Abstract
We evaluated the effect of optimized doses and dosing schedules of metronidazole, tetracycline, and bismuth-metronidazole-tetracycline (BMT) triple therapy with only 1 day of dosing on Helicobacter pylori SS1 titers in a mouse model. A reduction of bacterial titers was observable with 22.5 and 112.5 mg of metronidazole per kg of body weight (as well as BMT) given twice daily and four times daily (QID). Two hundred milligrams of tetracycline per kilogram, given QID, resulted in only a slight reduction of H. pylori titers in the stomach. We argue that optimization of doses based on antimicrobial drug levels in the animal and shortened (1 or 2 days) drug administration can be used to facilitate early evaluation of putative anti-H. pylori drug candidates in lieu of using human doses and extended schedules (7 to 14 days), as can be deduced from the results seen with these antimicrobial agents.
It has been established that Helicobacter pylori infection is a key factor in the pathogenesis of gastroduodenal ulcers that are not induced by nonsteroidal anti-inflammatory drugs (16). Optimal eradication of H. pylori occurs following combination antibiotic therapy with proton pump inhibitors (5), and new therapies are being continuously evaluated to avoid treatment failures. Since H. pylori therapies have been developed in an empirical fashion, limited systematic research is available to guide the development of novel H. pylori-specific agents. Additionally, although many attempts have been made to predict and quantitate drug levels in gastric mucus (11, 14) in order to address pharmacokinetic-pharmacodynamic issues, a prediction of clinical dose and efficacy through animal studies similar to those being established for commonly used broad-spectrum antimicrobials cannot be made for anti-H. pylori agents (3, 13, 21). This lack of predictive correlates of efficacy makes the selection of lead compounds for further optimization and potentially preclinical development difficult. Furthermore, to date H. pylori infection models have been designed to address questions of host-pathogen interactions, disease progression, or the importance of virulence factors and frequently use animal-specific Helicobacter species (8-10, 12, 15, 18, 20). One mouse model of H. pylori infection (12) uses a murine-adapted human clinical isolate of H. pylori, the SS1 strain, which produces robust colonization of the stomach of C57BL/6 mice.
The H. pylori SS1 mouse model, as well as models using larger animals, has great utility in determining the efficacy of H. pylori-specific antimicrobials at an advanced stage in pharmaceutical drug development. Compound administration in these models is usually based on human doses and regimens of 7 to 14 days and therefore requires drug quantities that are usually only available for compounds in the advanced stages of preclinical drug development. However, at the earlier stages in lead optimization, lead series need to be prioritized on the basis of reasonable criteria and representative compounds selected for further optimization. These criteria are difficult to establish for anti-H. pylori compounds, since in vitro H. pylori antimicrobial activity is not a reliable predictor of in vivo efficacy (6). Therefore, selection of promising lead candidates to be synthesized in sufficiently large quantities for evaluation in animal models of H. pylori infection often appears to be based solely on their biochemical target activity. We developed an in vivo model suitable for the selection of lead compounds, based on the premise that the antimicrobial activity of lead compounds is best evaluated against H. pylori grown in the host environment and under conditions of optimal compound-drug exposure in the target animals, while, at this stage in development, only limited amounts of synthetic material are available and a large number of compounds may have to be tested. This is not intended to replace animal models of H. pylori infection commonly used to determine the preclinical efficacy of drug candidates.
To test the feasibility of early evaluation of promising lead compounds in vivo, we evaluated whether a reduction of bacterial titers in the SS1 mouse model of H. pylori infection could be achieved with presently employed antimicrobial therapeutics (4) and with only 1 day of dosing, a strategy that would ultimately minimize material requirements for test compounds. To assure that only substances with detectable drug exposure in animals are tested (rather than blindly administering compounds), we first analyzed drug exposure profiles and confirmed that measurable drug levels are achieved in mice. Furthermore, we demonstrated that the levels of our test antimicrobials rapidly decreased in mice. To optimize drug exposure, we reasoned that higher and more frequent dosing for a limited duration should optimize drug levels in animals and result in quantifiable reduction of bacterial titers in the stomach, although drug administration would be done for 1 day only. Successful demonstration of reduction of bacterial titers at the site of infection would provide an in vivo screening model in which the effect of lead compounds could be estimated at an earlier stage of drug development and with reduced compound requirement.
