Abstract
Linezolid is a new oxazolidinone antibiotic with potent activity against gram-positive bacteria, including Streptococcus pneumoniae. The pharmacodynamic activity and in vivo efficacy of linezolid were compared to those of ceftriaxone in an immunocompetent rat model of pneumococcal pneumonia. Rats infected intratracheally with 8 × 107 CFU of a penicillin-sensitive (MIC, 0.032 μg/ml) strain of S. pneumoniae were treated for 5 days beginning 18 h postinfection. Groups of rats were sham treated with oral phosphate-buffered saline or received oral liquid linezolid at 25 or 50 mg/kg of body weight twice a day (b.i.d.) or subcutaneous ceftriaxone at 100 mg/kg once daily. Mortality was monitored for 10 days postinfection; blood culturing was performed on day 1 (pretreatment) and on days 3, 5, and 10 postinfection for the determination of bacteremia. Serum also was collected for the determination of pharmacokinetic and pharmacodynamic parameters at 30 min and at 3, 5, and 12 h (linezolid) or 3, 5, and 24 h (ceftriaxone) postdose. The cumulative mortality rates were 100% for the sham-treated group, 58.3% for the low-dose linezolid group, 8.3% for the high-dose linezolid group, and 0% for the ceftriaxone group. Rats in each of the antibiotic treatment groups had significantly fewer bacteria (P < 0.00001) in their bronchoalveolar lavage fluid (BALF) on day 3 postinfection than sham-treated rats. There also were significantly fewer organisms in the BALF of rats treated with ceftriaxone than in the BALF of rats treated with either dose of linezolid. Oral linezolid at 50 mg/kg b.i.d. therefore was as effective as ceftriaxone in experimental pneumococcal pneumonia, whereas the 25-mg/kg b.i.d. dose was significantly less effective. All pharmacodynamic parameters reflected efficacy and were significantly different for the two dosage regimens of linezolid (P < 0.01). However, the free-fraction pharmacodynamic parameters predictive of outcome were a value of >39% for the percentage of time in the experimental dosing interval during which the linezolid concentration exceeded the MIC and a value of >147 for the ratio of the area under the serum concentration-time curve to the MIC.
Linezolid is the first member of the novel class of oxazolidinone antibiotics to be licensed in the United States. These compounds bind to the 50S subunit of bacterial ribosomes, blocking the formation of the initiation complex without inhibiting elongation or termination (23). Linezolid has excellent activity in vitro against a number of gram-positive bacteria, including penicillin-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci (17, 27). It is rapidly and extensively absorbed after oral dosing and has an average absolute bioavailability of approximately 100% (6). Therefore, it may be given orally as well as intravenously.
S. pneumoniae (pneumococcus) is the most common bacterial agent of community-acquired pneumonia, causing 15 to 25% of all cases in adults (2, 12). Mortality in immunocompetent adults is ∼5% for uncomplicated pneumonia but rises to ∼20 to 25% if a patient becomes bacteremic (9, 16). Mortality associated with pneumococcal infections dropped dramatically in the 1940s following the discovery of penicillin, and the organism remained uniformly sensitive to that drug until the 1980s (13, 25). Today, however, the prevalence of penicillin-resistant pneumococci is rapidly increasing and is often accompanied by concomitant resistance to multiple additional antibiotic classes (3, 4). Because the oxazolidinones have a unique mechanism of action, they are not affected by cross-resistance of organisms possessing mechanisms of resistance to agents that inhibit ribosomal protein synthesis. These agents include the macrolides, lincosamides, tetracyclines, chloramphenicol, and aminoglycosides (5, 7, 11). Oxazolidinones, therefore, show great promise for the treatment of pneumococcal pneumonia. In the present study, a rat model of pneumococcal pneumonia was used to determine the pharmacokinetic and pharmacodynamic parameters of orally administered linezolid. Its efficacy also was compared to that of a proven dose of ceftriaxone.
(These data were presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2000, Toronto, Ontario, Canada.)
MATERIALS AND METHODS
Experimentally induced pneumococcal pneumonia.
Type 3 S. pneumoniae (ATCC 6303; American Type Culture Collection, Manassas, Va.) was grown to logarithmic phase in Todd-Hewitt broth containing 5% rabbit serum. The organisms were collected by centrifugation, washed twice, and resuspended in phosphate-buffered saline (PBS) to the appropriate concentration, as determined by spectrophotometric estimation at 540 nm. Actual numbers of bacterial CFU were determined retrospectively by a standard plate count procedure.
