Abstract
An in vitro method for determination of postantifungal effect (PAFE) in molds was developed by using three isolates each of Aspergillus fumigatus, A. flavus, A. terreus, A. nidulans, and A. ustus. MICs of amphotericin B and itraconazole were determined by using National Committee for Clinical Laboratory Standards guidelines (M38-P). The inoculum was prepared in RPMI 1640 broth buffered with MOPS (morpholinepropanesulfonic acid) at pH 7.0, and conidia were exposed to amphotericin B and itraconazole at concentrations of 4, 1, and 0.25 times the MIC, each for 4, 2, and 1 h at 37°C. The same procedure was followed for controls with drug-free medium. Following exposure, the conidia were washed three times in saline and the numbers of CFU per milliliter were determined. Exposed and control conidia were then inoculated into microtitration plates and incubated at 37°C for 48 h in a spectrophotometer reader. The optical density (OD) was measured automatically at 10-min intervals, resulting in growth curves. PAFE was quantified by comparing three arbitrary points in the control growth curve, the first increase of OD and the points when 20 and 50% of the maximal growth were reached, with the growth curve of drug-exposed conidia. Amphotericin B induced PAFE in A. fumigatus at four times the MIC after 2 and 4 h of exposure ranging from 1.83 to 6.00 h and 9.33 to 10.80 h, respectively. Significantly shorter PAFEs or lack of PAFE was observed for A. terreus, A. ustus, and A. nidulans. Itraconazole did not induce measurable PAFE in the Aspergillus isolates at any concentration or exposure time tested. Further studies are warranted to investigate the implications of PAFE in relation to clinical efficacy and dosing frequency.
Aspergillus species are documented as some of the most prevalent airborne molds, and the frequency of mycoses due to these fungi is rising worldwide, especially invasive infections that occur in immunocompromised patients, such as cancer patients, bone marrow transplant recipients, solid-organ transplant recipients (13), and human immunodeficiency virus-infected patients (18).
Aspergillus fumigatus and Aspergillus flavus are encountered most frequently, although other species have been documented to cause disease. Aspergillus terreus is an increasing cause of invasive infection in neutropenic patients and Aspergillus nidulans in patients with chronic granulomatous disease (22). Although amphotericin B is considered the drug of choice for treatment of invasive aspergillosis, the clinical response is limited, especially in infections caused by the latter two species. Alternatively, itraconazole is a triazole antifungal drug with good in vitro activity against Aspergillus and has been used successfully as first-line treatment in patients with invasive disease (2, 6).
Methods for in vitro susceptibility testing of molds have been proposed only recently by the National Committee for Clinical Laboratory Standards (NCCLS) (19), but the correlation between MIC and clinical response remains unclear, especially for amphotericin B. With the exception of A. terreus, MICs for clinical Aspergillus isolates of amphotericin B are normally below 1 μg/ml, and the distribution range of MICs is narrow. A correlation between treatment failure and MIC could not be demonstrated in an animal model of invasive aspergillosis (24). Only for itraconazole (7) and voriconazole was a good correlation found between MIC and response in animal models.
One explanation of discrepancies between MIC and clinical efficacy is post-drug-exposure effects. For instance, the success of intermittent dosing regimens for some antimicrobial agents has been attributed to delay in regrowth of microorganisms after drug concentrations in the tissues have fallen below the MIC. This so-called postantibiotic effect (PAE) was first described for bacteria (20). Post-drug-exposure effects in filamentous fungi have not been studied, mainly because of technical problems such as the nonhomogeneous growth of molds in liquid media. The aim of this study was to develop a system for studying postantifungal effects (PAFE) in filamentous fungi and to evaluate the PAFE of amphotericin B and itraconazole against Aspergillus species.
MATERIALS AND METHODS
Isolates.
Fifteen clinical strains from our private collection were evaluated: A. fumigatus AZN VO2-31, AZN VO2-32, and AZN VO2-33; A. terreus AZN 5914, AZN 2868, and AZN 515; Aspergillus ustus AZN 943, AZN 677, and AZN 678; A. nidulans AZN 8033, AZN 8950, and AZN 8958; and A. flavus AZN 2865, AZN 4094, and AZN 284. The strains were cultured from respiratory secretions (12), the external ear (2), and cerebrospinal fluid (1).
Antifungal agents.
Amphotericin B (Bristol-Myers Squibb, Woerden, The Netherlands) and itraconazole (Janssen-Cilag, Beerse, Belgium) were utilized for MIC determinations and PAFE studies. The drugs were dissolved in dimethyl sulfoxide (DMSO) and aliquots of the stock solution were stored at −70°C until used. Then they were diluted in RPMI 1640 medium (with l-glutamine and without bicarbonate) (GIBCO BRL, Life Technologies, Woerden, The Netherlands) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany).
Antifungal susceptibility testing.