To test the above hypotheses, we selected metronidazole, tetracycline, and bismuth-metronidazole-tetracycline (BMT) triple therapy as test compounds and evaluated their effect in a range of doses and schedules on H. pylori titers in vivo in a 1-day dosing model.
MATERIALS AND METHODS
All antibiotic powders and chemicals, except where noted, were purchased from the Sigma-Aldrich Chemical Co., St. Louis, Mo.
Microbiology.
H. pylori SS1 was obtained from Adrian Lee, University of New South Wales, Sydney, Australia, and was kept as a frozen stock in H. pylori broth (HPB) (brain heart infusion broth, 0.25% yeast extract, 10% horse serum) (7, 17) with 15% glycerol at −70°C. Cultures were prepared every 2 weeks from frozen stock on brucella agar plates containing 5% horse blood (Becton Dickinson, Franklin Lakes, N.J.). Urease (Selective Rapid Urea; Remel, Lenexa, Kans.), oxidase (SpotTest Oxidase Reagent; Difco Laboratories, Detroit, Mich.), and catalase (1) activities, along with Gram staining (carbol fuchsin counterstaining), were routinely confirmed from pure cultures according to the manufacturers' protocols.
All cultures were either incubated at 37°C in a microaerobic atmosphere using CampyPak Plus gas generator envelopes (Becton Dickinson) or in a humidified incubator in a 10% CO2 atmosphere. Broth microdilution susceptibility testing was performed in HPB with a final inoculum of 6.0 × 105 CFU/ml. MICs were determined visually after 72 h of incubation and were scored as the lowest test concentrations that completely inhibited the organism's growth. Control antibiotics routinely used to confirm the susceptibility of H. pylori SS1 were rifampin and amoxicillin.
Liquid cultures of H. pylori for animal infection were inoculated from stock plates into HPB and incubated for 3 days with shaking. H. pylori was cultured from stomach homogenates for up to 7 days on a modified Glaxo Selective Supplement A agar (15a), termed JQSM (J. Quispe, D. A. Stevenson, T. Modzelewski, J. Merrill, K. Amsler, L. Foster, A. Slee, and C. Sizemore, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. D-286, 2000): Blood Agar Base No. 2 (Becton Dickinson) with 5% (vol/vol) defibrinated horse blood (Becton Dickinson), 20 mg of bacitracin/liter, 10 mg of vancomycin/liter, 3 mg of polymyxin B/liter, 10 mg of trimethoprim/liter, 4 mg of nalidixic acid/liter, and 5 mg of amphotericin B/liter.
Animals.
Female C57BL/6 mice, Helicobacter-free (C57BL/6-H), weighing between 18 and 22 g were obtained from Charles River Laboratories (Wilmington, Mass.) and were maintained on a 12-h light cycle on wood chip bedding with water and food (PROLAB NIH-31 M/5% rodent diet; CHECKERS PMI Nutrition International, Inc., Brentwood, Mo.) ad libitum. We evaluated the potential of transfer of H. pylori from infected littermates to sentinel mice through coprophagous behavior and did not observe infection for the duration of our studies (3 to 20 days). All protocols used in this study were approved by the DuPont Pharmaceuticals Institutional Animal Care and Use Committee in accordance with animal welfare regulations and ethical standards of animal use in research.
Antibiotic preparations for use in mice.
