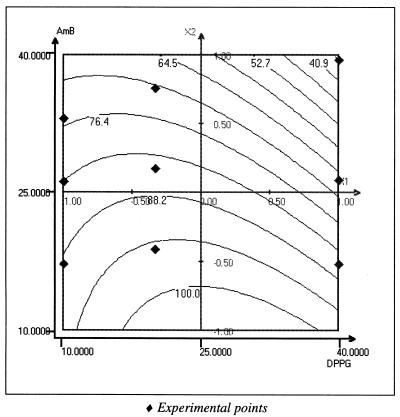
The ability of ABV to entrap and stabilize AmB was studied using a computer-generated experimental design and least-squares multiple regression software (Nemrod; LPRAI, Aix en Provence, France). AmB entrapped in the ABV was evaluated as the quantity of AmB passing through 0.2-μm-pore-size membrane filters and was determined spectrophotometrically at 340 nm. Figure 2 shows the two-dimensional response surface representing the entrapment yield in water as a function of DPPG percentage and AmB percentage. The yields were higher than 90% for all compositions. Particle size, measured on a Coulter N4D (Coultronics, Margency, France) Light Scattering system, was around 100 nm for all formulations. It is interesting to note that in a blank assayed using polysaccharide particles (PSP without DPPG), the particles were able to incorporate less than 20% AmB, indicating a strong solubility enhancement by the presence of lipids in the matrix. Figure 3 shows the filtration yield (0.2 μm) for particles incubated in phosphate-buffered saline (PBS) (room temperature, 30 min), indicating a clear instability of the system when the percentages of DPPG and AmB were increased. This suggests an important role for the excess cationic charge on particle and drug stabilization. Since DPPG and AmB are anionic, an increase in their concentration leads to particle neutralization and a decrease in stability.
FIG. 2.
Two-dimensional representation of AmB incorporation yield as a function of [DPPG] (10 to 40% [wt/wt]) and [AmB] (10 to 40% [wt/wt]) in water. Incorporation yield was calculated by the percentage of AmB passing through 0.2-μm-pore-size sterile filters and is indicated by the iso-response curves. Graphs were generated by NEMROD Software (LPRA).
FIG. 3.
Two-dimensional representation of AmB incorporation yield as a function of [DPPG] (10 to 40% [wt/wt]) and [AmB] (10 to 40% [wt/wt]) in PBS. Stability yield was calculated by the percentage of AmB passing through 0.2 μm sterile filters with respect to the quantity of AmB incorporated in water. Graphs were generated by NEMROD Software (LPRAI).
Two formulations were then chosen for in vivo and in vitro experiments; the formulations are referred to as ABV1/AmB and ABV2/AmB, and they had a different DPPG ratio and a constant load of AmB of 20% (wt/wt). The characteristics of the formulations and the starting ABV are given in Table 1.
TABLE 1.
Characteristics of AVB1 and ABV2 with or without AmBa
| Formulation | Concn (g/liter) of:
|
Size (nm) | AmB (% in water) | Size in PBS (nm) | AmB (% in PBS) | ||
|---|---|---|---|---|---|---|---|
| PSP | DPPG | AmB | |||||
| ABV1 | 10 | 2 | 0 | 91 | 0 | 97 | 0 |
| ABV2 | 10 | 5 | 0 | 97 | 0 | 111 | 0 |
| ABV1/AmB | 10 | 2 | 2 | 96 | 97 | 118 | 92 |
| ABV2/AmB | 10 | 5 | 2 | 107 | 95 | 121 | 90 |
The percentage of incorporated AmB was determined after sample filtration on 0.2-μm-pore-size filters. Under these conditions, nonformulated AmB did not pass through the filters (less than 1%).