The isolates were passaged twice at an interval of 5 to 7 days at 28°C by subculturing onto Sabouraud glucose agar (SGA) to obtain adequate sporulation. Conidia were collected with a cotton swab and suspended in saline with 0.05% Tween 20. After the heavy particles had been allowed to settle, the turbidity of supernatants was measured spectrophotometrically (Spectronic 20D; Milton Roy, Rochester, N.Y.) at 530 nm, and transmission was adjusted to 80 to 82%, corresponding to 0.5 × 106 to 4.5 × 106 CFU/ml. The viability was confirmed by plating serial dilutions onto SGA plates.
For inoculum preparation the conidial suspensions were diluted 1:100 in order to obtain a final inoculum of 0.5 × 104 to 4.5 × 104 CFU/ml. A broth microdilution method was performed according to NCCLS guidelines (M38-P) (19) using RPMI 1640 medium buffered to pH 7.0 with 0.165 M MOPS. Amphotericin B and itraconazole were dissolved in DMSO at concentrations of 3,200 μg/ml. Twofold serial dilutions of the drugs were made in RPMI 1640 medium in order to obtain final concentrations that ranged from 0.015 to 16 μg/ml for both drugs. A drug-free well containing 0.01% DMSO in the medium served as the growth control. The tests were performed in 96-well flat-bottom microtitration plates (Corning Incorporated, New York, N.Y.), which were kept at −70°C until the day of testing. Conidial suspensions prepared as described above were diluted 1:50 in RPMI 1640 to obtain twice the desired inoculum. After the inoculation, the microtitration plates were incubated at 35°C for 48 h. The MICs were read by spectrophotometric reader (Rosys Anthos ht3; Anthos Labtex Instruments GmbH, Salzburg, Austria). Background optical density (OD) was determined by spectrophotometric measurement of noninoculated wells processed in the same way as the inoculated wells. The relative ODs for each well based on measurements at 405 nm were calculated (in percentages) with the following formula: [(OD of drug-containing well − background OD)/(OD of drug-free well − background OD)] × 100. The MIC of amphotericin B was defined as the lowest concentration of the drug that resulted in at least 95% reduction of growth compared with the growth control, and that of itraconazole was defined as the lowest concentration of the drug that resulted in 50% of reduction of growth compared with the growth control.
PAFE assay.
The method used for determining PAFE in molds was based on methods used for bacteria and yeasts, with some modifications (4, 10, 12). Amphotericin B and itraconazole were dissolved in DMSO at initial concentrations of 400 μg/ml, and aliquots of the stock solution were stored at −70°C until used. Then they were diluted 50 times in RPMI 1640 medium (with l-glutamine without bicarbonate) buffered to pH 7.0 with 0.165 M MOPS. Serial dilutions of the drugs were made in RPMI 1640 in order to obtain final concentrations of 4, 1, and 0.25 times the corresponding MIC of each drug for each strain. Control conidial suspensions were made in RPMI 1640. The isolates were passaged twice at an interval of 5 to 7 days at 28°C by subculturing onto SGA to obtain adequate sporulation. Conidia were collected with a cotton swab and suspended in saline with 0.5% Tween 20. After the heavy particles were allowed to settle, the supernatant was transferred to another tube and vortexed for 10 s, and 10- and 100-fold dilutions were made. The concentration of conidia was established microscopically by Burker Turk hemocytometer chambers. Then the concentration was adjusted to obtain 4 × 105 conidia/ml. One milliliter of this suspension was added to tubes containing 9 ml of RPMI 1640 alone (control) or with amphotericin B or itraconazole at the concentrations mentioned above, resulting in a final volume of 10 ml. The hydrophobic nature of Aspergillus species made it necessary to add 0.5% Tween 20 to the media in order to keep the conidia in the solution during washing. The final inoculum therefore was 4 × 104 CFU/ml. Following this procedure, each strain was incubated for either 4, 2, or 1 h with continuous shaking at 37°C.
After incubation, the conidia were washed with saline 0.5% Tween 20, and centrifuged at 3,500 × g during 15 min. After three wash cycles, 98% of the supernatant was completely decanted and the pellets were resuspended in a final volume of 10 ml RPMI 1640 with 0.05% Tween 20. The procedure with two washings and removal of 90% of supernatant has been shown to reduce the antimicrobial concentration by 100-fold and with complete decanting, two washings can reduce concentrations as much as 10,000-fold (4). Following this step, 100 μl of sample was diluted 10-fold in sterile water, and 30-μl aliquots were plated onto SGA plates for colony count determination and incubated at 37°C for 24 and 48 h. The concentration of viable CFU per milliliter for drug-exposed conidia was determined in order to verify the concentration of viable conidia after drug exposure and to allow adjustment of the inoculum, if necessary, to match that of controls. From the resuspended suspension, 200 μl was placed in microtitration plates which were incubated at 37°C in a computerized spectrophotometric reader (Rosys Anthos ht3; Anthos Labtex Instruments GmbH). Growth was automatically monitored in terms of change in turbidity at 405 nm at 10-min intervals for 48 h. All assays were performed in duplicate.