All antibiotics evaluated during this study were prepared as suspensions in 0.25% (wt/vol) methylcellulose (methocel)/water and were given in 200-μl doses per mouse: (i) metronidazole (Sigma-Aldrich Chemical Co.), bead milled overnight and prepared as doses of 22.5 mg/kg of body weight for drug exposure studies and of 2.25, 22.5, and 112.5 mg/kg for the 1-day dosing model; (ii) tetracycline, diluted from Sumycin Syrup (Apothecon, a subsidiary of Bristol Myers-Squibb Co., Princeton, N.J.) and prepared as doses of 50 mg/kg for drug exposure studies and of 10, 50, and 200 mg/kg for the once-daily dosing model; and (iii) bismuth subsalicylate, diluted from Maximum Strength Pepto-Bismol (Procter & Gamble Pharmaceuticals, Cincinnati, Ohio) to achieve a dose of 6.25 mg/kg. These antibiotics were used either individually or in combination to prepare BMT triple therapy (6.15 mg of bismuth subsalicylate, 22.5 mg of metronidazole, and 50 mg of tetracycline per kg).
Drug exposure studies.
For each antibiotic dose, three mice were used per time point. Antibiotics were given once in a single dose orally as a 200-μl drug suspension (metronidazole, 22.5 mg/kg, and tetracycline, 50 mg/kg) using an 18-gauge metal gavage needle. Groups of three animals were sacrificed (CO2 asphyxiation) at 5, 15, 30, and 60 min and at 2, 3, 6, 8, and 24 h post-drug administration. Blood was collected via cardiac puncture and was pooled for each group of three animals into 4-ml SST Vacutainer serum separator tubes (Becton Dickinson). Serum was separated by centrifugation at 30 × g for 10 min and was frozen at −20°C. Stomachs were excised and opened along the greater curvature, and food particles were removed by briefly dipping the stomachs (three times) into sterile saline. To remove gastric mucus, the inner lining of the stomach was then gently scraped with a flat spatula, taking care not to disturb the cellular integrity of the mucosa. Gastric mucus from each group (three animals) was pooled. Mucus samples were transferred to preweighted, sterile, 1.5-ml polypropylene microcentrifuge tubes and were frozen at −20°C before analysis.
Analytical evaluation.
A Waters Alliance 2690 HPLC System with a dual-wavelength absorbance detector was used (Waters Corp., Milford, Mass.). Peak integration, calibration, and concentration determinations were performed using the Waters Millennium 32 Chromatography Manager. Thawed mucus samples were combined with 500 μl of distilled water and were homogenized in 1.5-ml polypropylene microcentrifuge tubes using a Teflon pestle (Kontes, Vineland, N.J.) before addition of internal standards. Calibration curves for the evaluation of unknown drug concentrations in serum samples were conducted in both the absence and presence of mouse serum.
Tetracycline.
Oxytetracycline in distilled water was used as the internal standard. One-hundred-microliter aliquots of thawed serum and mucus samples were mixed with 100 μl of releasing agent (water-acetonitrile-phosphoric acid, 78:20:2, vol/vol/vol), transferred to Microcon 10 filter tubes (Amicon Inc., Beverly, Mass.), and centrifuged at 23,500 × g for 30 min. One-hundred-microliter aliquots were analyzed on a Phenomenex LUNA 3 μ-phenyl-hexyl column (30 by 4.60 mm) in water-methanol-acetonitrile (65:25:10, vol/vol/vol) with 0.1 M oxalic acid at a flow rate of 1.0 ml/min at room temperature and were examined at 350 nm. The lower limits of quantification were 0.025 μg/mg in mucus and 0.0075 μg/ml in serum.
Metronidazole.
Tinidazole in acetonitrile was used as the internal standard. Two-hundred-microliter serum and mucus samples were extracted according to Salvesen et al. (19) with 400 μl of acetonitrile for 30 s. After centrifugation for 10 min at 3,000 × g, 100 μl of supernatant fluid was analyzed on a Waters μ-Bondapak C18 column (3.9 by 150 mm) in 0.02 M acetate buffer (pH 4)-acetonitrile (65:35, vol/vol) at a flow rate of 2.0 ml/min at room temperature and was examined at 313 nm. The lower limits of quantification were 0.22 μg/mg in mucus and 1.5 μg/ml in serum.
