Abstract
Licochalcone A was isolated from the roots of licorice, Glycyrrhiza inflata, which has various uses in the food and pharmaceutical industries; isolation was followed by extraction with ethanol and column chromatography with silica gel. In this study, the activities of licochalcone A against some food contaminant microorganisms were evaluated in vitro. The vegetative cell growth of Bacillus subtilis was inhibited in a licochalcone A concentration-dependent manner and was completely prevented by 3 μg of licochalcone A/ml. Licochalcone A showed a high level of resistance to heating at 80 to 121°C for 15 min. Licochalcone A did not inhibit the germination of heat-treated spores of B. subtilis induced by l-alanine. Licochalcone A showed effects against all gram-positive bacteria tested and especially was effective against all Bacillus spp. tested, with MICs of 2 to 3 μg/ml, but was not effective against gram-negative bacteria or eukaryotes at 50 μg/ml. Although the cationic antimicrobial peptides protamine and ɛ-poly-l-lysine resulted in the loss of antimicrobial activity in the presence of either 3% (wt/vol) NaCl or protease at 20 μg/ml, the antibacterial activity of licochalcone A was resistant to these conditions. Thus, licochalcone A could be a useful compound for the development of antibacterial agents for the preservation of foods containing high concentrations of salts and proteases, in which cationic peptides might be less effective.
The bacterial spores of the genera Bacillus and Clostridium act as a survival stage, which is characterized by a high level of resistance to heat and other adverse conditions typically used to kill vegetative cells and other microorganisms (2). In food, the spores themselves do not represent a hazard. However, despite being metabolically dormant, the spores have a functional environmental sensory mechanism that can trigger germination under favorable conditions (22). Thus, the processes of germination, outgrowth (vegetative growth after germination), and/or toxin formation can result in spoilage and/or food poisoning (17). In practice, conditions for outgrowth in many foods are suboptimal due to the presence of a combination of factors, such as water activity, reduced pH, preservatives, organic acids or salt, and pasteurization steps (2).
Generally, the surface charge of microbial cells is anionic at a neutral pH because of the dissociation of acidic groups, such as phosphate and carboxylate, on the cell surface (16). Recently, a great deal of attention has been paid to cationic antimicrobial peptides, such as protamine and ɛ-poly-l-lysine, as natural food preservatives with a low toxicity. Protamine found in salmon spermatozoan nuclei (salmine) is a basic peptide of 32 amino acids, of which 21 are arginine (6, 10). Protamine has activity against a broad spectrum of bacteria, yeasts, and fungi, and this activity is considered to be the result of its polycationic nature (6, 10, 16). The broad antimicrobial spectrum of protamine and the fact that protamine is naturally occurring and nontoxic to humans make it a promising biological alternative to chemical preservatives and disinfectants (6, 10, 16). Alternatively, ɛ-poly-l-lysine from culture filtrates of Streptomyces albulus also shows antimicrobial activity with a broad spectrum (14, 16, 20, 23). These peptides have the common features of being highly basic due to the presence of multiple arginine and lysine residues and of forming amphipathic structures (14, 16). However, the growth inhibitory effects of these peptides against food contaminant microorganisms are repressed by the presence of a high concentration of salt, which could interfere with the binding of cationic peptides to the cell surface (10, 16). Although these peptides carry many cationic residues required for antimicrobial activity, peptides bound to the cell surface are subject to protease digestion (16). In terms of practical applications, cationic antimicrobial peptides would be less effective in the preservation of salted foods and are not available for the preservation of foods with protease activity.
