Abstract
SCH-C (SCH 351125) is a small-molecule antagonist of the human immunodeficiency virus type 1(HIV-1) coreceptor CCR5. It has in vitro activity against R5 viruses with 50% inhibitory concentrations ranging from 1.0 to 30.9 nM. We have studied anti-HIV-1 interactions of SCH-C with other antiretroviral agents in vitro. Synergistic interactions were seen with nucleoside reverse transcriptase inhibitors (zidovudine and lamivudine), nonnucleoside reverse transcriptase inhibitors (efavirenz), and protease inhibitors (indinavir) at all inhibitory concentrations evaluated. We have also studied antiviral interactions between the HIV-1 fusion inhibitor T-20 and SCH-C against a panel of R5 HIV-1 isolates. We found synergistic interactions against all the viruses tested, some of which harbored resistance mutations to reverse transcriptase and protease inhibitors. Anti-HIV-1 synergy was also observed between SCH-C and another R5 virus inhibitor, aminooxypentane-RANTES. These findings suggest that SCH-C may be a useful anti-HIV drug in combination regimens and that a combination of chemokine coreceptor/fusion inhibitors may be useful in the treatment of multidrug-resistant viruses.
Great strides have been made in antiretroviral therapy for human immunodeficiency virus type 1 (HIV-1) infection over the past decade, with resultant decreases in mortality and morbidity in resource-rich countries (17). With these successes, however, have come new problems, including drug-associated toxicities and other medical complications such as pancreatitis and lipid redistribution syndromes (2). In addition, all currently approved antiretroviral drugs in North America and Europe fall into one of two major classes, reverse transcriptase inhibitors or protease inhibitors. With their increasing use has come increasing viral resistance to individual drugs as well as across classes. As many as 10 to 20% of HIV-1 isolates from untreated individuals may show some degree of drug resistance (10), and the percentages increase as individuals are exposed to varied treatment regimens.
It is clear that other sites in the HIV-1 replicative cycle should be targeted for antiretroviral drug development. One of the most promising is the complex process of viral attachment, chemokine coreceptor interactions, and fusion into susceptible cells (hereafter described as attachment/entry) (23). The process of HIV-1 attachment/entry begins with viral envelope protein (Env) attachment to the CD4 receptor, which itself may be a multistep process (3, 6). This leads to conformational changes in the glycoprotein 120 (gp120) subunit of Env, allowing interaction with cellular coreceptors, the most important of which are the seven transmembrane domain chemokine receptors, CCR5 and CXCR4. Most viruses involved in transmission use CCR5 (R5 viruses), whereas viruses using CXCR4 (X4 viruses) generally emerge later and may be associated with disease progression (5). Further conformational changes are induced by the interaction between gp120 and the chemokine receptor, exposing fusion peptides on the gp41 subunit of Env which subsequently fuse with cell membranes, allowing entry of the viral core into the cell cytoplasm. Agents have been developed which target each of these steps in the attachment/entry process, and several of these are now being studied in clinical trials. One fusion inhibitor, T-20, has shown considerable promise and is now being used in pivotal phase III clinical trials (12, 18).
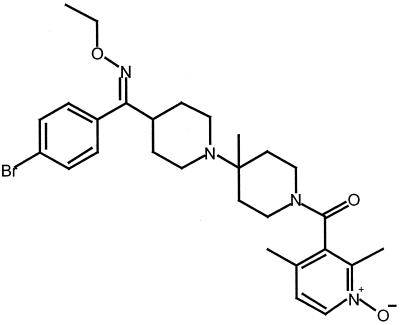
SCH-C (SCH 351125) is a small-molecule oxime-piperidine antagonist of the HIV-1 coreceptor CCR5 (Fig 1). It has potent activity against clinical HIV-1 R5 isolates, both in vitro and in a SCID-hu Thy/Liv mouse model, whereas it has no activity against HIV-1 X4 isolates (20). SCH-C has good oral bioavailability (50 to 60%) in rodents and primates, with a serum half-life of 5 to 6 h. It is now being studied in phase I clinical trials.
FIG. 1.
SCH 351125 chemical structure.
