Abstract
The production of histidine-rich protein II (HRP2), a histidine- and alanine-rich protein produced by Plasmodium falciparum, is closely associated with the development and proliferation of the parasite and therefore is perfectly suited to reflect growth inhibition as a measure of drug susceptibility. It was the aim of the present study to develop a malaria drug sensitivity assay based on the measurement of HRP2 in a simple enzyme-linked immunosorbent assay (ELISA). The new test proved to be as reliable as traditional in vitro assays, while it was considerably easier to establish and perform. Parasites are incubated at an initial level of parasitemia of 0.01 to 0.1% on microculture plates predosed with ascending concentrations of antimalarial drugs. After incubation for 48 to 72 h, the samples are freeze-thawed and transferred to ELISA plates. The complete ELISA takes about 2.5 h to perform, may be carried out with commercially available test kits, and requires relatively little technical equipment. In correlation analysis, the results closely paralleled those obtained by the isotopic assay (R = 0.892; P < 0.0001) and World Health Organization schizont maturation tests (R = 0.959; P < 0.0001). The novel HRP2 drug susceptibility assay proved to be very sensitive, simple to establish, and highly reproducible. It can be used for a wide range of applications, from epidemiological studies to the screening of new drugs, and may have the potential to replace traditional in vitro techniques. Standard operating procedures, updated information, and analytical software are available from http://malaria.farch.net.
Antimalarial drug resistance has become an issue of utmost importance for the control of falciparum malaria worldwide. Individual treatment regimens as well as malaria control strategies therefore need to be based on profound knowledge of drug sensitivity. There are basically two approaches to the assessment of the antimalarial drug susceptibility of Plasmodium falciparum: in vivo and in vitro assays. The most commonly used in vitro assays, the isotopic assay, the World Health Organization (WHO) microtest, and a number of parasite lactate dehydrogenase-based assays, have their advantages but also have a number of known drawbacks (1, 7, 8, 12, 14, 24, 26). It was the aim of this study to develop an in vitro assay that is easy to establish and to standardize, that has a high sensitivity, and that requires little technical equipment, similar to the WHO test, but that is also as reproducible and that can be performed as fast as the isotopic assay.
Histidine-rich protein II (HRP2) is a naturally occurring histidine- and alanine-rich protein localized in several cell compartments including the cytoplasm of P. falciparum. Recent studies have implicated HRP2 as an important factor in the detoxification of heme (11, 16, 17, 21). HRP2 was identified in all P. falciparum strains regardless of knob phenotype and was recovered from plasma and culture supernatants as a secreted water-soluble protein (20). It is found as concentrated packets in the host erythrocyte cytoplasm and on the infected erythrocyte membrane (9). HRP2 is a common target for rapid diagnostic tests for malaria (3). Its long half-life in vivo and its persistence in patients with successfully treated falciparum malaria, however, limit the application of HRP2-based dipstick or spot tests for the monitoring of therapeutic efficacy (13). For in vitro drug susceptibility assays, on the other hand, the stability of this protein may be a major advantage. Similar to schizont maturation and hypoxanthine uptake, levels of HRP2 production may vary between parasite strains. As drug sensitivity tests are internally controlled, however, this is not an issue in this context. The amount of HRP2 found in culture samples is closely associated with parasite growth and increases with parasite development and multiplication (6). We therefore concluded that HRP2 might be an excellent indicator of parasite growth and its inhibition by antimalarial drugs. The availability of commercial HRP2 enzyme-linked immunosorbent assay (ELISA) kits for the quantification of P. falciparum HRP2 is an additional distinct advantage and may make implementation of this assay faster than that of any other malaria in vitro drug sensitivity test.
MATERIALS AND METHODS
A total of 20 cryopreserved, culture-adapted strains of P. falciparum originating from three Asian countries (collected from 1994 to 2001 in Thailand, Myanmar, and Bangladesh) were subjected to the new HRP2 ELISA as well as to isotopic and morphological drug susceptibility assays. The strains were selected according to their previously established sensitivities to test isolates with a broad range of antimalarial drug susceptibilities. All tests were performed at the Department of Immunology and Medicine, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand. After the samples were thawed, they were washed and mixed with RPMI 1640 medium (with 10% human serum) at 5% hematocrit, and the mixture was transferred into cell culture flasks. The cell-medium mixture (CMM) was incubated at 37.5°C in a 5% CO2-5% O2-90% N2 gas mixture until a minimum parasite density of 0.5% (for the HRP2 and the morphological assays) or 2% (for the isotopic assay) was achieved (22).