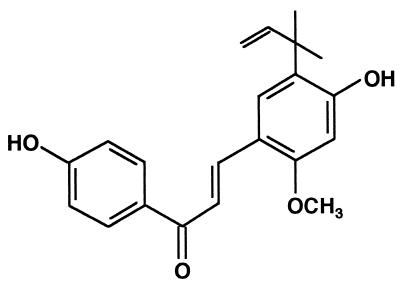
Licorice, the root and rhizome of the Glycyrrhiza spp. Glycyrrhiza uralensis (9, 19), Glycyrrhiza glabra (3, 5, 13, 15, 18, 19), and Glycyrrhiza inflata (7, 8, 11, 18, 19), is currently used in the tobacco, food, and pharmaceutical industries. It has been used for centuries as a medicine because of its wide-ranging therapeutic properties, including relief of rheumatic and other pain, and its healing effect on gastric ulcers (19). The crude extract of licorice has also found commercial use as a food additive in Japan, as it contains the sweetening principal glycyrrhizin (19). Chemical investigations have revealed the presence of a wide variety of bioactive phenolic constituents in licorice; these have attracted attention as a potential source of chemical leads (19). G. inflata is one of the main botanical sources of licorice and is chemically characterized by the presence of retrochalcones, which are distinguished from ordinary chalcones by the absence of oxygen functionality at position 2 (7, 8, 11, 18, 19). Five retrochalcones, licochalcones A, B, C, and D and echinatin, were isolated from G. inflata roots and characterized (7, 8, 11, 18); the content of licochalcone A (Fig. 1) was found to be very high (19). We chose to investigate licochalcone A simply because it was the most abundant component of the naturally occurring mixture.
FIG. 1.
Structure of licochalcone A.
Recently, from among the five retrochalcones, various biological activities of licochalcone A were reported, e.g., antiprotozoal (1), anti-inflammatory (19), anti-tumor promoting (19), antioxidative (7, 18), and antimicrobial (8, 18) activities, but little information has been reported for the other chalcones. Furthermore, the antimicrobial activity of licochalcone A has not been thoroughly investigated, and little is known about its activity against food contaminant bacteria, including spore-forming bacteria, such as the genera Bacillus and Clostridium, and toxin-producing bacteria, such as Bacillus cereus and Staphylococcus aureus. In this study, we purified licochalcone A from the roots of G. inflata and evaluated its in vitro activities against some food contaminant microorganisms and its effects in the presence of a combination of factors, such as pH, salt, protease, and heat, as food preservation conditions.
MATERIALS AND METHODS
Microorganisms.
The following 17 microorganisms, obtained from the stock culture collection at the Institute for Fermentation (Osaka, Japan), were used in this study: Escherichia coli K-12 IFO 3301, Pseudomonas aeruginosa IFO 3923, B. cereus IFO 3514, Bacillus coagulans IFO 12583, Bacillus subtilis IFO 3007, Bacillus stearothermophilus IFO 12550, Clostridium sporogenes IFO 13950, Enterococcus faecalis IFO 3989, Enterococcus faecium IFO 3826, Lactobacillus acidophilus IFO 13951, Lactobacillus plantarum IFO 12519, Leuconostoc mesenteroides IFO 3832, S. aureus 209P IFO 12732, Streptococcus lactis IFO 12546, Streptococcus mutans IFO 13955, Aspergillus niger IFO 6341, and Saccharomyces cerevisiae IFO 0203.
Extraction and isolation of licochalcone A.
The roots of licorice (G. inflata) used in this study were obtained from Kobayashi-Kei Co., Ltd. (Kobe, Japan). Licochalcone A was extracted and isolated from the crushed roots as described previously (7, 8, 11, 18). To remove water-soluble fractions from the roots of licorice, the roots were extracted with a 10-fold volume of hot water at 80°C for 3 h, and then the residue was dried at room temperature. The dried roots (1 kg) were extracted with 5 liters of ethanol to provide the extract (56 g). Silica gel (100 g, BW-200; Fuji Silysia Chemical Ltd., Kasugai, Japan) was added to the extract (56 g), and the mixture was concentrated in vacuo. Silica gel absorbed with the extract was added to a column (30 by 6.0 cm) and eluted with 2 liters of 70% (vol/vol) ethyl acetate in n-hexane. The concentrated eluent (30 g) in vacuo was chromatographed on a silica gel column (BW-200, 100 by 4.2 cm); elution was done with 0 to 10% (vol/vol) methanol (MeOH) in CHCl3 (a total of 4 liters) as the eluent to give five fractions, 1 (2 g), 2 (5 g), 3 (13 g), 4 (10 g), and 5 (5 g). The absorption at 380 nm was monitored by thin-layer chromatography (TLC) (silica gel, high-performance TLC plate, 0.2 mm; Merck) with a solvent system of 3% (vol/vol) MeOH in CHCl3. Fraction 3 (13 g) was rechromatographed on a silica gel column (BW-200, 100 by 4.2 cm); gradual elution was done with 0 to 10% (vol/vol) MeOH in CHCl3 (a total of 4 liters) and then 0 to 30% (vol/vol) acetone in n-hexane (a total of 4 liters). Each fraction was subjected to TLC (high-performance TLC plate, 0.2 mm; Merck) with a solvent system of 3% (vol/vol) MeOH in CHCl3. The absorption at 380 nm was monitored; subsequent recovery, evaporation, and recrystallization from MeOH-H2O furnished pure licochalcone A (3 g).