As with other classes of antiretroviral drugs, attachment/entry inhibitors are unlikely to be used in monotherapy, because of insufficient potency and the risk of emergence of resistant variants (10). Moreover, with coreceptor inhibitors in particular, another concern would be the potential for selecting viruses with different coreceptor specificities, because mixed infections with R5 and X4 viruses occur, as do infections with dualtropic R5X4 viruses (15). Combining antiretroviral drugs has many potential advantages over monotherapy, including delaying the emergence of resistant viral variants, increasing potency, and broadening coverage against existent variants in the population (21). Previous studies have shown that using antiretroviral drug combinations in vitro may lead to various outcomes ranging from synergy to antagonism and that these outcomes may be predictive of subsequent clinical results (9, 14).
To help define the role of SCH-C in the anti-HIV arsenal, we have evaluated its interactions in vitro with other antiretroviral drugs, including inhibitors of HIV-1 reverse transcriptase or protease, as well as other attachment/entry inhibitors. Activity was studied against a panel of clinical isolates, including one with a genotype coding for resistance to both reverse transcriptase and protease inhibitors.
MATERIALS AND METHODS
Design.
Peripheral blood mononuclear cells (PBMC) from HIV-1-seronegative donors were obtained by Ficoll-Hypaque density gradient centrifugation of heparinized venous blood. After 3-day photohemagglutinin assay (PHA) stimulation, PBMC were resuspended at a concentration of 106 cells/ml in RPMI-1640 culture medium (Sigma, St. Louis, Mo.) supplemented with 20% heat-inactivated fetal calf serum (Sigma), penicillin (50 U/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), HEPES buffer (10 mM), and 10% interleukin-2 in 24-well tissue culture plates (Becton Dickinson, San Jose, Calif.). Single drugs or combinations of drugs were added to each well, using a fixed ratio among drugs and serial dilutions. Drugs were dissolved using dimethyl sulfoxide for SCH-C and using phosphate-buffered saline for the other drugs. They were added simultaneously with the HIV-1 inoculum (1,000 to 5,000 50% tissue culture infective doses/106 cells), and plates were incubated at 37°C in a humidified 5% C02 atmosphere. Each condition was tested in duplicate, and each experiment was repeated at least twice. Cell-free culture supernatant fluids were harvested and analyzed by enzyme-linked immunosorbent assay (DuPont, Wilmington, Del.) for HIV-1 p24 antigen production on day 7 of culture. In addition, uninfected drug-treated cytotoxicity controls were maintained at the highest concentration of each agent tested. No toxicity was observed at concentrations up to 10 μM. Cell proliferation and viability were assessed by the trypan blue dye exclusion method.
Viruses.
A panel of R-5 clinical HIV-1 isolates was derived from a cohort of subjects with acute HIV-1 primary infection syndrome. The viruses were shown to be R-5 by replication in U87 MG-CD4 cell lines expressing CCR5 and absence of replication in U87 MG-CD4 cell lines expressing CXCR4. They were also non-syncytium inducing in MT-2 assays. Other characteristics of these viruses are shown in Table 1.
TABLE 1.
Characteristics of the HIV-1 isolates utilizeda
| Isolate | SCH-CIC50 (nM)b | Mutation(s) associated with reverse transcriptase and protease inhibitors |
|---|---|---|
| R5-08 | 8.7 ± 8.2 | Wild type |
| R5-02 | 2.2 ± 1.1 | Wild type |
| R5-16 | 1.2 ± 0.7 | PR 71T (polymorphism) |
| R5-18 | 1.0 ± 0.2 | PR 10V, 20R, 36I, 63P, 71T, 77I, 90M, RT 41L, 98G, 184V, 215Y |
| R5-06 | 2.1 ± 0.7 | PR 77I (polymorphism) |
| R5-01 | 14.7 ± 8.9 | Wild type |
| R5-19 | 30.9 ± 22.0 | PR 30N, RT 184V |
All isolates were characterized as R-5 and were derived from the peripheral blood of subjects with acute HIV-1 primary infection syndrome.
Average ± standard deviation (SD) of three to eight repeated experiments.
Compounds.
SCH-C was provided by Schering Plough Research Institute (Kenilworth, N.J.). T-20 was provided by Trimeris, Inc. (Durham, N.C.). Aminooxypentane (AOP)-RANTES was obtained from Gryphon Sciences (South San Francisco, Calif.). Zidovudine and lamivudine were provided by Glaxo Smith Kline, Inc. (Research Triangle Park, N.C.). Efavirenz was provided by DuPont Pharmaceuticals Co. (Wilmington, Del.). Indinavir was provided by Merck & Co, Inc. (West Point, Pa.).