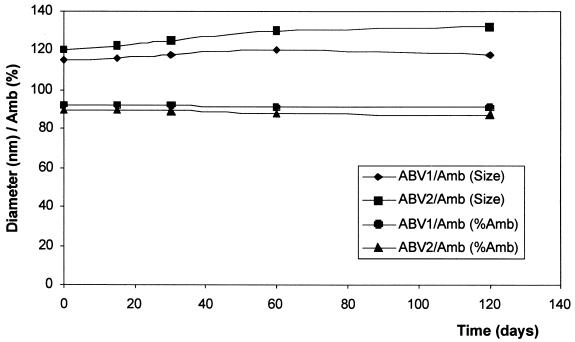
Both formulations were prepared in PBS, sterilized by 0.2-μm-pore-size filtration, and were ready to use. The formulations were stable in PBS for several months at 4°C, and no significant changes in size or AmB content were observed (Fig. 4).
FIG. 4.
Stability characteristics of ABV/AmB formulations in terms of average size stability (measured on a Coulter N4D Submicronic Particle Analyzer) or incorporation stability expressed as the percentage of AmB passing through 0.2-μm-pore-size filters (determined spectrophotometrically).
L. donovani (MHOM/IN/80/DD8), from the WHO strain collection in the London School of Hygiene and Tropical Medicine (London, United Kingdom), was used to obtain the AmB-resistant line by drug pressure, referred to as L. donovani DD8 AmB-R (6), which was used for in vitro experiments.
Peritoneal macrophages were harvested from female CD1 mice (Charles River, Cléon, France) 3 days after an intraperitoneal injection of 1.5 ml of sodium thioglycolate (Biomérieux) and were dispensed into eight-well chamber slides (LabTek Ltd.) at a concentration of 5 × 104/well (400 μl/well) in RPMI 1640 medium (Life Technologies, Cergy-Pontoise, France) supplemented with 10% heat-inactivated fetal calf serum, 25 mM HEPES, and 2 mM l-glutamine (Life Technologies, Cergy-Pontoise, France). Four hours after the macrophages were plated, they were washed in order to eliminate fibroblasts. After 24 h, the macrophages were infected with promastigote forms of L. donovani DD8 wild type (WT) and AmB-R in a stationary phase at a ratio of 10 and 20 parasites per macrophage, respectively, to obtain 86% of infected macrophages and 10 ± 3 amastigotes per macrophage for the WT line and 52% of infected macrophages and 7 ± 2 amastigotes per macrophage for the AmB-R line. At 18 h after the promastigotes had entered macrophages, the free promastigotes were eliminated and intramacrophagic amastigotes were treated at various concentrations of AmB, from 0.01 to 1 μM (day 1). Fungizone served as the reference compound and AmBisome served as the reference formulation. The experiment was performed in triplicate. The culture medium was renewed 48 h later and a new culture medium containing the drug was added. The experiment was stopped at day 5, and the percentage of infected macrophages was evaluated microscopically after Giemsa staining. The 50% inhibitory concentrations were determined by linear regression analysis, 95% confidence limits were calculated and the P value were calculated using the Student t test.
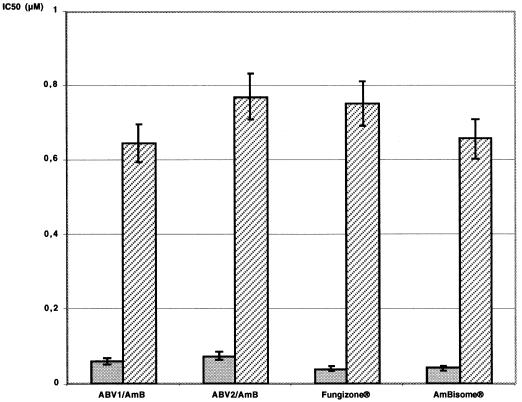
The in vitro activities of the two standard drugs, Fungizone and AmBisome, against L. donovani DD8 WT intramacrophagic amastigotes were similar to those of ABV1/AmB and ABV2/AmB (0.041 to 0.075 μM against WT and 0.657 to 0.771 μM against AmB-R), and no special activity enhancement was detected (Fig. 5).
FIG. 5.