Male Spraque-Dawley rats (Charles River Laboratories, Kingston, N.Y.) weighing 200 to 250 g were anesthetized with isoflurane (Aerrane, Fort Dodge, Iowa). A small incision was made in the skin overlying the trachea, which was exposed by blunt dissection (8). A 20-gauge catheter was inserted into the main stem bronchus, and through it was delivered 0.3 ml of the pneumococcal suspension containing 8 × 107CFU. This value represents ∼50 times the lethal dose for these animals, such that all untreated animals would be expected to die. All studies were conducted in accordance with standard animal experimentation guidelines and with the approval of the Animal Care and Use Committee at the Veterans Affairs Medical Center, Omaha, Nebr.
Antibiotic treatment.
Groups of 12 rats each were infected and then treated for 5 days, beginning 18 h postinfection. They were given oral PBS twice a day (b.i.d.), oral liquid linezolid (Zyvox pediatric suspension; Pharmacia Upjohn, Kalamazoo, Mich.) at 25 or 50 mg/kg of body weight b.i.d. (50 or 100 mg/kg/day), or subcutaneous ceftriaxone (Rocephin; Hoffmann-La Roche, Nutley, N.J.) at 100 mg/kg once daily (100 mg/kg/day). The MICs of the study antibiotics for the infecting strain were determined with E-test strips provided for linezolid by Pharmacia Upjohn and for ceftriaxone by AB Biodisk (Solna, Sweden). MICs were 0.75 μg/ml for linezolid and 0.016 μg/ml for ceftriaxone.
Survival studies.
Mortality was monitored for 10 days postinfection with quantitative blood cultures of samples obtained by aseptic foot puncture (24) on day 1 (pretreatment) and on days 3, 5, and 10 postinfection. The number of bacteria present per milliliter of blood was determined by a standard plate count technique.
Pharmacokinetic and pharmacodynamic study samples.
Rats (seven/group) not used in mortality studies were infected, treated for 3 days, and then killed by an intraperitoneal injection of pentobarbital (Nembutal; Abbott Laboratories, North Chicago, Ill.), followed by exsanguination. This meant that the rats were sacrificed 30 min after the fifth linezolid dose or 60 min after the third ceftriaxone dose. Serum was collected for the determination of antibiotic concentrations, and the lungs and trachea were removed immediately. A 20-gauge catheter was positioned above the carina. Cold PBS was injected into the lungs in 8-ml aliquots (∼60 ml total) and recovered by gravity drainage until a final volume of 50 ml was collected. Aliquots of bronchoalveolar lavage fluid (BALF) were cultured quantitatively by plate counting. Cells in the BALF (BAL cells) then were collected by centrifugation, resuspended in PBS, and counted with a hemacytometer. Differential counting was performed on Wright-stained cytospin slides of the cell preparations.
Additional blood samples for the determination of serum antibiotic concentrations were collected from rats infected and treated for 3 days as described above. Blood was obtained by cardiac puncture under isoflurane anesthesia immediately before (trough) and at 3, 5, and 12 h (linezolid) or 3, 5, and 24 h (ceftriaxone) after the final dose of antibiotic. Serum, BALF, and BAL cells were frozen at −80°C for subsequent antibiotic assays by high-performance liquid chromatography techniques as described below.
Concentrations of antibiotics in plasma, BALF, and BAL cells.
The concentrations of ceftriaxone in BALF, BAL cells, and plasma were determined by a previously published assay with UV detection (10). BAL cells were lysed by freeze-thaw cycles. Acetonitrile containing the internal standard (cephalexin; Sigma, St. Louis, Mo.) was added to the cells, along with plasma for deproteinization. The samples then were vortexed and centrifuged, and 50 μl of the supernatant was injected into the column. BALF samples were centrifuged and then filtered through a 0.22-μm-pore-size filter to remove bacteria prior to extraction and injection into the column. Separation was performed with a C18 reverse-phase column (70 by 4.6 mm; particle size, 3 μm; Phenomix, Torrance, Calif.). The mobile phase consisted of 24 mM hexadecyl trimethylammonium bromide-phosphate buffer (pH 7.0)-acetonitrile (45:5:52, vol/vol/vol) at a flow rate of 1.5 ml/min and a temperature of 35°C. Quality control samples were included in each analytical sequence to confirm the accuracy and precision of the assay, which was linear over the range of 0.25 to 100 μg/ml with the limit of quantification set at 10 ng/ml. Samples that exceeded the 100-μg/ml concentration were diluted and reinjected into the column. The inter- and intraday coefficients of variation were 7.7 and 3.8% for plasma, 8.0 and 5.2% for BAL cells, and 9.8 and 7.8% for BALF. The volume of BAL cells in the cell pellet was determined by methods described previously (1, 26).
Separation of free from protein-bound fractions of linezolid and ceftriaxone was accomplished by an ultrafiltration technique. Briefly, 0.375 ml of serum from each rat was divided into two aliquots, one of which was inserted into an ultrafiltration device (Amicon, Inc., Beverly, Mass.) and centrifuged at a fixed angle for 20 min at 1,000 × g. The resultant ultrafiltrate and the nonfiltered serum aliquot then were analyzed for the respective antibiotics. The ratio of the concentration in the ultrafiltrate to the total concentration in the nonfiltered sample was used to calculate the antibiotic free fraction.