Mathematical analysis.
The multiple-drug effect analysis of Chou, based on the median effect principle and the isobologram technique, was used to analyze combined-drug effects (4). This method involves plotting dose-effect curves for each drug and for multiply diluted fixed-ratio combinations of drug by using the median effect equation. The slope of the median effect plot, which signifies the shape of the dose-effect curve, and the x intercept of the plot, which signifies the potency of each compound and each combination, are used for a computerized calculation of a combination index (CI). A mutually exclusive model of analysis was used. A CI of <0.9 indicates synergy (i.e., greater than the expected additive effect when two agents are combined), 0.9 < CI < 1.1 indicates additive effects, and a CI of >1.1 indicates antagonism (i.e., less than the expected additive effect).
RESULTS
Use of SCH-C as monotherapy.
SCH-C had potent inhibitory activity against all R-5 isolates tested (Table 1). Values (50% inhibitory concentrations [IC50s]) ranged from 1.0 to 30.9 nm. No toxicity was observed in uninfected PBMC at the highest concentration of drug used.
Use of SCH-C in combinations.
SCH-C was evaluated in two drug combinations, using representative drugs from each of the currently FDA-approved antiretroviral classes: nucleoside reverse transcriptase inhibitors (zidovudine and lamivudine), nonnucleoside reverse transcriptase inhibitors (efavirenz), and protease inhibitors (indinavir). As illustrated in Table 2, all combinations tested showed synergistic interactions with mean CI values from repeated experiments ranging from 0.23 to 0.77. Combinations of SCH-C with other inhibitors of HIV-1 attachment/entry were also evaluated. These included the fusion inhibitor T-20 and the CCR5 antagonist, AOP-RANTES. T-20 and SCH-C exhibited mostly synergistic interactions against a broad panel of R5 clinical isolates, with CI values ranging from 0.33 to 0.98 (Table 3). The combination of SCH-C and AOP-RANTES was tested against only one R5 isolate (R5-08) and was synergistic against this virus (Table 2).
TABLE 2.
CIs for SCH-C and other antiretrovirals at various ICs against R5-01a
| Drug | IC
|
|||
|---|---|---|---|---|
| 50% | 75% | 90% | 95% | |
| Zidovudine | 0.56 ± 0.14 | 0.39 ± 0.05 | 0.28 ± 0.01 | 0.23 ± 0.00 |
| Lamivudine | 0.40 ± 0.10 | 0.29 ± 0.11 | 0.25 ± 0.16 | 0.24 ± 0.20 |
| Efavirenz | 0.77 ± 0.28 | 0.55 ± 0.06 | 0.45 ± 0.01 | 0.43 ± 0.05 |
| Indinavir | 0.74 ± 0.33 | 0.53 ± 0.05 | 0.50 ± 0.05 | 0.52 ± 0.08 |
| AOP-RANTES | 0.42 ± 0.02 | 0.37 ± 0.09 | 0.36 ± 0.19 | 0.37 ± 0.26 |
Concentrations were SCH-C, as follows: 1 to 54 nM; zidovudine, 1 to 54 nM; lamivudine, 0.03 to 1.62 μM; efavirenz, 0.2 to 36 nM; indinavir, 3 to 54 nM; and AOP-RANTES, 2 to 20 ng/ml. CI < 0.9 = synergy; 0.9 < CI < 1.1 = additivity; CI > 1.1 = antagonism. Values show the mean of two to three experiments ± SD.
TABLE 3.
CIs for SCH-C and T-20 at various ICs against a panel of R5 virusesa
| Virus | IC
|
|||
|---|---|---|---|---|
| 50% | 75% | 90% | 95% | |
| R5-08 | 0.61 ± 0.21 | 0.45 ± 0.14 | 0.37 ± 0.11 | 0.34 ± 0.11 |
| R5-02 | 0.77 ± 0.36 | 0.74 ± 0.17 | 0.74 ± 0.02 | 0.76 ± 0.16 |
| R5-16 | 0.81 ± 0.07 | 0.81 ± 0.13 | 0.82 ± 0.17 | 0.84 ± 0.19 |
| R5-18 | 0.98 ± 0.17 | 0.65 ± 0.06 | 0.43 ± 0.00 | 0.33 ± 0.02 |
| R5-06 | 0.84 ± 0.09 | 0.81 ± 0.12 | 0.80 ± 0.20 | 0.81 ± 0.27 |
| R5-01 | 0.52 ± 0.04 | 0.43 ± 0.04 | 0.37 ± 0.07 | 0.35 ± 0.09 |
Concentrations of SCH-C ranged from 0.25 to 160 nM and concentrations of T-20 ranged from 0.0025 to 0.18 μg/ml, depending on the viral isolate used. CI < 0.9 = synergy; 0.9 < CI < 1.1 = additivity; CI > 1.1 = antagonism. Values are the mean of two to three experiments ± SD.