H. pylori SS1 model.
Infection of C57BL/6-H mice was accomplished as described by Lee et al. (12). Briefly, 8 to 10 mice per group were infected orally three times during the course of 1 week with between 108 and 109 CFU of H. pylori SS1 in brucella broth-0.5% (wt/vol) bovine serum albumin (BSA) using an 18-gauge metal gavage needle. Each inoculum was plated on brucella agar-5% horse blood to verify the titer of the inoculum (CFU/milliliter values were available only retrospectively after 4 to 7 days). Experiments using lower-titer inocula were terminated and were not included in the evaluation of test compounds. Control animals were given 200 μl of brucella broth-0.5% (wt/vol) BSA. After a 7-day rest period to facilitate gastric colonization, mice were given 200 μl of various antibiotic preparations orally using an 18-gauge metal gavage needle for up to 14 days. To quantitate H. pylori titers, stomachs were excised and cut along the greater curvature and residual food particles were removed. Whole stomachs, rather than sections known to display maximum colonization with H. pylori (e.g., antrum), were used in this model. The necropsy of large number of animals, as would be the case in a study analyzing a series of lead compounds, was greatly facilitated by using whole, rather than subsectioned, stomach homogenates. Furthermore, using whole stomach homogenates greatly reduced necropsy time and allowed plate cultures to be transferred to a CO2-enriched environment within 90 min., which proved critical in the assurance of maximum viability of the recovered bacterial cultures. Individual stomachs were placed into 12-ml polypropylene test tubes (VWR International, West Chester, Pa.) and were weighed. Two milliliters of sterile brucella broth-0.5% (wt/vol) BSA was added, and the samples were homogenized for 20 s at 18,600 rpm using a Polytron 6000 tissue processor fitted with a 12-mm flat-bottom saw tooth generator (Kinematic AG, Lucerne, Switzerland). Between samples, the generator was rinsed in two consecutive changes of 0.9% sterile saline for 10 s each, flame sterilized for 5 s after immersion in 100% ethanol, and subsequently cooled for 10 s in chilled 0.9% saline. Stomach homogenates were plated on JQSM (described above) agar and were incubated for up to 7 days with strictly maintained humidity and temperature. Colonies were enumerated and were expressed as numbers of CFU/gram of tissue. All studies were repeated at least once.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Department of Health and Human Services.
RESULTS
Drug exposure studies.
To verify and later optimize antibiotic levels in the H. pylori mouse model, we first determined concentrations of metronidazole and tetracycline in both serum and gastric mucus after oral dosing. Dose selection was based upon human therapy (milligram/kilogram/dose based on a 70-kg human) (4), i.e., 200 to 500 mg of metronidazole (approximately 2.5 to 6.5 mg/kg/dose) in combination with the following antibiotics: tetracycline, 250 to 500 mg (approximately 3.3 to 6.5 mg/kg/dose); and bismuth, 120 mg (approximately 1.6 mg/kg/dose) as BMT triple therapy. Or the drugs were combined in the following amounts: 250 mg of metronidazole (3.3 mg/kg/dose); 500 mg of tetracycline (6.5 mg/kg/dose); and 320 mg of bismuth (4.2 mg/kg/dose, HELIDAC Therapy; Procter & Gamble Pharmaceuticals).
To maximize drug levels in mice, we used doses approximately 10 times those of the corresponding human doses. Metronidazole and tetracycline were given as a single 22.5- or 50-mg/kg oral dose, respectively. Serum and mucus samples were collected and analyzed. Although there is no established correlation among the MIC, serum or mucus levels, and clinical efficacy of anti-H. pylori agents, we nevertheless used MICs of metronidazole (0.5 μg/ml) and tetracycline (0.25 μg/ml) as a general guideline for minimum achievable drug levels.