Linezolid concentrations in all matrixes were determined by a sensitive high-performance liquid chromatography-UV assay described previously (19). Briefly, BALF and BAL cell samples were processed as described above. Linezolid was extracted from all samples by mixing an aliquot with methanol and centrifuging the mixture. The supernatant was collected, evaporated to dryness under a stream of nitrogen at 40°C, reconstituted with 200 μl of the mobile phase, and injected into the column. Separation was performed by using a C8 reverse-phase column (4.6 by 150 mm, 5 μm; Phenomix) with a mobile phase of acetonitrile-water (20:80, vol/vol) at a 1.25-ml flow rate and with the column heated to 40°C. The wavelength was set at 251 nm. The assay was linear over the concentration range of 5 to 1,000 ng/ml. Intra- and interday coefficients of variation were 4.9 and 6.7%, respectively.
Pharmacokinetics and pharmacodynamics.
Serum antibiotic concentrations versus time were analyzed with WinNonlin Software, Standard Edition, version 1.5 (Scientific Consulting, Inc., Cary, N.C.). The linezolid and ceftriaxone pharmacokinetic parameters were estimated by using a noncompartmental extravascular dose input model. The area under the serum-concentration time curve (AUC) from 0 to 12 h for linezolid and the AUC from 0 to 24 h for ceftriaxone were calculated by using the trapezoidal rule. Pharmacodynamic parameters were determined by dividing the MIC of the antibiotics for S. pneumoniae 6303 into the AUC (AUC/MIC) and the calculated maximum concentration of drug in serum (Cmax) (Cmax/MIC). The percentage of time during which the concentration in serum exceeded the MIC (%T>MIC) was calculated by using the equation Cpmin = Cpmax e−ket, where Cpmin equals the MIC, Cpmax equals the calculated maximum peak concentration in serum, and e−ket is the decay parameter.
Statistical analysis.
Differences in the development of bacteremia and mortality studies were analyzed by Fisher's exact test. The numbers of white blood cells (WBC) and bacteria in BALF samples were compared by a one-way analysis of variance with post hoc comparisons by Tukey's test. Mean pharmacokinetic and pharmacodynamic parameters were compared by a one-way analysis of variance with post hoc comparisons by the Newman-Keuls test. The level of significance was set at P < 0.05 for all analyses.
RESULTS
Survival studies.
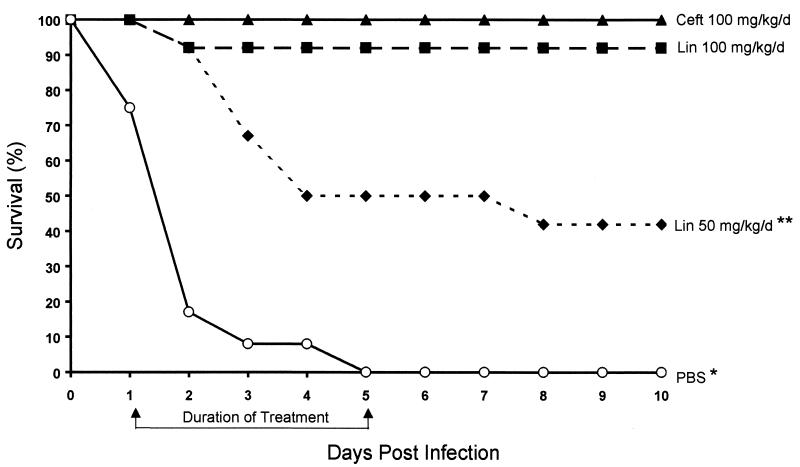
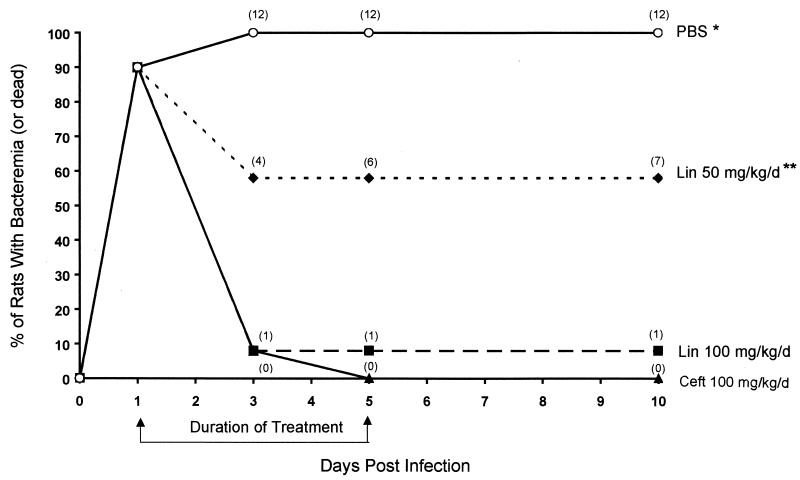
Two mortality trial experiments were performed with 6 rats/treatment group each (12 rats/group in total). The cumulative mortality for the PBS group was 100% (P < 0.05 for this group compared to all other groups), and that for the ceftriaxone group was 0%. One of the 12 rats (8.3%) treated with the high dose (100 mg/kg/day) of linezolid and 7 of the 12 rats (58.3%) treated with the low dose (50 mg/kg/day) of linezolid died (P < 0.05 for the low-dose linezolid group versus both the high-dose linezolid group and the ceftriaxone group). All but one of the deaths occurred during the antibiotic treatment regimens (days 2 to 5 postinfection) (Fig. 1). Resolution