In order to correlate OD changes with the morphology of the Aspergillus species, the microscopic morphology was examined in microtitration plates by a reverse microscope at different time points for each species for the control and the drug-exposed strains. Conidia were counted, and the percent germination was estimated in duplicate at 0, 4, 8, 12, 16, 24, and 36 h.
Data analysis.
Three points in the growth curve were considered to quantify the PAFE: (i) the first significant increase in the OD (repeatable increase in OD for three consecutive measurements; OD0) (16); (ii) the point where the OD reached 20% of the maximum of the growth curve at 48 h (OD20); and (iii) the point where the OD reached 50% of the maximum of the growth curve at 48 h (OD50). The PAFE was quantified by using the formula T − C, where T was the time of the first significant increase in OD0 of the drug-exposed conidia after removal of the drug and C was the time of the first significant increase in OD0 of the control. For OD20 and OD50, T was defined as the time required for the relative OD of the drug-exposed conidial suspension to reach the absorbance level calculated by the formula FC × (ODmax − ODmin) + ODmin after removal of the drug and C was the time required for the relative OD of the drug-free control conidial suspension to reach the same absorbance level calculated by the same formula, where ODmax is the maximum OD reached, ODmin is the baseline OD level reached, and FC is a variable of 0.2 and 0.5 that gives a point at 20% and 50% of the maximum of the curve, respectively. Thus, PAFE was defined as the difference in the time required to reach the defined point in the growth curves of drug-exposed conidia and controls and was expressed in hours.
The time to reach these chosen arbitrary points (OD0, OD20, and OD50) of at least eight controls for each species was determined, and the mean, range, upper 95% confidence interval (CI), and coefficient of variation (CV) were calculated in order to determine the reproducibility of the control curves at each point and to establish the reproducibility of the experiments.
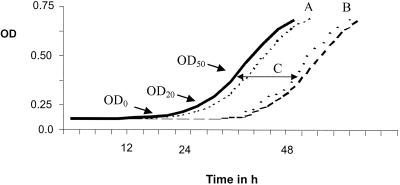
For each chosen point (OD0, OD20, and OD50) and for each species the upper 95% CI of the controls and the lower 95% CI of the drug-exposed isolates were chosen as the cutoff that distinguished between presence and absence of PAFE. When regrowth of the drug-exposed isolates occurred within the upper 95% CI time frame of the controls, PAFE was considered absent. Alternatively, if regrowth was delayed following drug exposure and the lower 95% CI of the drug-exposed isolates was delayed until beyond the upper 95% CI of the controls, PAFE was considered present (Fig. 1). Growth curves of each drug-exposed isolate were compared only with pooled controls from that same isolate.
FIG. 1.
Growth curve of control (—) and drug-exposed (---) molds showing PAFE. The mold was grown in RPMI 1640 medium incubated at 37°C during 48 h. Following drug exposure, the curve was shifted to the right when PAFE is present. PAFE was quantified by using OD0, OD20, and OD50. The time needed to reach each criterion for the control was subtracted from that for the drug-exposed conidia (C). PAFE was considered to be present when the upper 95% CI of the control growth curve (A) did not overlap with the lower 95% CI of the drug-exposed-conidia growth curve (B).
The inter- and intraspecies variation of PAFE were analyzed with all the raw data by analysis of variance using Tukey-Kramer multiple-comparison tests. P values of <0.05 were considered statistically significant.
RESULTS
For all Aspergillus isolates tested the amphotericin B MIC was 1 μg/ml and that of itraconazole ranged between 0.125 and 2 μg/ml (A. fumigatus, 0.125 to 0.5 μg/ml; A. flavus, 0.125 to 0.25 μg/ml; A. terreus, 0.125 μg/ml; A. nidulans, 0.125 to 0.25 μg/ml; and A. ustus, 2 μg/ml).
Inoculum.
Based on the inoculum preparation procedure of the NCCLS M38-P guideline, the range of numbers of viable CFU per milliliter recovered in the colony counts was too great to achieve reproducible growth curves. Therefore, a more stringent range was used based on inoculum preparation by hemocytometer. By this method, a limited range of 2 × 104 to 4 × 104 viable CFU/ml was achieved. Exposure of conidia to amphotericin B and itraconazole for 1 to 4 h had no effect on the viability of the Aspergillus species based on subculture of serial twofold dilutions (data not shown). Therefore adjustment of the inoculum following drug exposure to match that of the controls was not necessary.
Microscopic morphology.