In vitro activity of AmB formulations on L. donovani intramacrophagic amastigotes. After WT strain infection (shaded bars), 86% of the macrophages were infected, and nontreated infected macrophages contained 10.2 ± 3.1 amastigotes 4 days postinfection. After AmB-R strain infection (hatched bars), 52% of the macrophages were infected, and nontreated infected macrophages contained 7.4 ± 2.6 amastigotes 4 days postinfection. IC50, 50% inhibitory concentration.
L. donovani (MHOM/ET/1967/L82, LV9 strain) was used for the in vivo evaluation. This strain was kindly provided by S. L. Croft (London School of Hygiene and Tropical Medicine, London, United Kingdom) and was routinely maintained in male golden hamsters (Mesocricetus auratus; Charles River). After 6 to 8 weeks of infection, amastigotes were isolated from the spleen of a previously infected golden hamster for the in vivo studies. Female BALB/c mice (Charles River) weighed 19.25 ± 0.68 g before infection. They were infected i.v. with 107 amastigotes obtained from the spleens of infected golden hamsters at day 1. One week after infection, the mice were randomly divided into groups of 10 mice each. Treatments were performed via the i.v. route at a single dose (day 8), and the infected mice were treated with the same concentration of AmB. All mice were autopsied at day 15, and drug activity was estimated by counting the number of amastigotes/500 liver cells in Giemsa-stained impression smears prepared from the weighed livers of treated and untreated mice.
The two formulations (ABV1/AmB, ABV2/AmB) were compared to AmBisome in a challenge against L. donovani LV9 on BALB/c-infected mice at the single doses of 5 and 0.25 mg/kg of body weight i.v. administered. Three groups were compared using the Kruskall-Wallis test, and two groups were compared using the U-Mann and Whitney tests.
The results are presented in Table 2 as parasite elimination efficacy after i.v. injection. No difference, as evidenced by leishmanicidal activity in vivo, at 0.25 mg of AmB/kg was detectedbetween ABV1/AmB and AmBisome, whereas ABV2/AmB was slightly less efficient than AmBisome (P < 0.03). At the higher AmB dosage, 5 mg/kg, AmBisome was significantly more efficient than ABV1/AmB and ABV2/AmB (P values of <0.01 and <0.02, respectively). No difference was observed between ABV1/AmB and ABV2/AmB, although ABV2/AmB was more toxic, provoking a decrease in the weight of the mice at the end of the experiment.
TABLE 2.
Effect of Ionic Amphiphile Biovector systems on the L. donovani LV9/BALB/c mouse model compared to AmBisomea
| Drug formulation | Treatment dose (mg of AmB/kg) | Stauber count (mean ± SEM) (108)b | % Parasite inhibition | Mouse weight (g) at autopsy (mean ± SEM) |
|---|---|---|---|---|
| ABV1/AmB | 0.25 | 2.55 ± 0.29∗ | 57.62 | 19.86 ± 0.56 |
| ABV1/AmB | 5 | 0.036 ± 0.011∗ | 99.39 | 19.92 ± 0.35 |
| ABV2/AmB | 0.25 | 3.33 ± 0.37∗ | 44.55 | 20.10 ± 0.39 |
| ABV2/AmB | 5 | 0.019 ± 0.0011∗ | 99.69 | 18.40 ± 2.23 |
| AmBisome | 0.25 | 2.15 ± 0.15∗ | 64.19 | 20.24 ± 0.74 |
| AmBisome | 5 | 0.015 ± 0.0013∗ | 99.77 | 20.20 ± 0.39 |
| Control | 5.94 ± 0.35 | 0 | 19.98 ± 0.63 |
There were 10 mice per batch, and i.v. treatments were given in a single dose.