of bacteremia essentially mirrored mortality, with negative blood cultures being obtained in 0% of the PBS, 100% of the ceftriaxone, 82% of the high-dose linezolid, and 42% of the low-dose linezolid groups when the treatments were discontinued on day 5 postinfection (Fig. 2). Death occurred on day 2 for one rat receiving high-dose linezolid and one rat receiving low-dose linezolid after they had received only two doses of the drug. The remainder of the antibiotic-treated rats that died had received at least four doses before succumbing.
FIG. 1.
Mortality study. Survival of rats for 10 days after experimental challenge with 8 × 107 CFU of S. pneumoniae 6303. Rats were treated on days (d) 1 to 5 with ceftriaxone (Ceft), linezolid (Lin), or PBS. A single asterisk indicates a P value of 0.0001 for comparisons with ceftriaxone and linezolid at 100 mg/kg/day and a P value of 0.04 for a comparison with linezolid at 50 mg/kg/day. A double asterisk indicates a P value of 0.005 for a comparison with ceftriaxone and a P value of 0.03 for a comparison with linezolid at 100 mg/kg/day.
FIG. 2.
Bacteremia development and resolution during treatment. Rats were infected on day 0 with 8 × 107 CFU of S. pneumoniae 6303. Treatment with the indicated antibiotics occurred on days 1 to 5. All rats that remained bacteremic after 3 days of treatment died. See the legend to Fig. 1 for definitions of abbreviations. Numbers in parentheses are cumulative deaths for 12 rats/group. A single asterisk indicates a P value of <0.05 for all comparisons. A double asterisk indicates a P value of 0.03 for comparisons with ceftriaxone and linezolid at 100 mg/kg/day.
Bacterium and cell counts in BALF.
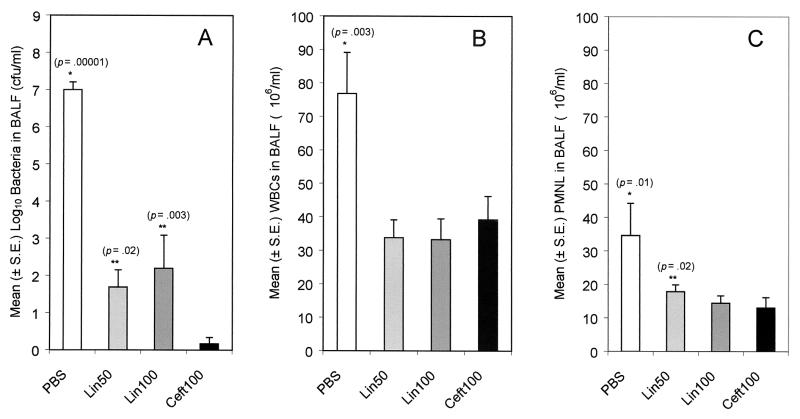
On day 3 of treatment, there were significantly more live S. pneumoniae organisms in the BALF of PBS-treated rats than in the BALF of rats treated with any of the antibiotics (P < 0.00001 for all comparisons) (Fig. 3A). There also were significantly fewer organisms in the BALF of ceftriaxone-treated rats than in the BALF of rats treated with either dose of linezolid (P = 0.02 and 0.003 for the low-dose and high-dose linezolid groups, respectively).
FIG. 3.
Numbers of bacteria, total WBC, and PMNL in BALF. Rats were infected with 8 × 107 CFU of S. pneumoniae 6303. On day 3 postinfection (after five doses of linezolid or three doses of ceftriaxone), the rats were sacrificed and bronchoalveolar lavage was performed until 50 ml of fluid was obtained. Bacteria in the fluid were quantified by a plate count technique, total cells were counted with a hemacytometer, and PMNL numbers were determined by multiplying the number of total cells by the percentage of cells that were neutrophils. The lower limit of bacterial detection was 10/ml. Ceft100, ceftriaxone at 100 mg/kg/day; Lin100, linezolid at 100 mg/kg/day; Lin50, linezolid at 50 mg/kg/day; S.E., standard error. A single asterisk indicates that the mean was significantly higher than that for each of the antibiotic-treated groups. A double asterisk indicates that the mean was significantly higher than that for the ceftriaxone-treated group.