Microscopic examination of the conidia before and after drug exposure showed that germination did not occur within the maximal exposure period of 4 h for any species except A. flavus, where germ tubes were visible within 4 h of incubation. At 8 h, germination had not occurred for any species except A. flavus. For A. fumigatus and A. ustus, germination of conidia occurred after 12 h for 95% of the controls and drug-exposed conidia in strains where PAFE was not present. When PAFE was present, germination of conidia was further delayed. A. terreus and A. nidulans germinated after 16 and 20 h, respectively. After 36 h, mycelia were observed for all the species for both controls and drug-exposed conidia.
There were no microscopic differences between drug-exposed conidia and controls. During incubation, the morphology of the hyphae and the frequency of branching appeared to be similar for both drug-exposed and non-drug-exposed strains. The only difference between drug-exposed and non-drug-exposed strains was the time at which germination commenced.
PAFE assay.
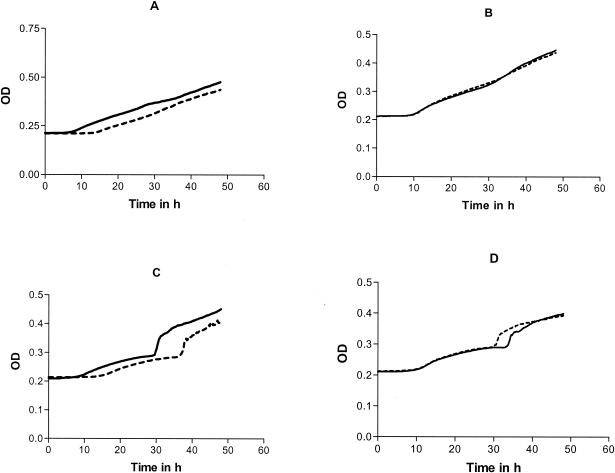
The shapes of the growth curves were different depending on the species tested; however, they were reproducible among the replicates. For the same species, the shapes of the control and the drug-exposed strains were identical. When PAFE was present, the growth curve of the drug-exposed conidia was shifted to the right compared with that of the control. Examples of growth curves for A. fumigatus and A. ustus are shown in Fig. 2. A sharp increase in OD was observed for A. ustus after 30 h of incubation which was found to be related to the formation of crystals in the well (Fig. 2C and D).
FIG. 2.
Growth curves of A. fumigatus and A. ustus following exposure to amphotericin B (---) compared with controls (—). (A and B) A. fumigatus after 4 h of incubation at four times the MIC (A) and at the MIC (B). (C and D) A. ustus after 4 h of incubation at four times the MIC (C) and at the MIC (D). (A and C) PAFE is present; (B and D) PAFE is absent.
The variability in OD for each of the chosen points for all tested species was calculated by analyzing 180 growth curves in total. In general the variability, expressed as the CV, was not significantly different for the three criteria analyzed, although the CV was the lowest for OD0 (Table 1). When four replicates were used for both control and drug-exposed isolates, the CV was lower than 13% for OD0 and lower than 25% for OD20 and OD50.
TABLE 1.
Variability in time of one Aspergillus strain of each species to reach the three arbitrary growth curve criteriaa
| Strain | Criterion | 4-h incubation
|
2-h incubation
|
||
|---|---|---|---|---|---|
| Mean time (range)b | Upper 95% CI (CV) | Mean time (range)b | Upper 95% CI (CV) | ||
| A. fumigatus AZN VO2-33 | OD0 | 8.1 (7.5-9.6) | 9.8 (12.7) | 8.9 (8.2-10.6) | 10.7 (12.4) |
| OD20 | 11.7 (11.1-12.6) | 12.9 (6.6) | 11.7 (11.1-12.6) | 14.4 (4.7) | |
| OD50 | 24.4 (22.5-26.5) | 27.1 (6.7) | 24.3 (22.5-26.5) | 27.1 (6.7) | |
| A. terreus AZN 5914 | OD0 | 12.4 (12-12.7) | 13.1 (5.8) | 15 (14.2-15.7) | 16.4 (5.8) |
| OD20 | 21.7 (16.6-25.8) | 29.3 (22.0) | 24.2 (18.2-29.7) | 33.8 (25.1) | |
| OD50 | 28.9 (24.1-34.0) | 36.6 (16.6) | 30.2 (25.3-34.7) | 38.3 (16.8) | |
| A. ustus AZN 677 | OD0 | 12.7 (12-133.3) | 13.9 (6.1) | 8.5 (7.7-9.3) | 10 (10.7) |
| OD20 | 18.6 (14.7-24.9) | 25.9 (24.7) | 18 (15.6-24.3) | 24.7 (23.3) | |
| OD50 | 29.3 (20.9-35.3) | 40.7 (24.3) | 32.1 (23.8-38.2) | 43.4 (22.2) | |
| A. nidulans AZN 8950 | OD0 | 27.9 (27.2-28.7) | 29.3 (3.1) | 26.5 (25-28) | 29.3 (6.5) |
| OD20 | 25.4 (20-29.7) | 31.9 (16.1) | 26.8 (10.9-38.7) | 45.3 (43.2) | |
| OD50 | 34.7 (27.3-42.1) | 44.7 (18.2) | 38.2 (31.1-44.7) | 48.4 (16.7) | |
| A. flavus AZN 4094 | OD0 | 7.5 (6.2-9.9) | 12.7 (27.6) | 11 (10.3-11.4) | 12.5 (5.5) |
| OD20 | 14 (10.6-17.6) | 19.6 (25.3) | 18.8 (13.8-21.9) | 24.8 (20.1) | |
| OD50 | 25.3 (17.5-29) | 33.7 (21) | 25.2 (18.8-28.1) | 32.2 (17.3) | |
Strains were incubated for 2 or 4 hours to serve as controls for the drug-exposed isolates.