∗, values are significantly different from those of the control (P < 0.001). At the dose of 5 mg/kg, the values obtained after the AmBisome, ABV1/AmB, and ABV2/AmB treatments were significantly lower than those of the control (P < 0.001), the values obtained after the AmBisome treatment were significantly lower than those obtained after the ABV1/AmB (P < 0.01) and ABV2/AmB (P < 0.03) treatments, and the values obtained after the ABV1/AmB and ABV2/AmB treatments were not significantly different. At the dose of 0.25 mg/kg, the values obtained after the AmBisome, ABV1/AmB, and ABV2/AmB treatments were significantly lower than those of the control (P < 0.001), the values obtained after the AmBisome treatment were significantly lower than those obtained after the ABV2/AmB treatment (P < 0.02), and the values obtained after the AmBisome and ABV1/AmB treatments were not significantly different.
The two AmB lipid formulations used in this study had major differences in the size and composition of the lipid vehicles compared to those of Fungizone and AmBisome. Despite these differences, the in vitro and in vivo activities remained in the same range of doses and concentrations, suggesting that their interaction with host cells, uptake, and intracellular distribution were efficient.
On the basis that the antiparasitic activities of the AmB formulations are dependent on the delivery of the drug to the infected phagocytes of the mononuclear phagocytic system, it appears difficult to classify the formulations as a function of their activity in comparison to AmBisome. The use of ABV2 led to a 5% decrease in the weight of the mice after treatment at a dose level of 5 mg/kg, which might be a drawback to its further use. Considering both toxicity and antileishmanial activity, AmBisome seems slightly more active and safer than the ABV formulations tested.
Ionic Amphiphile Biovectors are, however, in the early stages of development. Size, charge density, lipid ratio, and lipid type could all be optimized in order to improve formulation efficacy.
In this work, a new ionic amphiphile particulate drug delivery system was used to formulate AmB for i.v. administration. The system consisted of an ionic polymer network into which an opposite charge lipid phase was included, which gave amphiphilic properties to the ionic polymer-lipid complex matrix. The system showed quantitative yields of incorporation with good stability in saline conditions, resulting in 20% (wt/wt) AmB being loaded into 100-nm-diameter sterile nanoparticles ready for injection. The evaluation of these particles in a challenge against L. donovani in infected mice yielded results similar to those of the commercially available system AmBisome.
The major advantage of the ABV formulations is their capacity to incorporate AmB actively with quantitative yields by using a simple mixing procedure and subsequently facilitating the formulation and manufacturing process. They have a high stability in saline and can be sterilized by filtering on 0.2-μm-pore-size filters without any significant loss of material, which could lead to stable ready-to-use suspensions. These results are very promising, since ABV can be optimized in terms of par-ticle size, charge, lipid composition, and AmB loading. In further studies we plan to consider these physico-chemical parameters as well as the pharmacokinetics and the optimization of a treatment regimen.
Ionic Amphiphile Biovector technology appears to be a promising tool for the formulation of poorly soluble drugs since their solubilizing properties are not limited to AmB and could be extended to other drugs by using a single technology platform.
REFERENCES
- 1.Betbeder, D., A. Etienne, I. De Miguel, R. Kravtzoff, and M. Major. 1998. Mucosal administration of substances to mammals. Patent PCT WO 98/29099.
- 2.Bryceson, A. 1987. Therapy in man, p. 847-907. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine, vol. II. Academic Press Ltd., London, United Kingdom. [Google Scholar]
- 3.Davidson, R. N., S. L. Croft, A. Scott, M. Maini, A. H. Moody, and A. D. M. Bryceson. 1991. Liposomal amphotericin B in drug-resistant visceral leishmaniasis. Lancet 337:1061-1062. [DOI] [PubMed] [Google Scholar]
- 4.Espuelas, S., P. Legrand, P. M. Loiseau, C. Bories, G. Barratt, J. M. Irache. 2000. In vitro reversion of amphotericin B resistance in Leishmania donovani by poloxamer 188. Antimicrob. Agents Chemother. 44:2190-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imbertie, I., D. Betbeder, F. Lescure, P. Von Hoegen, I. De Miguel. 2001. New Ionic Amphiphile BiovectorTM as carrier of poor solubility drugs. J. Control Release 72:249-252. [Google Scholar]
- 6.Mbongo, N., P. M. Loiseau, M. A. Billion, M. Robert-Gero. 1998. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 42:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]