The PBS-treated group had significantly larger numbers of total WBC (Fig. 3B) and polymorphonuclear leukocytes (PMNL) (Fig. 3C) in their BALF than rats treated with any of the antibiotics (P = 0.003 and 0.01, respectively). The numbers of WBC and PMNL were nearly equivalent for the antibiotic-treated groups (with the exception of slightly larger numbers of PMNL in the low-dose linezolid group).
Pharmacokinetic and pharmacodynamic parameters.
The mean Cmax (milligrams per liter) was highest for the ceftriaxone group (P < 0.001). The Cmax values for the low-dose linezolid group were approximately half those for the high-dose group (Table 1). The BALF and WBC concentrations for the high-dose linezolid group were not quite double those of the low-dose group, although they were significantly higher (P = 0.012). Levels of ceftriaxone were below the detection limits of the assay in both the BALF and WBC.
TABLE 1.
Pharmacokinetic and pharmacodynamic properties of the treatment regimensa
| Antibiotic (dose) | Cmaxb (mg/liter) | BALF (μg/ml) | WBC (μg/ml) | AUC from 0 to 24 h (mg · h/liter) |
|---|---|---|---|---|
| Linezolid (25 mg/kg b.i.d.) | 12.7 ± 2.9 | 2.3 ± 1.8 | 1.2 ± 0.5 | 89.2 ± 17.8 |
| Linezolid (50 mg/kg b.i.d.) | 24.6 ± 5.2 | 3.9 ± 2.4c | 1.9 ± 0.6 | 148.6 ± 32.8 |
| Ceftriaxone (100 mg/kg q.i.d.) | 97.2 ± 14.6d | Tr | ND | 65.1 ± 12.5 |
Values are given as means and standard deviations. ND, not detected.
At 30 min after the dose.
The P value for a comparison with the low-dose linezolid group was 0.012.
The P value for comparisons with both linezolid groups was <0.001.
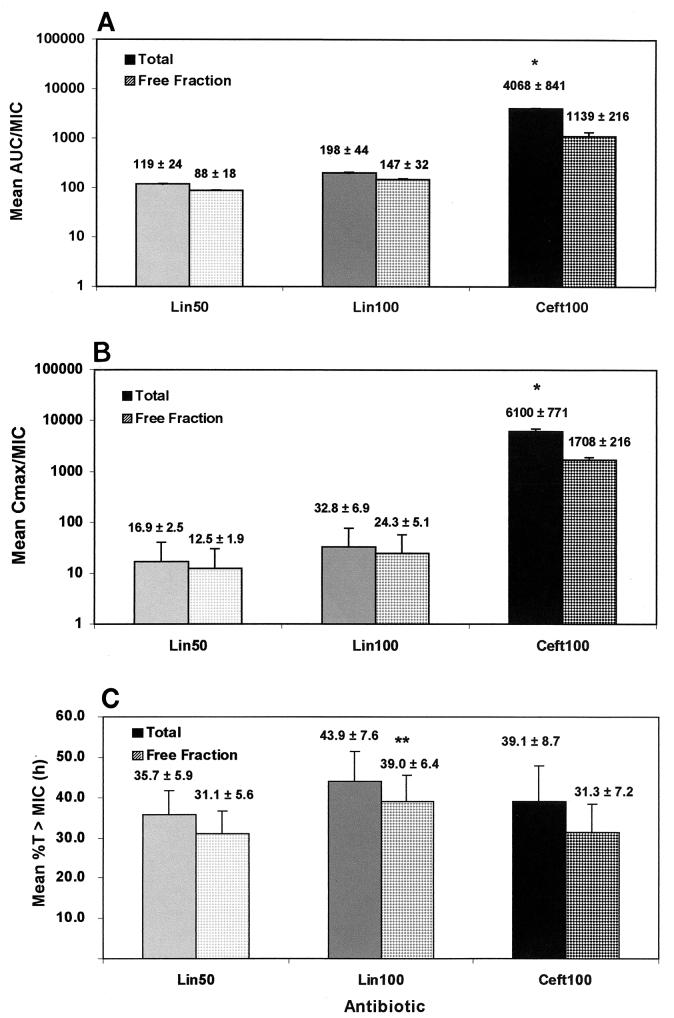
The ceftriaxone pharmacodynamic parameters of total and free-fraction AUC/MIC and Cmax/MIC ratios (Fig. 4A and B) were quite high, a result which was a reflection of the low MIC of this compound against S. pneumoniae 6303. Both parameters were significantly higher than those observed with either dose of linezolid (P < 0.001 for all comparisons). However, the free-fraction pharmacodynamic parameter of %T>MIC, which reflects the antibiotic half-life, concentration in serum, and MIC against the organism, was significantly higher (39.0%) for the high-dose linezolid group than for the low-dose linezolid group (31.1%) (P < 0.01) or the ceftriaxone group (31.3%) (P < 0.01) (Fig. 4C).