In hours.
Amphotericin B.
In general, PAFE was not observed after 1 h of exposure to amphotericin B at any concentration for all the species and for any of the three criteria used (data not shown). The magnitude of the PAFEs induced by amphotericin B at the chosen points in the growth curve (OD0, OD20, and OD50) after 2 and 4 h of exposure and at four times the corresponding MIC and at the MIC in RPMI 1640 broth are shown in Table 2.
TABLE 2.
PAFEs induced by exposure of Aspergillus species to amphotericin B
| Strain | PAFE (h) obtained with indicated criterion, exposure time, and drug concna
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD0
|
OD20
|
OD50
|
||||||||||
| 4 h
|
2 h
|
4 h
|
2 h
|
4 h
|
2 h
|
|||||||
| 4× | 1× | 4× | 1× | 4× | 1× | 4× | 1× | 4× | 1× | 4× | 1× | |
| A. fumigatus | ||||||||||||
| AZN VO2-31 | 9.3b | 3.6b | 1.8b | 0.8 | 10.5b | 5.6b | 2.5 | 0.9 | 4.2b | 2.8b | 2.5 | 0.9 |
| AZN VO2-32 | 9.7b | 3.3b | 6.0b | 2.2b | 8.9b | 1.5 | 5.4b | 2.5b | 5.3b | 3.7b | 1.9 | 1.8 |
| AZN VO2-33 | 10.8b | 5.2b | 4.3b | 2.7b | 7.1b | 3.7b | 6.5b | 1.7b | 6.5b | 1.6 | 4.4b | 0.1 |
| A. terreus | ||||||||||||
| AZN 5914 | 1.2 | 0.5 | 0.7 | 0.5 | 1.2 | 0.7 | 0.9 | 0.4 | 0.4 | 0.3 | 0.7 | 0.5 |
| AZN 515 | 2.1b | 0.4 | 0.2 | 0.1 | 4.2b | 2.3b | 0.8 | 0.6 | 4.3b | 2.1b | 0.4 | 0.6 |
| AZN 2868 | 4.3b | 2.1b | 1.1b | 0.4b | 1.2b | 0.8b | 0.7b | 0.2 | 1.4b | 1.5b | 0.6 | 0.7 |
| A. ustus | ||||||||||||
| AZN 677 | 4.2b | 2.3b | 1.3b | 0.2b | 2.9 | 1.9 | 1.5 | 0.3 | 3.2 | 2.3 | 3.3 | 0.3 |
| AZN 678 | 5.8b | 1.8b | 1.2b | 0.5b | 3.1 | 2.1 | 1.2 | 0.1 | 4.8b | 2.8b | 0.1 | 0.3 |
| AZN 943 | 1.8 | 1.2 | 0.5 | 0.5 | 5.2 | 2.1 | 1.8 | 0.8 | 2.9 | 1.5 | 2.9 | 1.3 |
| A. nidulans | ||||||||||||
| AZN 8950 | 0.3 | −0.2 | 0.2 | 0.5 | 0.5 | 0.1 | 0.8 | 0.8 | 0.1 | 0.1 | 2.9 | 1.2 |
| AZN 8033 | 0.8b | 0.1b | 0.2b | 2.2b | 1.5 | 3.1 | 4.9 | 0.9 | 4.6b | 1.8b | 2.0 | 0.5 |
| AZN 8958 | 0.8b | 0.7b | 4.7 | 0.1 | 2.4 | 1.7 | 0.9 | 0.7 | 2.1 | 4.7 | 0.8 | 0.6 |
| A. flavus | ||||||||||||
| AZN 4094 | 1.7 | 0.1 | −2.5 | −3.2 | 2.1 | 0.1 | −2.4 | 2.6 | 0.1 | 0.2 | 0.7 | 1.6 |
| AZN 2865 | 3.5 | −0.7 | 0.2 | 0.8 | 2.9 | 0.9 | −4.5 | 1.7b | 1.2 | 0.1 | 0.1 | 3.3 |
| AZN 284 | 3.5b | 1.0 | 0.2 | 1.8 | 2.8 | −0.8 | −3.0 | 1.4b | 0.1 | 0.1 | 0.1 | 0.1 |
4×, 4 times the MIC; 1×, MIC.