HRP2 drug susceptibility assay.
The samples from the continuous cultures were washed and resuspended with RPMI 1640 (with 10% human serum) and uninfected erythrocytes to obtain 0.05% parasitemia and 1.5% hematocrit. The samples should contain predominantly ring forms and generally do not require synchronization. Stock solutions (1 mg/ml) in 70% ethanol were prepared from mefloquine hydrochloride (Mr, 414.778), chloroquine diphosphate (Mr, 515.867), quinine sulfate dihydrate (Mr, 785.06), and artesunate (Mr, 384.425). These were diluted with RPMI 1640 in order to obtain the desired test concentrations (mefloquine hydrochloride concentrations, 9.39 to 601.28 nM; chloroquine diphosphate concentrations, 27.70 to 1772.55 nM; quinine sulfate dihydrate concentrations, 54.61 to 3495.27 nM; artesunate concentrations, 0.34 to 22.02 nM). Serial twofold dilutions (seven concentrations and one drug-free control) of the drugs (25 μl/well) were dispensed into standard 96-well microculture plates (Costar 3599 plates) manually or by a semiautomated microdilution technique, and 200 μl of CMM was added to each well. The plates were then incubated for 72 h in a gas mixture (5% CO2, 5% O2, 90% N2) at 37.5°C. They were subsequently frozen-thawed twice to obtain complete hemolysis. After the incubation the plates were processed immediately or were stored at −30°C until further processing.
HRP2 ELISA.
A commercial HRP2 ELISA kit (Malaria Ag CELISA; Cellabs Pty. Ltd., Brookvale, New South Wales, Australia) was used for the quantification of HRP2 in the culture samples. One hundred microliters of each of the hemolyzed culture samples was transferred to the ELISA plates, which are precoated with monoclonal antibodies against P. falciparum HRP2 (capture antibody of the immunoglobulin M class; code CPF4), and the plates were incubated at room temperature for 1 h. Subsequently, the plates were washed four times with the washing solution provided with the test kit, and 100 μl of the diluted antibody conjugate (an indicator antibody of the immunoglobulin G1 isotype; code CPF6) was added to each well. After incubation for an additional 1 h, the plates were washed four times and 100 μl of diluted (1:20) chromogen (tetramethylbenzidine) was added to each well. The plates were then incubated for another 15 min in the dark, and 50 μl of the stop solution was added. Spectrophotometric analysis was performed with an ELISA plate reader (SpectraMAX 340 Microplate spectrophotometer; Molecular Devices, Sunnyvale, Calif.) at an absorbance maximum of 450 nm. The optical density values correspond to the amount of HRP2 found in the culture samples and provide consistent indicators of parasite growth. The complete ELISA may easily be performed within less than 3 h, largely independent of the number of samples to be tested.
Potential modifications to the HRP2 assay.
The HRP2 assay may be adjusted to meet the requirements of individual laboratories (however, all test parameters should be kept constant within a study). In its current form the basic procedure for the assay was developed with culture-adapted, cryopreserved P. falciparum strains. Although essentially the HRP2 assay may also be performed with fresh isolates, so far there are no data from field trials with fresh isolates. The validation of the assay with fresh isolates will therefore be an important issue for a future application in field studies. From our experience the assay may also be performed with an incubation time of 48 h. However, the 72-h incubation allows the testing of slowly acting drugs without the need for changes to the protocol and gives a success rate close to 100%, even with slowly growing parasite strains. We successfully conducted experiments with initial parasite densities as low as 0.01%. However, the range that yielded the best results was 0.02 to 0.07%. Samples with an initial level of parasitemia of more than 0.1% need to be diluted accordingly, either before incubation (with uninfected erythrocytes and RPMI 1640) or before the ELISA is performed (with RPMI 1640). We used 200 μl of CMM and 25 μl of drug solution per well throughout this study. As the ELISA requires only a 100-μl sample volume, the assay can also be performed with a reduced amount of CMM. A sample of the CMM may be set aside and frozen before the start of the incubation to assess the amount of preexisting HRP2. This sample is also tested in the ELISA, and its optical density is subtracted from those for the test samples. This allows a very precise estimate of the rise in HRP2 levels to be obtained. If full inhibition of parasite growth can be expected, however, it may be sufficient to use the lowest value in each test instead.
Isotopic assay.