The crystals of licochalcone A were orange needles, and the melting point was 101°C. The main absorption maxima of licochalcone A in MeOH were 254, 312, and 377 nm. Mass spectrometry of purified licochalcone A was carried out on a JMS-AM II apparatus (Joel Ltd., Tokyo, Japan) recording at 70 eV and with a source temperature of 250°C: m/z 338 (M+, 17%), 323 (4%), 307 (100%), and 121 (88%). The purity of licochalcone A was examined by high-pressure liquid chromatography and mass spectrometry as described previously (11, 18). Purified licochalcone A was dissolved in the mobile-phase solvent and applied to an octyldecyl silane column (YMC-Pack ODS-A312, 5-μm particle size, 6 by 150 mm; YMC Co. Ltd., Kyoto, Japan). Licochalcone A was eluted with 65 to 100% (vol/vol) MeOH at 1.5 ml/min and analyzed with a UV detector (SPD-6AU; Shimadzu, Kyoto, Japan) at 310 nm. Pure licochalcone A (>99%) was dissolved in dimethyl sulfoxide (DMSO) at 5% (wt/vol), and the licochalcone A solution was used as an antimicrobial agent in the experiments described below.
Activity against B. subtilis.
B. subtilis IFO 3007 was inoculated into nutrient broth (NB) (pH 7.0; Difco) in test tubes, grown with shaking for 24 h at 30°C, and then subcultured as vegetative cultures in fresh NB with shaking for 18 h at 30°C to approximately 109 cells/ml (2, 22). To determine the activity of licochalcone A against vegetative cells of B. subtilis, aliquots of 10 μl of the cultures were inoculated into 100 ml of fresh NB containing licochalcone A at 0, 1.0, 2.0, 3.0, 4.0, or 5.0 μg/ml in 200-ml Erlenmeyer flasks (12). The 5% (wt/vol) licochalcone A solution in DMSO was diluted in medium and added to the flasks. DMSO controls were included, although no adverse effects were observed at the highest concentration used. Culturing was continued at 30°C, and at each incubation time point, aliquots of the broth containing licochalcone A were inoculated into 10 ml of fresh NB agar on plates to determine viable cell counts in the broth. The plates were incubated for 48 h at 30°C, and CFU were counted as described previously (12). All analyses were performed with triplicate cultures, and values are given as means of three measurements.
To prepare spores of B. subtilis (2, 22), the vegetative cultures were spread onto NB agar supplemented with 50 μg of MnSO4/ml (21); the plates were incubated at 30°C for 7 days, until at least 95% of the population consisted of phase-bright spores under a phase-contrast light microscope. For harvesting of spores, the plates were washed with cold, sterile distilled water (4°C) to detach the spores, followed by centrifugation at 4,000 × g for 20 min at 4°C (2). Spore suspensions were cleaned of vegetative cells and debris by discarding the uppermost layers of the pellets obtained by centrifugation (22). The clean spore suspensions were stored at −20°C. A spore suspension (1 ml; 109 to 1010 cells/ml) was diluted in 3 ml of 0.1 M phosphate buffer (Na2HPO4-KH2PO4; pH 7.0) and activated by heating in a water bath at 70°C for 30 min (2, 22). Aliquots of the heat-activated spore suspension were transferred to fresh buffer in a final volume of 3 ml to adjust the turbidity at 610 nm to 1.0 (22). After both 10 mM l-alanine and 10 mM d-glucose, inducers of germination, were added to the buffer (2, 22), the germination of spores was determined by measuring the turbidity at 610 nm. To examine the effect of licochalcone A on the germination of B. subtilis, 10 μg of licochalcone A/ml was added to the spores with both l-alanine and d-glucose. All analyses were performed with triplicate cultures, and values are given as means of three measurements.