PAFE was significant.
A. fumigatus.
By the OD0 criterion, the PAFE induced by amphotericin B was longer for A. fumigatus than for the other Aspergillus species, with mean PAFEs of 9.9 and 4.0 h after 4 h of exposure at four times the MIC and at the MIC, respectively, and 4.0 h after 2 h of exposure to four times the MIC. Also, at concentrations of amphotericin B of four times the MIC and equal to the MIC, after 4 h PAFE was observed for A. fumigatus, with means of 8.9 and 3.6 h, respectively, using the OD20 criterion. At OD50 the A. fumigatus isolates also displayed PAFE, with means of 5.3 h after 4 h of exposure and four times the MIC and 2.7 h at the MIC. Amphotericin B induced PAFE against A. fumigatus after 2 h of incubation at four times the MIC, with a mean of 4.0 h using the OD0 criterion.
Other Aspergillus species.
For A. terreus and A. nidulans, after 4 h of exposure to concentrations of amphotericin B of four times the MIC, the mean PAFEs were 2.5 and 0.7 h at OD0. Amphotericin B induced PAFE against A. ustus at concentrations of four times the MIC and equal to the MIC after 4 and 2 h of exposure, with mean PAFEs of 3.9, 1.8, 1.0, and 0.4 h, respectively, using the OD0 criterion. The results for A. flavus were inconsistent and did not allow conclusions to be drawn.
At OD0, PAFE was induced by amphotericin B after 4 h of exposure for all three A. fumigatus isolates and for two of three strains of A. ustus, A. terreus, and A. nidulans. After 2 h of exposure, PAFE was observed only for A. fumigatus for all the chosen criteria (Table 2). A statistically significantly longer PAFE was induced by amphotericin B after 4 h of exposure at four times the MIC for A. fumigatus than for the other Aspergillus species (P < 0.05). For A. fumigatus, significantly longer PAFEs were found after 4 h of exposure to amphotericin B at the MIC compared with A. terreus, after 2 h of exposure at four times the MIC compared with A. nidulans, and after 2 h of exposure at the MIC compared with A. terreus and A. ustus (P < 0.05). No statistically significant intraspecies variation was observed.
Itraconazole.
Itraconazole failed to induce a PAFE at any concentration tested and for any exposure time. Even exposure to itraconazole concentrations as high as 10, 20, and 50 times the MIC failed to induce a PAFE (data not shown).
DISCUSSION
PAFE assay.
In this study a method that allows the quantification and study of post-antifungal-drug-exposure effects in molds was developed. Since the use of viability counts to monitor microbial growth kinetics following drug removal is not feasible with molds due to their nonhomogeneous growth, OD measurements were used. Although the growth curves thus obtained allow quantification of PAFE, microscopic examination of the fungal growth in the wells of the microtitration plates is of great importance. Post-drug-exposure effects other than growth suppression could have an impact on the OD measured. For instance, alteration of the frequency of hyphal branching could result in changes in the OD. However, careful microscopic examination of the molds at different times postexposure revealed no differences in morphology compared with unexposed controls. The reliability of measurement of fungal growth using this spectrophotometric system has been described before, and even small changes in morphology can be detected (16).
It has been demonstrated that Aspergillus conidia germinate when incubated at 37°C in RPMI 1640 after 6 h or more (15, 16), with exception only of A. flavus, which germinates within 4 h. Therefore, a maximum exposure period of 4 h was chosen. The exposure of Aspergillus conidia to amphotericin B did not affect the viability of the conidia at any of the concentrations or incubation times used. Consequently, adjustment of the inoculum to match that of the controls was not necessary.
The criterion used to define PAFE is arbitrary, although most workers have used the difference between time required for the control and the drug-exposed bacteria or yeasts to increase 1 log10 compared with the initial CFU count (4, 10) or, if a spectrophotometric procedure was used, time to reach a 0.05 absorbance level (9). In the present study, PAFE was not evaluated with CFU counts because of the nonhomogeneous growth of molds. Therefore, we used a spectrophotometric procedure that was originally developed to study growth characteristics of molds (16). Since viability counts were not performed, several arbitrary chosen criteria were analyzed to calculate the PAFE, namely, OD0, OD20, and OD50. The variability between replicates of control isolates with these three criteria was not significantly different. Furthermore, for each criterion, only small differences in PAFE were observed, especially for A. fumigatus and A. terreus. However, using OD0 to quantify PAFE has several advantages. First, the variability among the controls was lower when OD0 was used than with the other two criteria. When four replicates were used, the CV was below 13% for both drug-exposed and control strains with OD0. Second, OD0 is reached earlier than the other criteria, leaving less impact of variables such as production of metabolites, crystals, or cellular debris that might interfere with the OD reading (12). Third, in order to calculate OD20 and OD50, the OD that corresponds to maximal growth is required, thereby prolonging the time before results become available, especially in slow-growing fungi. RPMI 1640 medium was chosen since it is recommended by the NCCLS for MIC determination of conidium-forming fungi. However, it has been shown that RPMI 1640 poorly supports the growth of Aspergillus species (16). The optimal growth curve, which contains a lag phase, a log phase, and a plateau, is not obtained when Aspergillus species are cultured in RPMI 1640, but a linear curve was found with the same spectrophotometric system (16). Maximal growth of Aspergillus is not reached within 99 h, and therefore we defined maximal growth as the OD that was reached after 48 h of incubation. Alternative media that support the growth of Aspergillus more adequately could be useful in this respect, although further studies are needed.