The isotopic assay followed the routine established at the AFRIMS laboratory based on the method of Desjardins et al. (7, 23, 25). The samples from continuous culture were diluted to 0.5% parasitemia and 1.5% hematocrit. The plates were dosed with antimalarial drugs, and 200 μl of CMM was added. The plates were incubated in a gas mixture (5% CO2, 5% O2, 90% N2) for 24 h at 37.5°C, pulsed with 25 μl of [3H]hypoxanthine solution (hypoxanthine monohydrochloride), and reincubated for an additional 18 h. The samples were harvested onto filter paper with a harvesting machine (Filtermate Harvester; Packard Instruments, Meriden, Conn.), dried, and mixed with 25 μl of scintillation fluid (Microscint 20; Packard Instruments). The scintillation was determined in a microplate scintillation counter (Top Counter NXT; Packard Instruments).
Morphological assay.
A modified WHO schizont maturation assay was used to morphologically assess parasite development (26). The culture samples were diluted in the same way as those used for the HRP2 assay (1.5% hematocrit, 0.05% parasitemia). As the morphological assay requires a high degree of synchronism, the growth in the samples was synchronized with 5% sorbitol (10). Subsequently, 200 μl of CMM was incubated for 24 h at 37.5°C on the same plates used for the HRP2 assay. After removal of the sediment, thick films were prepared from the cell sediment of each well. These were microscopically evaluated by counting the number of schizonts with three or more chromatins per 200 asexual parasites.
Statistical analysis.
Individual inhibitory concentrations (the 50% inhibitory concentration [IC50], IC90, IC99) for all three assays were determined by nonlinear regression analysis. The software used is based on a polynomial regression model and is freely available from http://malaria.farch.net. The results (IC50) closely parallel those obtained with other nonlinear estimation programs or log-probit analysis. Bland-Altman plots were prepared to assess the levels of agreement between two methods (4). Standard correlation analysis was used to establish associations between the ICs obtained by the different assays with the various drugs. The t test was used to evaluate differences between group means. Nonparametric procedures were used for data that were not normally distributed.
RESULTS
In total, 20 strains were culture adapted and successfully tested by all three assays. The novel HRP2 drug susceptibility assay was found to be easy and rapid to perform. It produced results comparable to those obtained by the other assays.
The geometric mean IC50s determined by the HRP2 drug susceptibility assay were 65.32 nM (95% confidence interval [CI], 38.97 to 109.48 nM), 343.86 nM (95% CI, 239.93 to 492.80 nM), 217.39 nM (95% CI, 162.70 to 290.46 nM), and 2.06 nM (95% CI, 1.57 to 2.70 nM) for mefloquine, quinine, chloroquine, and artesunate, respectively. The geometric mean ICs and confidence intervals for all three assays are shown in Table 1. A number of samples (n = 4) were also tested after 48 h of incubation, and the ICs were similar. The mean difference in the IC50 at 48 h of incubation compared to that at 72 h of incubation was −0.05 on the log scale (limits of agreement, −0.368 and 0.267).
TABLE 1.
Geometric mean IC50s, IC90s, and IC99, with 95% CIs, for mefloquine, quinine, chloroquine, and artesunate determined by the HRP2 drug susceptibility assay, a modified WHO schizont maturation assay, and the isotopic assaya
| Drug and assay (n = 20) | IC (nM [95% CI])
|
||
|---|---|---|---|
| 50% | 90% | 99% | |
| MEF | |||
| HRP2 | 65.32 (38.97-109.48) | 142.29 (91.73-220.71) | 189.73 (123.93-290.45) |
| MORPH | 46.20 (28.43-75.08) | 95.23 (58.55-154.89) | 122.06 (75.29-197.88) |
| RADIO | 70.03 (43.88-112.77) | 152.36 (100.49-231.00) | 206.83 (144.83-295.38) |
| QNN | |||
| HRP2 | 343.86 (239.93-492.80) | 903.13 (712.46-1,144.83) | 1,218.11 (952.20-1,558.29) |
| MORPH | 364.84 (258.76-514.40) | 763.97 (571.30-1,021.61) | 950.98 (718.54-1,258.61) |
| RADIO | 345.32 (260.43-457.89) | 847.99 (661.11-1,087.71) | 1,210.67 (970.68-1,509.98) |
| CHL | |||
| HRP2 | 217.39 (162.70-290.46) | 528.89 (395.00-708.15) | 739.08 (577.50-945.88) |
| MORPH | 221.28 (160.68-304.74) | 421.93 (298.46-596.46) | 515.62 (367.13-724.17) |
| RADIO | 117.98 (97.32-143.04) | 248.88 (206.11-300.53) | 313.51 (259.39-378.93) |
| ARS | |||
| HRP2 | 2.06 (1.57-2.70) | 7.55 (5.00-11.40) | 15.60 (10.85-22.43) |
| MORPH | 0.96 (0.82-1.13) | 1.48 (1.16-1.90) | 2.21 (1.58-3.08) |
| RADIO | 2.82 (2.16-3.67) | 5.85 (4.77-7.16) | 7.45 (6.27-8.85) |
Abbreviations: MEF, mefloquine; QNN, quinine; CHL, chloroquine; ARS, artesunate; HRP2, HRP2 drug susceptibility assay; MORPH, a modified WHO schizont maturation assay; RADIO, isotopic assay.