FIG. 4.
Pharmacodynamic parameters for all antibiotic groups. Pharmacodynamic parameters (mean and standard deviation) for each of the antibiotic groups were calculated from the values shown in Table 1. The %T>MIC was calculated by using MICs of 0.75 and 0.016 μg/ml and half-lives of 1.02 and 0.7 h for linezolid and ceftriaxone, respectively. See the legend to Fig. 3 for definitions of abbreviations. A single asterisk indicates a P value of <0.001 for a comparison with either dose of linezolid. A double asterisk indicates a P value of <0.01 for a comparison with ceftriaxone or the low dose of linezolid.
DISCUSSION
Linezolid, a new oxazolidinone antibiotic, is active against a number of gram-positive bacteria. It has excellent activity against both penicillin-sensitive and penicillin-resistant S. pneumoniae (14, 15, 17). In the present study, the pharmacokinetic and pharmacodynamic properties of linezolid, as well as its efficacy against a penicillin-sensitive (MIC, 0.032 μg/ml) S. pneumoniae isolate, were compared to those of ceftriaxone, an expanded-spectrum parenteral cephalosporin. Ceftriaxone has been approved for the therapy of pneumococcal infections, including pneumonia, bacteremia, and meningitis. It was shown previously to be uniformly effective in preventing fatal pneumococcal pneumonia in our rat model (22). The two linezolid dosage regimens used in this study were chosen to help determine the important pharmacokinetic and pharmacodynamic properties of an effective dose versus a substandard dose of the antibiotic.
When the antibiotic treatment was initiated at 18 h postinfection, 90% of the rats were found to have developed bacteremia. By the time the antibiotic treatment was completed on day 5 postinfection, all 12 ceftriaxone-treated, 11 of 12 high-dose linezolid-treated, and 5 of 12 low-dose linezolid-treated rats had completely resolved the bacteremia. Each of those rats survived the infection. In contrast, all of the PBS (sham)-treated rats remained bacteremic, and all of them died. A large number of the control rats (83%) succumbed to the infection by the end of day 2, demonstrating the severity of the bacterial challenge. This result calls into question the significance of the single death that occurred in the high-dose (50 mg/kg b.i.d.) linezolid group after only two doses of the antibiotic. One low-dose (25 mg/kg b.i.d.) linezolid-treated rat also died on day 2 postinfection, but five additional rats in the same treatment group died on days 3 to 8, demonstrating that the lower dose was not sufficient to cure this severe bacterial challenge. This failure of the low-dose treatment is contrary to the findings of a recently published study with a chinchilla model of otitis media due to a penicillin-resistant strain of S. pneumoniae (18). In that study, oral b.i.d. dosing with 25 mg/kg for 5 days was effective in sterilizing middle ear fluid cultures and eradicating the organism from the nasopharynges of the animals. However, the bacterial inoculum used in that study was 100 to 1,000 CFU/chinchilla, as opposed to the inoculum of 8 × 107 CFU used in the present study.
On day 3 postinfection, the BALF from the PBS-treated rats contained a mean of 7.0 logs of S. pneumoniae/ml of fluid retrieved. Treatment with any of the antibiotic regimens resulted in a mean 4.8- to 6.8-log decrease in the number of organisms per milliliter of fluid collected from the rat lungs. There was no significant difference in the numbers of organisms collected by lung lavage from rats receiving the high dose versus the low dose of linezolid. However, rats in both linezolid groups had significantly more bacteria in their lungs than those treated with ceftriaxone. These differences were due apparently to the actions of the antibiotics themselves rather than to increased clearance by pulmonary phagocytes, since all groups of antibiotic-treated animals had essentially equivalent numbers of WBC and PMNL in their lungs. The PBS-treated rats, on the other hand, had significantly more total WBC and more PMNL in their lungs than rats in any of the antibiotic treatment groups but still succumbed to the infection.