PAFE.
PAEs have been found to be dependent on several factors, such as the concentration of the antibiotic agent, the time during which the bacteria were exposed to the drug, and the characteristics of the drug used to induce the effect (20). PAFE also appears to depend on drug, concentration, and duration of exposure, as was described for Candida species (10). In the present study, the PAFE displayed by amphotericin B showed dependence on the concentration and the exposure period. This is partly consistent with a previous published study with Candida, where longer PAFEs were found following exposure to amphotericin B at concentrations above the MIC compared with shorter PAFEs following exposure to sub-MIC drug concentrations (10). The echinocandin caspofungin (MK-0991) also induced a PAFE against Candida, and the magnitude of the effect depended on the concentration of the drug. Both amphotericin B and caspofungin exhibit fungicidal activity in vitro against Candida (10, 21, 23).
Amphotericin B.
Overall, the magnitude of the PAFE induced by amphotericin B in vitro was the greatest against A. fumigatus. The range found in our study was similar to that found in a previous study with Candida species, where the PAFE ranged between 0.5 and 10 h (9, 11). However, significantly shorter PAFEs were observed for A. terreus and A. nidulans, where only exposure to high concentrations of amphotericin B could induce a PAFE. Invasive aspergillosis caused by A. terreus and A. nidulans is often clinically unresponsive to treatment with amphotericin B (5, 22). The short PAFE or lack of PAFE we found in A. terreus and A. nidulans isolates could contribute to the poor clinical response.
Itraconazole.
In our study, exposure to the triazole drug itraconazole did not induce PAFE in any of the Aspergillus species tested. Even exposure of Aspergillus isolates to a concentration of 50 times the MIC for 4 h was not sufficient to induce PAFE. The inability of itraconazole to induce PAFE in Aspergillus is in accordance with observations reported for Candida, where no measurable PAFE was observed following exposure to fluconazole (10, 17, 23). The lack of PAFE induced by azoles could be due to the exposure period being too short to cause sufficient damage to the mold. However, longer exposure periods could not be investigated with our system, since germination of the control strains occurred after 6 h of incubation.
Implications.
Two characteristics determine the time course of antimicrobial activity: the effect of increasing drug concentrations on the extent of killing of the microorganism and the presence or absence of postexposure effects. Our results indicate that post-antifungal-drug-exposure effects occur in Aspergillus. The consequences of PAFE of amphotericin B in several Aspergillus species for the dosing regimen of the drug remain unclear. Due to the pharmacokinetics of amphotericin B, the concentrations in plasma do not normally exceed 2 μg/ml (14), and after leaving the circulation, the drug slowly accumulates in some tissue compartments (3). Consequently, the concentrations of amphotericin B in the blood do not accurately reflect the concentrations in tissues (14). The concentration in mouse kidneys can be up to four times greater than the level in serum, and it may appear that antifungal activity persists despite little to no measurable drug in serum (8). The MICs for strains tested in this study were 1 μg/ml, indicating that four times the MIC is a concentration that is achievable in vivo.
Given the fact that amphotericin B is administered once daily, our results suggest that growth suppression is achieved for a significant period for A. fumigatus depending on the concentration at the site of infection. However, a larger collection of isolates should be tested and in vivo models are required to confirm our findings. In a murine model of invasive candidiasis, the pharmacodynamics of amphotericin B were best predicted by the peak serum level/MIC ratio (1). Furthermore, high infrequent doses of amphotericin B were as effective as lower, more frequently administered doses in treating invasive candidiasis (1). Since PAFEs of amphotericin B for A. fumigatus were similar to those observed with Candida albicans, infrequent dosing might be a useful strategy in the treatment of invasive aspergillosis due to A. fumigatus.
In conclusion, we have developed an assay that allows the study of PAFE of different antifungal agents in Aspergillus species and other molds. Amphotericin B induced PAFE in A. fumigatus, but in A. terreus and A. nidulans short PAFEs or a lack of PAFE was observed. However, further studies are warranted, including in vivo experiments, to study the impact of PAFE in Aspergillus on dosing regimens of amphotericin B.