Determination of MICs against food microorganisms.
The media used for food contaminant microorganisms in this study were as follows: NB (pH 7.0; Difco) for E. coli K-12 IFO 3301, P. aeruginosa IFO 3923, B. cereus IFO 3514, B. coagulans IFO 12583, B. subtilis IFO 3007, B. stearothermophilus IFO 12550, and S. aureus 209P IFO 12732; brain heart infusion broth (pH 6.0; Difco) for E. faecalis IFO 3989, E. faecium IFO 3826, L. acidophilus IFO 13951, L. plantarum IFO 12519, L. mesenteroides IFO 3832, S. lactis IFO 12546, and S. mutans IFO 13955; thioglycolate medium (pH 7.0; Wako, Osaka, Japan) for C. sporogenes IFO 13950; and yeast extract-peptone-dextrose (pH 5.0) for A. niger IFO 6341 and S. cerevisiae IFO 0203. All microorganisms except for C. sporogenes IFO 13950 were inoculated into medium in test tubes and grown to stationary phase for 24 h at 30°C to up to 2.0 × 108 to 1.0 × 109 CFU/ml. C. sporogenes IFO 13950 was grown anaerobically for 72 h at 30°C to up to 108 CFU/ml.
MICs were estimated as described by Katsura et al. (12). The culture of each food microorganism was diluted 10-fold with sterile water. Aliquots of 100 μl of the diluted culture of each food microorganism were inoculated at 105 to 106 CFU/ml into 10 ml of each medium containing licochalcone A at 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0, 9.0, 10, 15, 20, 25, or 50 μg/ml in test tubes, serially diluted licochalcone A, and the respective growth medium. The MICs of licochalcone A against all microorganisms except for C. sporogenes IFO 13950 were determined by measuring the turbidity at 610 nm after 48 h of incubation at 30°C. The MIC against C. sporogenes IFO 13950 was determined by measuring the turbidity at 610 nm after 7 days of incubation at 30°C. The MIC for each microorganism was defined as the minimum concentration of licochalcone A limiting turbidity to <0.05 absorbance unit at 610 nm (12). All analyses were performed with triplicate cultures, and values are given as means of three measurements.
Stability of antimicrobial activity.
The MIC of licochalcone A against B. subtilis IFO 3007 was determined under the following conditions: temperature, 80, 100, and 121°C for 15 min; pH, 5.0 to 7.0; NaCl, 0 to 3.0% (wt/vol); and protease digestion, 20 μg/ml (Pronase-P; Nacalai Tesque, Kyoto, Japan). To evaluate the antimicrobial activity of licochalcone A, the antimicrobial peptides protamine (salmine; Wako) and ɛ-poly-l-lysine (Wako) were used for control experiments. All analyses were performed with triplicate cultures, and values are given as means of three measurements.
RESULTS
Activity against B. subtilis.
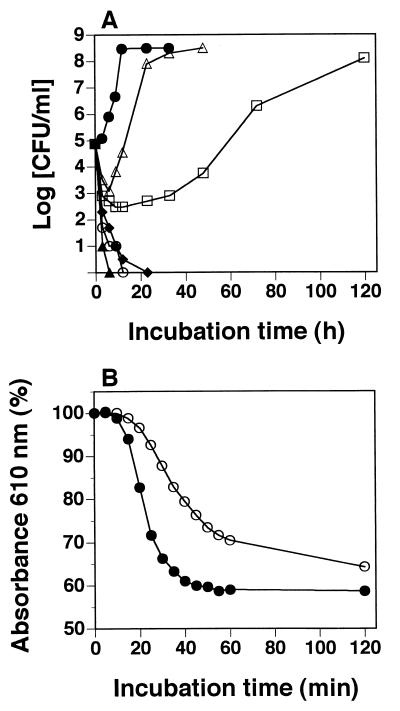
Figure 2A shows the inhibitory effect of licochalcone A on the vegetative growth of B. subtilis IFO 3007. Vegetative cell growth was inhibited in a licochalcone A concentration-dependent manner. At 2 μg/ml, licochalcone A inhibited the growth of B. subtilis IFO 3007 for 12 h before cell growth started. At 3 μg/ml, licochalcone A completely inhibited cell growth.