DISCUSSION
Combination antiretroviral therapy has become standard management for individuals requiring treatment for HIV-1 infection (1). The benefits of monotherapy include greater potency, increased durability of virus suppression, and delayed development of drug resistance, resulting in increased disease-free survival among infected subjects. Studies of in vitro antiviral interactions among different antiretroviral agents have been helpful in predicting clinical results. For example, given favorable drug-drug interactions, it was shown that three drugs were better than two and that two were better than one (13, 16), findings that have been duplicated in clinical trials (7, 8). Moreover, the use of simultaneous combinations is preferable to alternating combinations, both in vitro and in vivo (13, 16). In addition, combinations that are synergistic in vitro, e.g., zidovudine plus lamivudine, are clinically useful, whereas those that are antagonistic in vitro, e.g., zidovudine and stavudine, fail in the clinic (9, 14). Thus, understanding potential antiviral drug interactions in cell culture is important when considering the addition of new agents to the clinical HIV-1 arsenal.
Novel inhibitors of the complex HIV-1 attachment-chemokine coreceptor-fusion process are receiving considerable attention, particularly as viruses resistant to reverse transcriptase inhibitors and protease inhibitors increase in the population (10). T-20, an inhibitor of fusion between viral envelope gp's and cell membranes, has already shown activity in clinical trials conducted in subjects with advanced HIV-1 infection (12, 18). Agents that act at other steps in the attachment/entry pathway are also under development.
SCH-C is a small-molecule, potent, and specific CCR5 receptor antagonist that has anti-HIV activity in nanomolar concentrations against most primary R5 viral isolates (20). Because it also has favorable pharmacokinetic profiles in animals, including good oral bioavailability and absorption, it is now being evaluated in phase I clinical studies.
The gp120 regions responsible for attachment to the coreceptor CCR5 are quite variable and could affect the responses of clinical isolates to CCR5 inhibitors (11, 19, 23). We observed a greater than 30-fold variation in IC50s for SCH-C among the clinical isolates we have tested, although these IC50s remained in the nanomalor range. This is consistent with results obtained by others (20). Viral determinants responsible for differences in response to coreceptor inhibitors are being studied. Whether the range of IC50s will have an impact on clinical effectiveness remains to be evaluated. However, the broad range of susceptibilities may support the use of these compounds in combination regimens.
Our studies indicate that SCH-C demonstrates strong synergy with representative drugs from all classes of currently used antiretroviral agents across a broad range of inhibitory concentrations. We also evaluated combinations of SCH-C with other inhibitors of HIV-1 attachment/entry, including the fusion inhibitor T-20 and the R5 virus inhibitor AOP-RANTES. T-20 is a 36-amino-acid peptide that binds to gp41, thereby preventing conformational changes necessary for viral fusion. AOP-RANTES is an amino terminally modified analogue of the natural beta-chemokine RANTES that inhibits HIV-1 R5 isolates and induces coreceptor internalization. SCH-C and T-20 showed anti-HIV synergy against a panel of seven primary R5 isolates across a broad range of inhibitory concentrations. This is consistent with other studies of fusion inhibitor and coreceptor inhibitor combinations, where strong synergy has been observed (22). One hypothesis to explain this synergy is that partial blockage of the coreceptor affects the conformational changes leading to membrane fusion and allows a more efficient action of fusion inhibitors. This hypothesis requires experimental support. In addition to T-20 synergy with SCH-C, two inhibitors of R-5 viruses, SCH-C and AOP-RANTES, also synergistically inhibited an R5 HIV-1 isolate.
As more and more drug-resistant HIV-1 isolates emerge, new classes of potent antiretroviral agents targeting different steps of the HIV replicative cycle are a welcome addition to the HIV arsenal. Some of the isolates we used in this study showed mutations associated with resistance to both reverse transcriptase and protease inhibitors, suggesting that a combination of chemokine coreceptor-fusion inhibitors might be particularly useful in the treatment of multi-drug resistant viruses. The benefits of such attachment/entry inhibitors and combinations thereof will need to be evaluated in clinical trials.