No statistically significant differences in the results of the HRP2 assay in relation to those of the schizont maturation assay and the isotopic assay were found for mefloquine and quinine. The chloroquine IC50 determined by the isotopic assay and the artesunate IC50 determined by the morphological assay were significantly lower (P < 0.001) than the corresponding values obtained by the other two assays.
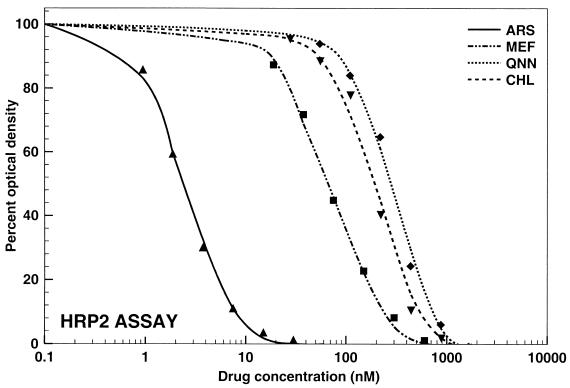
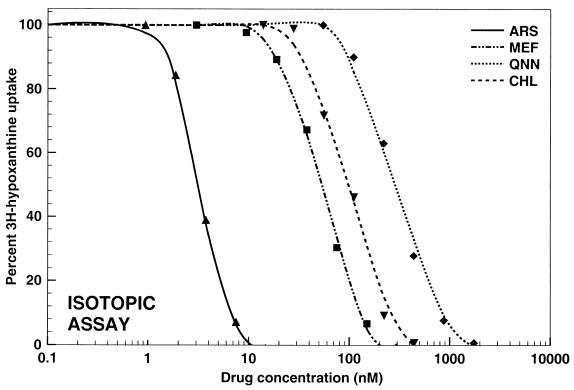
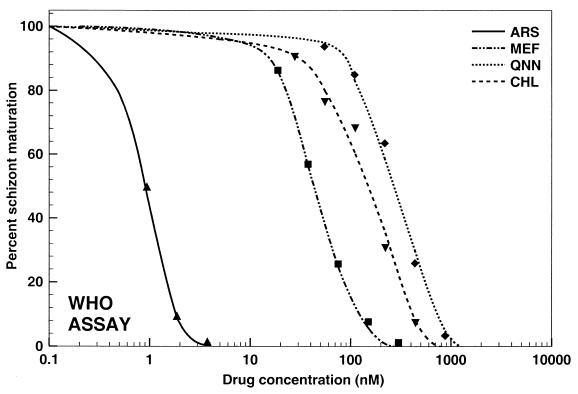
The data obtained by all three assays were analyzed by nonlinear regression analysis. Representative graphs illustrating the pooled data for the responses of all 20 P. falciparum strains to mefloquine, quinine, chloroquine, and artesunate are shown in Fig. 1 to 3 . An excellent fit of the data to the polynomial regression model was observed with all regressions (all regressions resulted in squared correlation coefficients higher than 0.989).
FIG. 1.
Activities of mefloquine (MEF; R2 = 0.9974), quinine (QNN; R2 = 0.9951), chloroquine (CHL; R2 = 0.9903), and artesunate (ARS; R2 = 0.9977) against all P. falciparum strains tested (n = 20) by the new HRP2 drug susceptibility assay.
FIG. 3.
Activities of mefloquine (MEF; R2 = 0.9985), quinine (QNN; R2 = 0.9927), chloroquine (CHL; R2 = 0.9926), and artesunate (ARS; R2 = 0.9933) against all P. falciparum strains tested (n = 20) by the isotopic assay.