The higher concentrations in serum and lower MIC for ceftriaxone resulted in superior AUC/MIC and Cmax/MIC pharmacodynamic parameters for this drug relative to those for either dose of linezolid. However, the differences in these two parameters were not reflected in the efficacies of the high-dose linezolid and ceftriaxone groups, both of which were protective. The use of serum free-fraction values in the calculation of pharmacodynamic parameters appears to be a better representation of in vivo antibiotic responses. The protein binding values for ceftriaxone and linezolid in our rat model (72 and 26%, respectively) are similar to those found in other animal and human studies (21; K. Chiba, K. L. Feenstra, J. G. Slatter, P. T. Daley-Yates, J. N. Duncan, et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-123, 1998). Our data suggest that when linezolid achieved free-fraction AUC/MIC, Cmax/MIC, and %T>MIC values of 147, 24.3, and 39.0, respectively, the resulting efficacy was similar to that of ceftriaxone. Based on the pharmacokinetic parameters achieved for linezolid in humans (T. L. Sisson, G. L. Jungbluth, D. J. Stalker, and N. K. Hopkins, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1194, 1999), all of these values should be exceeded with a linezolid dose of 600 mg b.i.d.
The magnitude of the differences in certain pharmacodynamic parameters between the two drugs used in this study could have been reduced by using an S. pneumoniae strain for which the ceftriaxone MIC was higher (similar to that of linezolid). The highly ceftriaxone-sensitive strain used for this investigation was originally isolated from a clinical source. However, it may not be representative of the more resistant strains often encountered today in patients with pneumonia. A shift to higher MICs of ceftriaxone would suggest that it might perform less well in clinical practice than in this study, particularly since it has such high protein binding levels (>90%) at doses commonly used in humans (21). This hypothesis is supported by clinical studies that resulted in similar efficacies for linezolid and ceftriaxone against pneumococcal bacteremia and pneumonia (20).
Ceftriaxone is referred to as a time-dependent killing cephalosporin for most pathogens. However, it acts in a concentration-dependent manner against pneumococci at very high AUC/MIC ratios. This fact was validated in this study by free-fraction AUC/MIC ratios exceeding 1,000 and a %T>MIC of approximately 31. If we had used a pneumococcal strain for which the MIC was 0.75 μg/ml, the free-fraction AUC/MIC ratios generated would have fallen well below 100, making them more comparable to those observed with linezolid. Linezolid is a slowly cidal drug at low AUC/MIC ratios. It is therefore not surprising that it performed more poorly at the lower dose. Our results also could suggest that linezolid should not be used for pneumococci that are very susceptible to beta-lactam antibiotics because the drug would kill the organisms more slowly. With the current pricing structure, linezolid may need to be reserved primarily for patients for whom beta-lactam therapy has failed or who have isolates known or suspected of being penicillin intermediate or penicillin resistant prior to initiation of therapy.
The pharmacodynamic activity of linezolid against pneumococci was described previously in an animal thigh infection model, in which achieving a pharmacokinetic goal of greater than 40% T>MIC significantly enhanced bacterial killing (D. Andes, M. L. Van Ogtrop, and W. A. Craig, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-9, 1998). Although the present study was not designed in a format (i.e., maximum effect model) to determine the critical %T>MIC value that must be achieved for linezolid efficacy, the value likely lies between the 35.7% obtained with the 50-mg/kg/day dose and the 43.9% achieved with the 100-mg/kg/day treatment. Thus, the findings obtained in our rat model of pneumococcal pneumonia are consistent with those observed in previous studies.
In conclusion, oral linezolid at 50 mg/kg b.i.d. was as effective as subcutaneous ceftriaxone at 100 once daily in the treatment of pneumococcal pneumonia in an immunocompetent rat model. Both agents drastically reduced the numbers of organisms in the rat lungs, produced resolution of bacteremia, and prevented mortality from a fatal infection. The low dose of 25 mg/kg b.i.d. approximates the 50% effective dose in this model. When linezolid free-fraction pharmacodynamic parameters (AUC/MIC ratio, Cmax/MIC ratio, and %T>MIC) reached 147, 24.3, and 39, respectively, the drug became highly efficacious in enhancing survival.
Acknowledgments
We thank Mary U. Snitily, Kristina M. Haase, and Heather P. Hoopes for excellent technical assistance in performing these studies.
These studies were funded in part by a grant from Pharmacia Upjohn.
REFERENCES
- 1.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob. Agents Chemother. 36:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., and L. M. Mundy. 1995. Community-acquired pneumonia. N. Engl. J. Med. 333:1618-1624. [DOI] [PubMed] [Google Scholar]
- 3.Breiman, R. F., J. C. Butler, F. C. Tenover, J. A. Elliott, and R. R. Facklam. 1994. Emergence of drug-resistant pneumococcal infections in the United States. JAMA 271:1831-1835. [PubMed] [Google Scholar]
- 4.Butler, J. C., J. Hofmann, M. S. Cetron, J. A. Elliott, R. R. Facklam, R. F. Breiman, and the Pneumococcal Sentinel Surveillance Working Group. 1996. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J. Infect. Dis. 174:986-993. [DOI] [PubMed] [Google Scholar]
- 5.Daly, J. S., G. M. Eliopoulos, S. Willey, and R. C. Moellering, Jr. 1988. Mechanism of action and in vitro and in vivo activities of S-6123, a new oxazolidinone compound. Antimicrob. Agents Chemother. 32:1341-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema, D. J., and R. N. Jones. 2000. Oxazolidinones: a review. Drugs 59:7-16. [DOI] [PubMed] [Google Scholar]
- 7.Fines, M., and R. Leclercq. 2000. Activity of linezolid against Gram-positive cocci possessing genes conferring resistance to protein synthesis inhibitors. J. Antimicrob. Chemother. 45:797-802. [DOI] [PubMed] [Google Scholar]
- 8.Gentry, M. J., and L. C. Preheim. 1999. Models of pneumonia in ethanol-treated rats, p. 501-507. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Academic Press, Inc., San Diego, Calif.