FIG. 2.
Viable counts of vegetative cells (A) and germination of spores (B) of B. subtilis IFO 3007 in the presence and absence of licochalcone A. Symbols (licochalcone A concentration): for panel A, ▵, 1 μg/ml; □, 2 μg/ml; ⧫, 3 μg/ml; ○, 4 μg/ml; ▴, 5 μg/ml; and •, none; and for panel B, ○, 10 μg/ml; and •, none.
To examine the effects of licochalcone A on the germination of B. subtilis IFO 3007, 10 μg of licochalcone A/ml was added to heat-treated spores with both 10 mM l-alanine and 10 mM d-glucose (Fig. 2B). Heat-treated spores of B. subtilis IFO 3007 germinated to vegetative cells after the addition of l-alanine as an inducer of germination. Germination was slightly inhibited by licochalcone A but reached the same level as in controls without licochalcone A after 120 min of incubation. Therefore, licochalcone A did not inhibit germination, as shown in Fig. 2B, but inhibited vegetative growth after the germination of B. subtilis spores, as shown in Fig. 2A.
MICs of licochalcone A against food microorganisms.
To evaluate the activity of licochalcone A against food contaminant microorganisms, the MICs were determined (Table 1). The bacteriostatic effects of licochalcone A were shown by MICs of 2 to 15 μg/ml for all gram-positive bacteria tested, including spore-forming bacteria, such as the genera Bacillus and Clostridium, and toxin-producing bacteria, such as B. cereus and S. aureus. In particular, licochalcone A was effective for all tested Bacillus spp., with MICs of 2 to 3 μg/ml. However, licochalcone A was not effective for gram-negative bacteria, such as E. coli and P. aeruginosa, or eukaryotes, such as fungi, at 50 μg/ml.
TABLE 1.
Activity of licochalcone A against food contaminant microorganisms
| Microorganism | MIC (μg/ml) |
|---|---|
| Escherichia coli K-12 IFO 3301 | >50 |
| Pseudomonas aeruginosa IFO 3923 | >50 |
| Bacillus cereus IFO 3514 | 3.0 |
| Bacillus coagulans IFO 12583 | 2.0 |
| Bacillus subtilis IFO 3007 | 2.0 |
| Bacillus stearothermophilus IFO 12550 | 2.0 |
| Clostridium sporogenes IFO 13950 | 8.0 |
| Enterococcus faecalis IFO 3989 | 6.0 |
| Enterococcus faecium IFO 3826 | 6.0 |
| Lactobacillus acidophilus IFO 13951 | 5.0 |
| Lactobacillus plantarum IFO 12519 | 5.0 |
| Leuconostoc mesenteroides IFO 3832 | 15.0 |
| Staphylococcus aureus 209P IFO 12732 | 3.0 |
| Streptococcus lactis IFO 12546 | 8.0 |
| Streptococcus mutans IFO 13955 | 5.0 |
| Aspergillus niger IFO 6341 | >50 |
| Saccharomyces cerevisiae IFO 0203 | >50 |
From the roots of G. inflata, we also purified two chalcones as reported by Hatano et al. (9) and examined the MICs against B. subtilis IFO 3007; these were 40 μg/ml for isoliquiritigenin and 50 μg/ml for echinatin.
Stability of antimicrobial activity.
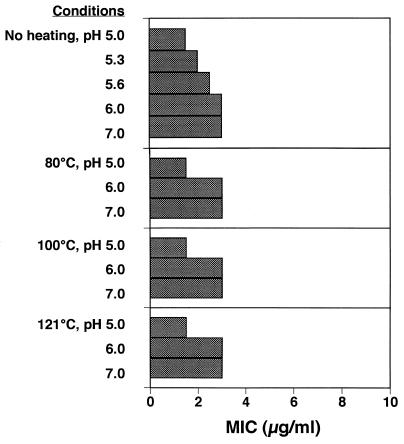
Figure 3 shows the heat stability of the effect of licochalcone A against B. subtilis IFO 3007 after holding at 80, 100, or 121°C for 15 min at a pH of between 5.0 and 7.0, conditions representative of the practical conditions encountered in food processing. The bacteriostatic activity of licochalcone A was resistant to heating at 80 to 121°C for 15 min and stable from pH 5.0 to pH 7.0, although the antibacterial activity at an acidic pH was higher than that at a neutral or an alkaline pH.