Acknowledgments
This work was supported by NIH grant CA12464 and by a grant from the Schering-Plough Research Institute. C.L.T. was a Research Fellow supported by the Fonds de Recherche en Santé du Québec.
REFERENCES
- 1.Carpenter, C. C. J., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. G. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy for HIV infection in 2000: updated recommendations of the International AIDS Society-USA panel. JAMA 283:381-390. [DOI] [PubMed] [Google Scholar]
- 2.Carr, A., K. Samaras, A. Thorisdottir, G. R. Kaufmann, D. J. Chisholm, and D. A. Cooper. 1999. Diagnosis, prediction, and natural course of HIV-1 protease inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes: a cohort study. Lancet 353:2093-2099. [DOI] [PubMed] [Google Scholar]
- 3.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 4.Chou, T. C. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-102. In T. C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, San Diego, Calif.
- 5.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammer, S. M., D. A. Katzenstein, M. D. Hughes, H. Gundacker, R. T. Schooley, R. H. Haubrich, W. K. Henry, M. M. Lederman, J. P. Phair, M. Niu, M. S. Hirsch, and T. C. Merigan. 1996. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N. Engl. J. Med. 335:1081-1090. [DOI] [PubMed] [Google Scholar]
- 8.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 9.Havlir, D. V., C. Tierney, G. H. Friedland, R. B. Pollard, L. Smeaton, J.-P. Sommadossi, L. Fox, H. Kessler, K. H. Fife, and D. D. Richman. 2000. In vivo antagonism with zidovudine plus stavudine combination therapy. J. Infect. Dis. 182:321-325. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch, M. S., F. Brun-Vezinet, R. T. D'Aquila, S. M. Hammer, V. A. Johnson, D. R. Kuritzkes, C. Loveday, J. W. Mellors, B. Clotet, B. Conway, L. M. Demeter, S. Vella, D. M. Jacobsen, and D. D. Richman. 2000. Antiretroviral drug resistance testing in adults with HIV infection: recommendations of an International AIDS Society-USA panel. JAMA 283:2417-2426. [DOI] [PubMed] [Google Scholar]
- 11.Hwang, S. S., T. J. Boyle, H. K. Lyerly, and B. R. Bullen. 1991. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science 253:71-74. [DOI] [PubMed] [Google Scholar]
- 12.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 13.Mazzuli, T., S. Rusconi, D. P. Merrill, R. T. D'Aquila, M. Moonis, T.-C. Chou, and M. S. Hirsch. 1994. Alternating versus continuous drug regimens in combination chemotherapy of human immunodeficiency virus type 1 infection in vitro. Antimicrob. Agents Chemother. 38:656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill, D. P., M. Moonis, T.-C. Chou, and M. S. Hirsch. 1996. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type 1 replication in vitro. J. Infect. Dis. 173:355-364. [DOI] [PubMed] [Google Scholar]
- 15.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, E. R. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh, M.-D., D. P. Merrill, L. Sutton, and M. S. Hirsch. 1997. Sequential versus simultaneous combination antiretroviral regimens for the treatment of human immunodeficiency virus type 1 infection in vitro. J. Infect. Dis. 176:510-514. [DOI] [PubMed] [Google Scholar]
- 17.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 18.Pilcher, C. D., J. Eron, Jr., L. Ngo, A. Dusek, P. Sista, J. Gleavy, D. Brooks, T. Venetta, E. DiMassimo, and S. Hopkins. 1999. Prolonged therapy with the fusion inhibitor T-20 in combination with oral antiretroviral agents in an HIV-infected individual. AIDS 13:2171-2173. [DOI] [PubMed] [Google Scholar]
- 19.Rizzuto, C. D., R Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 20.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C.-C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay, C. L., J. C. Kaplan, and M. S. Hirsch. 2001. Combination antiretroviral therapy, p. 313-337. In E. DeClercq (ed.), Antiretroviral therapy. ASM Press, Washington, D.C.
- 22.Tremblay, C. L., C. Kollmann, F. Giguel, T. C. Chou, and M. S. Hirsch. 2000. Strong in vitro synergy between the fusion inhibitor T-20 and the CXCR4 blocker AMD-3100. J. Acquir. Immune Defic. Syndr. 25:99-102. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]