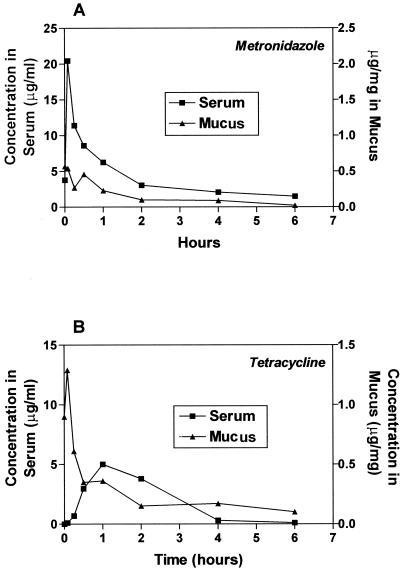
From these analyses, it was determined that adequate serum and/or mucus exposures were obtained. However, in order to provide maximum drug levels, both metronidazole and tetracycline needed to be administered more than twice daily (BID) to provide good drug concentrations in any given 24-h dosing interval (Fig. 1A and B). Furthermore, since neither metronidazole (11% ± 3%) nor tetracycline (65% ± 3%) is significantly protein bound (2), a correction for free drug levels in serum was not required. The duration of drug exposure in the gastric mucus is most likely the result of the combination of topical (oral delivery) and resecreted antibiotic (11, 14). Hence, we chose to view mucus concentration solely as an indication that drug is available in this milieu (the site of H. pylori infection) and to show exposure patterns distinct from those of serum levels. From the evaluation of serum and mucus samples, we concluded that increased dose frequencies and drug amounts given over the course of 1 day should provide sufficient drug exposure to affect bacterial titers.
FIG. 1.
Concentration of metronidazole or tetracycline in either serum or mucus after a single oral dose. Serum and gastric mucus samples are pooled for three animals for each data point. (A) Metronidazole, 22.5 mg/kg. (B) Tetracycline, 50 mg/kg.
One-day dosing model.
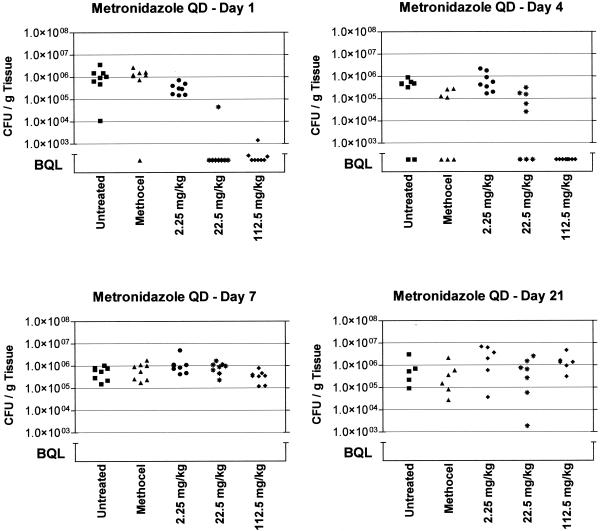
To test the hypothesis that an effect on in vivo bacterial counts can be meaningfully demonstrated after only 1 day of antibiotic administration, we first followed the reduction of H. pylori titers in the stomachs of mice treated with metronidazole and BMT over the course of several days to determine the optimal time to terminate the study. Groups of eight mice were given doses of metronidazole (2.25, 22.5, and 112.5 mg/kg) after the initial 1-week rest period after infection. On the next day (day 1) and days 4, 7, and 21, one group for each dose, as well as methocel control groups and untreated animals, was sacrificed and its stomachs were processed to determine H. pylori titers. Figure 2 summarizes the results from this experiment and clearly demonstrates that, for up to 4 days after the administration of 112.5 and 22.5 mg of metronidazole/kg, significant reductions of bacterial titers in the stomach can be observed.
FIG. 2.
Time course of H. pylori titers in mice after single oral doses of metronidazole and methocel (control) (vehicle) compared to untreated, infected animals. The number of CFU/gram of gastric tissue is plotted for each animal within a dose group. Groups of animals were sacrificed on days 1, 4, 7, and 21 after the dosing day. BQL = approximately 103 CFU/g of tissue. QD, once daily.