FIG. 3.
Activity of licochalcone A against B. subtilis IFO 3007 after heating at 80, 100, or 121°C for 15 min at a pH of between 5.0 and 7.0.
To compare the antibacterial activity of licochalcone A in the presence of NaCl or protease with those of two cationic antimicrobial peptides, protamine and ɛ-poly-l-lysine, the inhibitory actions of these compounds on B. subtilis IFO 3007 and S. aureus IFO 12732 were examined by using media supplemented with NaCl or protease (Table 2). The MICs of protamine and ɛ-poly-l-lysine for the two bacterial strains increased in both an NaCl concentration-dependent manner and an incubation time-dependent manner. After only 5 h of incubation of the two antimicrobial peptides with 3% (wt/vol) NaCl, the MICs of these peptides increased rapidly (data not shown). In contrast, the MIC of licochalcone A was stable even in the presence of 3% (wt/vol) NaCl after 7 days. Moreover, digestion of the two antimicrobial peptides with protease resulted in the partial loss of antimicrobial activities, but licochalcone A was resistant to protease digestion.
TABLE 2.
MICs of licochalcone A, protamine, and ɛ-poly-l-lysine against food contaminant bacteria in the presence of either NaCl or protease
| Compound | Incubation time (days)a | MIC (μg/ml) against the following organism under the indicated conditions:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. subtilis IFO 3007
|
S. aureus IFO 12732
|
||||||||||
| % NaCl (wt/vol)
|
Protease at 20 μg/ml | % NaCl (wt/vol)
|
Protease at 20 μg/ml | ||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||||
| Licochalcone A | 2 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| 7 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | |
| Protamine | 2 | 6.0 | 15.0 | 100 | 300 | 25.0 | 3.0 | 6.0 | 6.0 | 600 | 6.0 |
| 7 | 6.0 | 25.0 | 300 | 300 | 25.0 | 3.0 | 15.0 | 300 | >600 | 25.0 | |
| ɛ-Poly-l-lysine | 2 | 3.0 | 3.0 | 6.0 | 15.0 | 6.0 | 2.0 | 2.0 | 15.0 | 300 | 2.0 |
| 7 | 3.0 | 3.0 | 6.0 | 100 | 6.0 | 3.0 | 6.0 | 600 | >600 | 2.0 | |
MICs were determined by measuring the turbidity at 610 nm after 2 or 7 days of incubation at 30°C.
DISCUSSION
A recent trend in food processing is to avoid the use of chemical preservatives; thus, natural antimicrobial alternatives are required. It is of interest to determine if spores occurring in foods will germinate after heat treatment and if they will grow during storage. The heat resistance of spores is influenced by the environment and varies for different Bacillus species (17). Generally, food manufacturers rely on preservation by moist heat to produce food products stable under ambient conditions (2). However, if heat activation of germination of the spores were to occur in this process, subsequent outgrowth might result in spoilage and/or food poisoning during preservation (2). Therefore, it is important to evaluate activity against spore-forming bacteria to determine whether antimicrobial compounds inhibit spore germination or vegetative growth. In particular, food preservatives would need to have antibacterial effects against outgrowth after pasteurization. Licochalcone A did not inhibit germination but completely inhibited the outgrowth of B. subtilis spores (Fig. 2). Two cationic antimicrobial peptides, protamine and ɛ-poly-l-lysine, showed similar effects (14, 16, 20), but oleuropein purified from olive extract inhibited both the germination and the subsequent outgrowth of spores of B. cereus (22).