Acknowledgments
Eric Dannaoui and Joseph Meletiadis are acknowledged for their helpful discussions.
We thank the Mycology Research Center of Nijmegen for financial support.
REFERENCES
- 1.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boogaerts, M., D. J. Winston, E. J. Bow, G. Garber, A. C. Reboli, A. P. Schwarer, N. Novitzky, A. Boehme, E. Chwetzoff, and K. De Beule. 2001. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy. A randomized, controlled trial. Ann. Intern. Med. 135:412-422. [DOI] [PubMed] [Google Scholar]
- 3.Collette, N., P. van der Auwera, A. P. Lopez, C. Heymans, and F. Meunier. 1989. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob. Agents Chemother. 33:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig, W. A., and S. Gudmunsson. 1991. Postantibiotic effect, p. 403-431. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, Md.
- 5.Dannaoui, E., E. Borel, F. Persat, M. A. Piens, and S. Picot. 2000. Amphotericin B resistance of Aspergillus terreus in a murine model of disseminated aspergillosis. J. Med. Microbiol. 49:601-606. [DOI] [PubMed] [Google Scholar]
- 6.Dannaoui, E., F. Persat, M. F. Monier, E. Borel, M. A. Piens, and S. Picot. 1999. In-vitro susceptibility of Aspergillus spp. isolates to amphotericin B and itraconazole. J. Antimicrob. Chemother. 44:553-555. [DOI] [PubMed] [Google Scholar]
- 7.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodds, E. S., R. H. Drew, and J. R. Perfect. 2000. Antifungal pharmacodynamics: review of the literature and clinical applications. Pharmacotherapy 20:1335-1355. [DOI] [PubMed] [Google Scholar]
- 9.Ellepola, A. N., and L. P. Samaranayake. 1999. The in vitro post-antifungal effect of nystatin on Candida species of oral origin. J. Oral Pathol. Med. 28:112-116. [DOI] [PubMed] [Google Scholar]
- 10.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia, M. T., M. T. Llorente, F. Minguez, and J. Prieto. 2000. Influence of pH and concentration on the postantifungal effect and on the effects of sub-MIC concentrations of 4 antifungal agents on previously treated Candida spp. Scand. J. Infect. Dis. 32:669-673. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson, S. M., H. Hoffman, E. J. Ernst, M. A. Pfaller, and M. E. Klepser. 2000. In vitro pharmacodynamic characteristics of nystatin including time-kill and postantifungal effect. Antimicrob. Agents Chemother. 44:2887-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser, L., T. Huguenin, P. D. Lew, B. Chapuis, and D. Pittet. 1998. Invasive aspergillosis. Clinical features of 35 proven cases at a single institution. Medicine (Baltimore) 77:188-194. [DOI] [PubMed] [Google Scholar]
- 14.Khoo, S. H., J. Bond, and D. W. Denning. 1994. Administering amphotericin B—a practical approach. J. Antimicrob. Chemother. 33:203-213. [DOI] [PubMed] [Google Scholar]
- 15.Manavathu, E. K., J. Cutright, and P. H. Chandrasekar. 1999. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J. Clin. Microbiol. 37:858-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meletiadis, J., J. F. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minguez, F., M. L. Chiu, J. E. Lima, R. Nique, and J. Prieto. 1994. Activity of fluconazole: postantifungal effect, effects of low concentrations and of pretreatment on the susceptibility of Candida albicans to leucocytes. J. Antimicrob. Chemother. 34:93-100. [DOI] [PubMed] [Google Scholar]
- 18.Mylonakis, E., T. F. Barlam, T. Flanigan, and J. D. Rich. 1998. Pulmonary aspergillosis and invasive disease in AIDS: review of 342 cases. Chest 114:251-262. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. Document M-38P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Odenholt, I. 2001. Pharmacodynamic effects of subinhibitory antibiotic concentrations. Int. J. Antimicrob. Agents 17:1-8. [DOI] [PubMed] [Google Scholar]
- 21.Scalarone, G. M., Y. Mikami, N. Kurita, Y. Ichihara, K. Yazawa, and M. Miyaji. 1991. Turbidometric characterization of the postantifungal effect: comparative studies with amphotericin B, 5-fluorocytosine and miconazole on Candida albicans. Mycoses 34:297-302. [DOI] [PubMed] [Google Scholar]
- 22.Segal, B. H., E. S. DeCarlo, K. J. Kwon-Chung, H. L. Malech, J. I. Gallin, and S. M. Holland. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 77:345-354. [DOI] [PubMed] [Google Scholar]
- 23.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]
- 24.Verweij, P. E., K. L. Oakley, J. Morrissey, G. Morrissey, and D. W. Denning. 1998. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 42:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]