Occasionally, we observed animals with undetectable H. pylori titers in the control groups. The lack of infection may have been caused by an inefficient infection technique at the beginning of the study or may constitute normal variability in infection that may be seen with this model. We also observed that oral dosing of a 200-μl volume four times daily (QID) results in transient reduction of bacterial counts in the stomachs of control animals (data not shown). After 2 days, values for numbers of CFU/gram of tissue in control animals returned to the levels of infected but untreated animals. Based on these results, we evaluated the effect of the above metronidazole doses on H. pylori titers in mice when given once daily, BID, and QID during the course of 16 h and when mice were sacrificed on day 3 after the last dose. We selected to terminate the experiment after 3 days, which produced results (data not shown) similar to those for longer observation times. Additionally, we included BMT triple therapy (6.15 mg of bismuth subsalicylate, 22.5 mg of metronidazole, and 50 mg of tetracycline per kg), as well as the respective methocel controls given once daily, BID, and QID during the 16-h period.
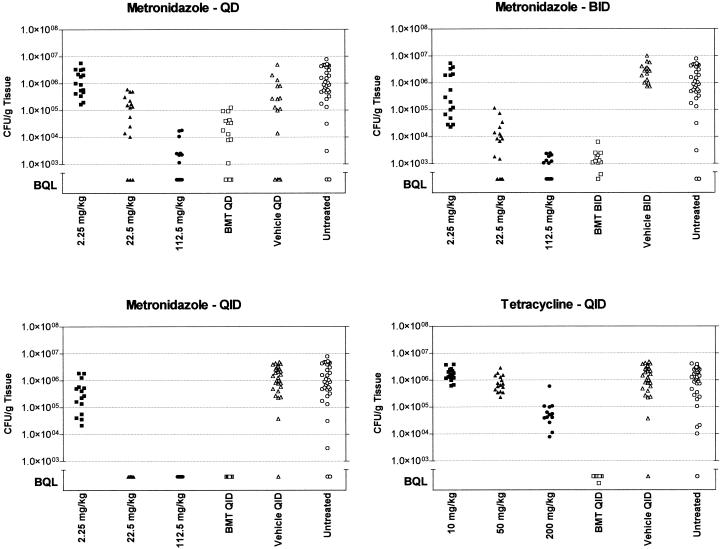
Figure 3 shows that metronidazole, when administered either frequently (QID) or at a sufficiently high dose, led to a reduction of H. pylori titers to below quantifiable levels (BQL). The BMT regimen also showed titer reductions below the level of quantitation in a pattern similar to that observed for the 22.5-mg/kg dose of metronidazole. This result suggests that the primary effect of BMT triple therapy in the SS1 mouse model may be attributed to the action of metronidazole. To evaluate the effect of tetracycline on H. pylori titers, a parallel study was conducted using 10, 50, and 200 mg of tetracycline/kg once daily, BID, and QID (Fig. 3). In contrast to metronidazole, tetracycline produced only a slight but reproducible reduction in H. pylori titers with the 200-mg/kg dose when given four times during 16 h. All other schedules yielded bacterial counts equivalent to those for untreated and vehicle-treated controls (data not shown).
FIG. 3.
Effects of selected doses and schedules of metronidazole and tetracycline administration on H. pylori titers in the mouse stomach. Metronidazole doses were administered once daily (QD), BID, and QID during the course of 16 h. Data for tetracycline given QID are shown. BMT, bismuth subsalicylate (6.15 mg/kg)-metronidazole (22.5 mg/kg)-tetracycline (50 mg/kg). The number of CFU/gram of gastric tissue is plotted for each animal within a dose group and is compared to those for methocel-treated controls (vehicle treated) and untreated, infected animals. Groups of animals were sacrificed on day 3 after drug administration. BQL = approximately 103 CFU/g of tissue.