The cationic antimicrobial peptides caused the release of calcium from the spores of Bacillus spp. (16), and the growth inhibitory effect was repressed by the addition of calcium salts (10) and NaCl, as shown in Table 2. These findings suggested that the presence of a high concentration of salt could interfere with the binding of cationic peptides to the cell surface. Furthermore, the peptides were not resistant to protease digestion; thus, the macromolecules carried many cationic residues required for activity (16). Recently, it was reported that a series of hybrid cationic peptides were resistant to salt and had activity against P. aeruginosa in the presence of up to 300 mM NaCl (4). The ability to resist salt (NaCl is the most predominant salt in vivo) is important for cationic peptides to function under physiological conditions. Interactions with food components, such as salts and proteases of endogenous or microbial origin, might limit the scope of the use of cationic antimicrobial peptides as preservatives in foods. The antibacterial activity of licochalcone A was stable even in the presence of 3% (wt/vol) NaCl, and this compound was resistant to protease digestion. Therefore, licochalcone A would be a useful compound for the development of antibacterial agents for the preservation of foods containing high concentrations of salt, such as soup, and proteases, such as fermentative foods, in which the cationic peptides might be less effective.
Some antimicrobial flavonoids from the licorice species G. uralensis (9), G. glabra (3, 5, 13, 15, 18), and G. inflata (8, 18) were reported previously. The content of licochalcone A in G. inflata was the highest (ca. 0.8%) in these three licorice species (19). We examined the MICs of chalcones purified from the roots of G. inflata against B. subtilis IFO 3007; the MICs (in micrograms per milliliter) were as follows: licochalcone A, 2.0; isoliquiritigenin, 40; and echinatin, 50. Among these compounds, licochalcone A showed the strongest antibacterial activity. Furthermore, licochalcone A also showed potent effects on both methicillin-resistant S. aureus and methicillin-sensitive S. aureus, with an MIC of 16 μg/ml (9). It was reported that licochalcone A inhibited oxygen consumption in Micrococcus luteus cells, and the site of inhibition was thought to be between CoQ and cytochrome c in the bacterial respiratory electron transport chain (8). The presence of a hydrophobic prenyl moiety in licochalcone A would be important, since it would provide sufficient hydrophobicity for molecules to penetrate the cell membrane. Recently, Chen et al. (1) reported that chalcones exhibited potent activities (antileishmanial and antimalarial) against Leishmania major and Leishmania donovani and that licochalcone A selectively inhibited fumarate reductase in the respiratory chain of the parasites.
In the present study, salt-, heat-, and protease-resistant licochalcone A was suggested to be a promising lead compound for the development of agents against spore-forming bacteria, such as those of the genera Bacillus and Clostridium. The oil extract from G. inflata in which licochalcone A is the main constituent has been used for the preservation of foods. Higashimaru Shoyu Co., Ltd. (Tatsuno, Japan) is the only commercial source for the extract containing licochalcone A as an antibacterial agent because it holds the patent (22a). The mechanism of the antibacterial activity of licochalcone A and its application as a food additive are currently under investigation in our laboratory.
REFERENCES
- 1.Chen, M., L. Zhai, S. B. Christensen, T. G. Theander, and A. Kharazmi. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciarciaglini, G., P. J. Hill, K. Davies, P. J. McClure, D. Kilsby, M. H. Brown, and P. J. Coote. 2000. Germination-induced bioluminescence, a route to determine the inhibitory effect of a combination preservation treatment on bacterial spores. Appl. Environ. Microbiol. 66:3735-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demizu, S., K. Kajiyama, K. Takahashi, Y. Hiraga, S. Yamamoto, Y. Tamura, K. Okada, and T. Kinoshita. 1988. Antioxidant and antimicrobial constituents of licorice: isolation and structure elucidation of a new benzofuran derivative. Chem. Pharm. Bull. 36:3474-3479. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. W. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukui, H., K. Goto, and M. Tabata. 1988. Two antimicrobial flavanones from the leaves of Glycyrrhiza glabra. Chem. Pharm. Bull. 36:4174-4176. [DOI] [PubMed] [Google Scholar]