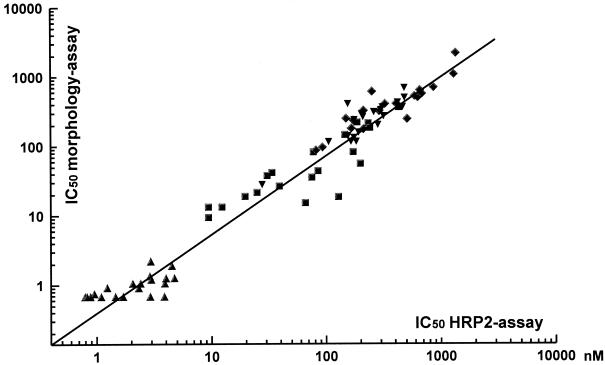
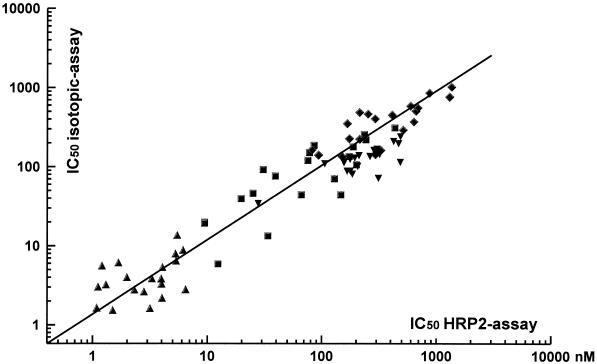
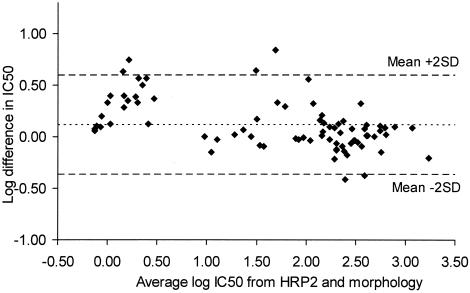
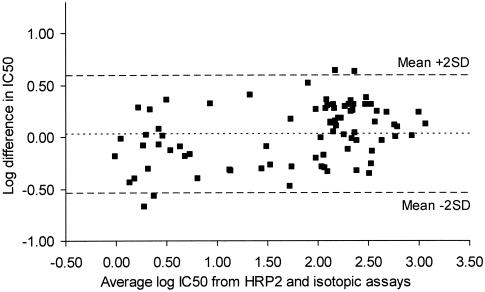
By correlation analysis the pooled results obtained by the HRP2 drug sensitivity tests with all four antimalarials showed a distinct association with those obtained by the schizont maturation assay (n = 20; R = 0.959; P < 0.001) and the isotopic assay (n = 20; R = 0.892; P < 0.001) (Fig. 4 and 5, respectively). The Bland-Altman plots for the agreement of the results obtained by the HRP2 assay in relation to those obtained by the other two techniques as well as the mean difference and limits of agreement are shown in Fig. 6 and 7, respectively.
FIG. 4.
Scatter plot for the association of individual IC50s (in nanomolar) for mefloquine (squares), quinine (diamonds), chloroquine (inverted triangles), and artesunate (triangles) determined by the HRP2 drug susceptibility assay and a modified WHO schizont maturation (morphology) assay (n = 20; R = 0.959; P < 0.001).
FIG. 5.
Scatter plot for the association of individual IC50s (in nanomolar) for mefloquine (squares), quinine (diamonds), chloroquine (inverted triangles), and artesunate (triangles) determined by the HRP2 drug susceptibility assay and the isotopic assay (n = 20; R = 0.892; P < 0.001).
FIG. 6.
Bland-Altman plot of the difference in log IC50s for mefloquine, quinine, chloroquine, and artesunate determined by the HRP2 drug susceptibility assay and a modified WHO schizont maturation (morphology) assay plotted against their mean values. The mean difference in IC50s on the log scale was 0.112 (limits of agreement, −0.370 and 0.594).
FIG. 7.
Bland-Altman plot of the difference in log IC50s for mefloquine, quinine, chloroquine, and artesunate determined by HRP2 drug susceptibility assay and the isotopic assay plotted against their mean values. The mean difference in IC50s on the log scale was 0.024 (limits of agreement, −0.538 and 0.578).