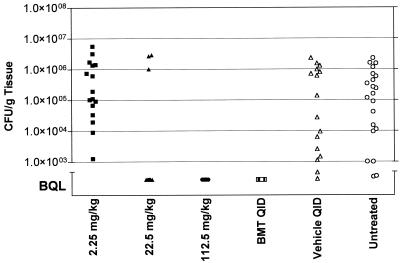
Metronidazole produced a reduction of bacterial titers BQL at doses of 22.5 and 112.5 mg/kg QID, which may either mean eradication of bacteria from the sites of infection or may simply mean reduction of bacteria below the level of detection of our assay. To distinguish between these possibilities, a second treatment group, dosed over the course of 16 h with 2.25, 22.5, and 112.5 mg of metronidazole/kg QID, was retained for twenty additional treatment-free days and was sacrificed on day 21. The same metronidazole doses that had previously shown a reduction in bacterial counts BQL also resulted in H. pylori titers BQL in this extended experiment (Fig. 4.). This result suggests that these doses of metronidazole given QID reduce bacterial counts in the stomach to such a degree that regrowth cannot be observed even after twenty additional days. For use of this model in the evaluation of drug candidates, one may consider distinguishing between eradication and suppression by performing H. pylori-specific PCR on stomach tissue samples.
FIG. 4.
Effect of metronidazole on H. pylori titers in the stomach after a 20-day treatment-free period. Metronidazole doses (2.25, 22.5, and 112.5 mg/kg) were administered four times during the course of 16 h. BMT, bismuth subsalicylate (6.15 mg/kg)-metronidazole (22.5 mg/kg)-tetracycline (50 mg/kg). The number of CFU/gram of gastric tissue is plotted for each animal within a dose group and is compared to those for methocel-treated controls (vehicle treated) and untreated, infected animals. BQL = approximately 103 CFU/g of tissue.
DISCUSSION
Early evaluation of lead candidates in animal models of infection is a prerequisite in cases where no appropriate in vitro assays are available to provide a reasonable estimate of activity in vivo. Considering eradication of H. pylori from the stomach, neither antimicrobial activity in vitro (MIC), rate-of-kill analyses, nor proposed optimal drug exposure profiles in serum are suitable to select which lead candidate series to advance for further optimization. Presently, to determine whether a research compound is active against H. pylori in vivo, mice are given scaled human doses once or twice daily for 7 to 14 days. This regimen, however, requires the availability of significant quantities of chemical material that are usually not provided during early stages of lead evaluation.
We hypothesized that, given appropriate drug exposure profiles in mice, preferably similar to those achievable in humans, dosing for 1 day only could still result in the reduction of bacterial titers. This paradigm would then allow development of a rapid lead selection model on the basis of an infection model, rather than a disease model, that would be sufficient to provide host-specific growth characteristics of the pathogen.
Our studies demonstrated that, in the SS1 mouse model of H. pylori infection, significant reduction in bacterial titers using metronidazole or BMT with only 1 day of dosing is indeed achievable. While metronidazole and BMT both reduce bacterial titers dramatically, the effect of tetracycline was modest. Thus, it may be possible to use our method to distinguish between antimicrobials with different levels of activity in vivo . However, to detect weakly active compounds, an extension of dosing to 2 or 3 days may be necessary. Furthermore, analysis of gastric titers after an extended treatment-free period may provide information about the eradication potential of investigational compounds, since the maximized metronidazole regimen still resulted in undetectable bacterial counts even after 20 days.
This adaptation of the classical H. pylori SS1 infection model as an early-stage lead identification model may have great utility in the development of H. pylori-specific antimicrobials. As it is rapid and requires only relatively small amounts of research compound and a minimized workload in animal husbandry, it can be used as an early-stage screening model for candidate compounds. We have also recently applied the paradigm of short-term dosing with optimized drug exposure to an animal model of Mycobacterium infection (M. S. DeStefano and M. H. Cynamon, personal communication) and were able to demonstrate similar results. This suggests that the above strategy may be applied to the lead selection process for a variety of pathogens.
Acknowledgments
We thank D. P. Martin and his staff for their dedication and support of our studies and Eric Wexler for his technical expertise and help using imaging technology.
REFERENCES
- 1.Baron, E. J., and S. M. Finegold (ed.). 1990. Conventional and rapid microbiological methods for identification of bacteria and fungi, p. 100-126. In Bailey and Scott's diagnostic microbiology, 8th ed. The C.V. Mosby Co., St. Louis, Mo.