- 6.Hansen, L. T., J. W. Austin, and T. A. Gill. 2001. Antibacterial effect of protamine in combination with EDTA and refrigeration. Int. J. Food Microbiol. 66:149-161. [DOI] [PubMed] [Google Scholar]
- 7.Haraguchi, H., H. Ishikawa, K. Mizutani, Y. Tamura, and T. Kinoshita. 1998. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg. Med. Chem. 6:339-347. [DOI] [PubMed] [Google Scholar]
- 8.Haraguchi, H., K. Tanimoto, Y. Tamura, K. Mizutani, and T. Kinoshita. 1998. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 48:125-129. [DOI] [PubMed] [Google Scholar]
- 9.Hatano, T., Y. Shintani, Y. Aga, S. Shiota, T. Tsuchiya, and T. Yoshida. 2000. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanonem and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 48:1286-1292. [DOI] [PubMed] [Google Scholar]
- 10.Johansen, C., A. Verheul, L. Gram, T. Gill, and T. Abee. 1997. Protamine-induced permeabilization of cell envelopes of gram-positive and gram-negative bacteria. Appl. Environ. Microbiol. 63:1155-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajiyama, K., S. Demizu, Y. Hiraga, K. Kinoshita, K. Koyama, K. Takahashi, Y. Tamura, K. Okada, and T. Kinoshita. 1992. Two prenylated retrochalcones from Glycyrrhiza inflata. Phytochemistry 31:3229-3232. [Google Scholar]
- 12.Katsura, H., R. Tsukiyama, A. Suzuki, and M. Kobayashi. 2001. In vitro antimicrobial activity of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 45:3009-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, W., Y. Asada, and T. Yoshikawa. 1998. Antimicrobial flavonoids from Glycyrrhiza glabra hairy root cultures. Planta Med. 64:746-747. [DOI] [PubMed] [Google Scholar]
- 14.Liang, J. F., and S. G. Kim. 1999. Not only the nature of peptide but also the characteristics of cell membrane determine the antimicrobial mechanism of a peptide. J. Pept. Res. 53:518-522. [DOI] [PubMed] [Google Scholar]
- 15.Mitscher, L. A., Y. H. Park, and D. Clark. 1980. Antimicrobial agents from higher plants. Antimicrobial isoflavanoids and related substances from Glycyrrhiza glabra L. var. typica. J. Nat. Prod. 43:259-269. [DOI] [PubMed] [Google Scholar]
- 16.Nishihara, T., M. Kosugi, Y. Matsue, J. Nishikawa, A. Takasaki, Y. Takubo, and M. Kondo. 1992. Antimicrobial activity of positive colloids against food-poisoning bacteria. J. Antibact. Antifung. Agents 20:241-245. [Google Scholar]
- 17.Nissen, H., H. Holo, L. Axelsson, and H. Blom. 2001. Characterization and growth of Bacillus spp. in heat-treated cream with and without nisin. J. Appl. Microbiol. 90:530-534. [DOI] [PubMed] [Google Scholar]
- 18.Okada, K., Y. Tamura, M. Yamamoto, Y. Inoue, R. Takagaki, K. Takahashi, S. Demizu, K. Kajiyama, Y. Hiraga, and T. Kinoshita. 1989. Identification of antimicrobial and antioxidant constituents from licorice of Russian and Xinjiang origin. Chem. Pharm. Bull. 37:2528-2530. [DOI] [PubMed] [Google Scholar]
- 19.Shibata, S. 2000. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 120:849-862. [DOI] [PubMed] [Google Scholar]
- 20.Shima, S. H. Matsuoka, T. Iwamoto, and H. Sakai. 1984. Antimicrobial action of ɛ-poly-l-lysine. J. Antibiot. 37:1449-1455. [DOI] [PubMed] [Google Scholar]
- 21.Sneath, P. H. A. 1986. Endospore-forming gram-positive rods and cocci, p.1104-1207. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md.
- 22.Tassou, C. C., G. J. E. Nychas, and R. G. Board. 1991. Effect of phenolic compounds and oleuropein on the germination of Bacillus cereus T spores. Biotechnol. Appl. Biochem. 13:231-237. [PubMed] [Google Scholar]
- 22a.Tsukiyama, R., E. Miura, and S. Moriguchi. 11April1994. Antibacterial agent. Japanese patent 1,837,978.
- 23.Wang, Y.-B., and G. R. Germaine. 1991. Effect of lysozyme on glucose fermentation, cytoplasmic pH, and intracellular potassium concentrations in Streptococcus mutans 10449. Infect. Immun. 59:638-644. [DOI] [PMC free article] [PubMed] [Google Scholar]