- 9.Hook, E.W. III, C. A. Horton, and D. R. Schaberg. 1983. Failure of intensive care unit support to influence mortality from pneumococcal bacteremia. JAMA 249:1055-1057. [PubMed] [Google Scholar]
- 10.Jehl, F., C. Gallion, and H. Monteil. 1990. High performance liquid chromatography assay of antibiotics. J. Chromatogr. 531:509-548. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, A. P., M. Warner, and D. M. Livermore. 2000. Activity of linezolid against multi-resistant Gram-positive bacteria from diverse hospitals in the United Kingdom. J. Antimicrob. Chemother. 45:225-230. [DOI] [PubMed] [Google Scholar]
- 12.Johnston, R. B., Jr. 1991. Pathogenesis of pneumococcal pneumonia. Rev. Infect. Dis. 13(Suppl. 6):S509-S517. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen, J. H., G. V. Doern, L. A. Maher, A. W. Howell, and J. S. Redding. 1990. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob. Agents Chemother. 34:2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzor, O., J. Pawlak, and L. Saravolatz. 1999. In-vitro activity of 29 antimicrobial agents against penicillin-resistant and -intermediate isolates of Streptococcus pneumoniae. J. Antimicrob. Chemother. 43:31-36. [DOI] [PubMed] [Google Scholar]
- 15.Mason, E. O., L. B. Lamberth, and S. L. Kaplan. 1996. In vitro activities of oxazolidinones U-100592 and U-100766 against penicillin-resistant and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:1039-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mufson, M. A., D. M. Kruss, R. E. Wasil, and W. I. Metzger. 1974. Capsular types and outcome of bcteremic pneumococcal disease in the antibiotic era. Arch. Intern. Med. 134:505-510. [PubMed] [Google Scholar]
- 17.Noskin, G. A., F. Siddiqui, V. Stosor, D. Hacek, and L. R. Peterson. 1999. In vitro activities of linezolid against important gram-positive bacterial pathogens, including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelton, S. I., M. Figueira, R. Albut, and D. Stalker. 2000. Efficacy of linezolid in experimental otitis media. Antimicrob. Agents Chemother. 44:654-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng, G. W., R. P. Stryd, S. Murata, M. Igarashi, K. Chiba, H. Aoyama, M. Aoyama, T. Zenki, and N. Ozawa. 1999. Determination of linezolid in plasma by reverse-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 20:65-73. [DOI] [PubMed] [Google Scholar]
- 20.Plouffe, J. F. 2000. Emerging therapies for serious gram-positive bacterial infections: a focus on linezolid. Clin. Infect. Dis. 31(Suppl. 4):S144-S149. [DOI] [PubMed] [Google Scholar]
- 21.Popick, A. C., W. G. Crouthamel, and I. Bekersky. 1987. Plasma protein binding of ceftriaxone. Xenobiotica 17:1139-1145. [DOI] [PubMed] [Google Scholar]
- 22.Preheim, L. C., K. M. Olsen, M. Yue, M. U. Snitily, and M. J. Gentry. 1999. Ethanol feeding does not affect the efficacy or pharmacokinetics of azithromycin, trovafloxacin, or ceftriaxone in a rat model of pneumococcal pneumonia. Alcohol. Clin. Exp. Res. 23:842-849. [PubMed] [Google Scholar]
- 23.Shinabarger, D. L., K. R. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snitily, M. U., M. J. Gentry, M. A. Mellencamp, and L. C. Preheim. 1991. A simple method for collection of blood from the rat foot. Lab. Anim. Sci. 41:285-287. [PubMed] [Google Scholar]
- 25.Spika, J. S., R. R. Facklam, B. D. Plikaytis, M. J. Oxtoby, and the Pneumococcal Surveillance Working Group. 1991. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979-1987. J. Infect. Dis. 163:1273-1278. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox, M., A. Kervitsky, L. C. Watter, and T. E. King, Jr. 1988. Quantification of cells recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 138:74-80. [DOI] [PubMed] [Google Scholar]
- 27.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]