- 2.Benet, L. Z., S. Øie, and J. B. Schwartz. 1996. Design and optimization of dosage regimens: pharmacokinetic data, p. 1707-1792. In J. G. Hardman, L. E. Limbird, P. B. Molinoff, R. W. Ruddon, and A. G. Gilman (ed.), Goodman & Gilman's the pharmacological basis of therapeutics, 9th ed. McGraw-Hill, New York, N.Y.
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 4.De Boer, W. A., and G. N. J. Tytgat. 2000. Treatment of Helicobacter pylori infection. Br. Med. J. 320:31-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fennerty, M. B., D. A. Lieberman, N. Vakil, N. Margaret, D. O. Faigel, and M. Helfand. 1999. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch. Intern. Med. 159:1562-1566. [DOI] [PubMed] [Google Scholar]
- 6.Goddard, A. F. 1998. Getting to the route of Helicobacter pylori treatment. J. Antimicrob. Chemother. 42:1-3. [PubMed] [Google Scholar]
- 7.Hartzen, S. H., L. P. Andersen, A. Bremmelgaard, H. Colding, M. Arpi, J. Kristiansen, T. Justesen, F. Espersen, N. Frimodt-Møller, and O. Bonnevie. 1997. Antimicrobial susceptibility testing of 230 Helicobacter pylori strains: importance of medium, inoculum, and incubation time. Antimicrob. Agents Chemother. 41:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeno, T., H. Ota, A. Sugiyama, K. Ishida, T. Katsuyama, R. M. Genta, and S. Kawasaki. 1999. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am. J. Pathol. 154:951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krakowka, S., K. A. Eaton, and M. Rings. 1995. Occurrence of gastric ulcers in gnotobiotic piglets colonized by Helicobacter pylori. Infect. Immun. 63:2352-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krakowka, S., K. A. Eaton, and R. D. Leunk. 1998. Antimicrobial therapies for Helicobacter pylori infection in gnotobiotic piglets. Antimicrob. Agents Chemother. 42:1549-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert, J. R. 1996. Pharmacology of the gastric mucosa: a rational approach to Helicobacter polytherapy. Gastroenterology 111:521-523. [DOI] [PubMed] [Google Scholar]
- 12.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 13.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogekamn, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 14.Lozniewski, A., J. D. de Korwin, F. Muhale, and F. Jehl. 1988. Gastric diffusion of antibiotics used against Helicobacter pylori. Int. J. Antimicrob. Agents 9:181-193. [DOI] [PubMed] [Google Scholar]
- 15.Marchetti, M., B. Aricò, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 15a.McColm, A. A., J. Bagshaw, C. O’Malley, and A. McLaren. 1995. Development of a mouse model of gastric colonisation with Helicobacter pylori. Gut 37(Suppl. 1):A50. [Google Scholar]
- 16.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. 1994. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA 272:65-69. [PubMed] [Google Scholar]
- 17.Pavičić, M. J. A. M. P., F. Namavar, T. Verboom, A. J. van Winkelhoff, and J. de Graaff. 1993. In vitro susceptibility of Helicobacter pylori to several antimicrobial combinations. Antimicrob. Agents Chemother. 37:1184-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi, G., M. Rossi, C. G. Vitali, D. Fortuna, D. Burroni, L. Pancotto, S. Capecchi, S. Sozzi, G. Renzoni, G. Braca, G. Del Giudice, R. Rappuoli, P. Ghiara, and E. Taccini. 1999. A conventional beagle dog model for acute and chronic infection with Helicobacter pylori. Infect. Immun. 67:3112-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvesen, B., O. Leinebo, and T. Bergan. 1984. Assay of metronidazole by HPLC compared with microbial method. Scand. J. Gastroenterol. Suppl. 91:31-43. [PubMed] [Google Scholar]
- 20.Solnick, J. V., D. R. Canfield, S. Yang, and J. Parsonnet. 1999. Rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific-pathogen-free monkeys. Lab. Anim. Sci. 49:197-201. [PubMed] [Google Scholar]
- 21.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]