Highly significant drug-to-drug interactions were found by the HRP2 assay as well as by the other two assays. The IC50s of mefloquine obtained by the HRP2 assay were closely correlated with those of artesunate (R = 0.794; P < 0.001) and quinine (R = 0.733; P < 0.001). Quinine and artesunate activities were similarly correlated (R = 0.892; P < 0.001). The patterns of the drug-to-drug correlations were identical by the WHO schizont maturation assay (for mefloquine-artesunate, R = 0.767 and P < 0.001; for mefloquine-quinine, R = 0.555 and P = 0.011; for quinine-artesunate, R = 0.556 and P = 0.011) and by the isotopic assay (for mefloquine-artesunate, R = 0.695 and P = 0.001; for mefloquine-quinine, R = 0.635 and P = 0.003; for quinine-artesunate, R = 0.594 and P = 0.006).
DISCUSSION
The value of in vitro drug susceptibility data for the development of new drugs as well as for epidemiological purposes is undisputed. The quickening pace of antimalarial drug resistance in recent years and the need for the monitoring of drug sensitivity have necessitated the development of new, user-friendly assays for antimalarial drug sensitivity testing to complement or replace techniques that have been in use for more than two decades.
The new HRP2 drug sensitivity assay provides a rapid and simple method for the quantification of antimalarial drug action. It combines the advantages of the other two assays, while it omits most of their disadvantages. In relation to the other two assays, the HRP2 assay was found to be easier and faster to perform. The availability of commercial ELISA kits for the quantification of P. falciparum HRP2 eliminates the need for standardization of the ELISA procedure, thereby making its implementation faster than that of any other assay.
Even though all three assays use different end points, the results of the HRP2 assay closely paralleled those obtained by the isotopic and the schizont maturation assays. The element common to all three assays is that they provide measures of parasite development and growth. Owing to the peculiarities of each assay, it is virtually impossible to perform all three assays under identical conditions. In the isotopic assay the parasites are incubated for 24 h before being pulsed with [3H]hypoxanthine (7). After incubation for an additional 18 h the amounts of incorporation of the tracer into the parasite DNA and RNA are measured. The isotopic assay therefore quantifies the metabolic activity in the second half of the erythrocytic life cycle. However, it requires a relatively high level of parasitemia (about 0.5%) to obtain adequate readings. Furthermore, the isotopic assay has the disadvantage of requiring expensive, highly specialized equipment and the handling of radioactive substances.
The schizont maturation assay, on the other hand, determines the percentage of parasites that develop from rings to schizonts within 24 h, thus requiring parasites whose development is highly synchronized (19). It measures a much earlier phase of development than the isotopic assay. The microscopic evaluation of the test results makes the assay considerably more sensitive than the isotopic assay. While it is very economical, the WHO microtest is particularly labor-intensive and requires highly trained personnel in order to limit individual variability caused by human factors.
The HRP2 assay measures the increase in HRP2 levels over the incubation time, which is most marked during the ring and trophozoite stages (6). Owing to the sensitivity of the ELISA, the HRP2 assay is very sensitive but requires a certain minimum incubation time to obtain significant increases in HRP2 levels. A 48-h incubation covers a full erythrocytic life cycle and, consequently, leads to an increase in the HRP2 concentration that corresponds to the rise in the level of parasitemia. Synchronism of the growth of the culture samples is therefore not as crucial. In contrast to the other assays, it does not involve particularly expensive equipment or the handling of radioactive substances and leaves little room for error caused by individual variability.
All three assays consistently differentiated strains sensitive to mefloquine, quinine, and chloroquine from strains resistant to these drugs. The distinct parallels between the individual results obtained by the new assay and both of the conventional assays reflect the reliabilities of the three test systems when they are applied to various drugs. The parallels in the drug-to-drug correlations, such as those found between mefloquine, artesunate, and quinine, demonstrate the ability of the new test system to quantify the extent of cross-resistance among antimalarial drugs. This is an important issue for in vitro testing, as cross-resistance may hardly be established in clinical trials (15). Such activity correlations, especially the correlation between the activities of artesunate and mefloquine, were evident in all three assays and are also a common finding in other drug sensitivity studies, irrespective of the method used (2, 15, 18).
Another major advantage of using HRP2 levels as an indicator of parasite growth and biomass lies in the stability of the protein (5). The resulting stable background enables the assay to detect even minor variations in parasite development. We used a commercial HRP2 ELISA kit for the quantification of HRP2 in the culture samples. Basically, the assay can be performed with any P. falciparum HRP2-specific ELISA. Numerous laboratories and companies produce monoclonal antibodies specific for HRP2. The major advantage of using commercial test kits lies in the simple standardization and reproducibility of the assay. Its high sensitivity accounts for the low level of parasitemia used in the drug susceptibility assay. With initial parasite densities as low as 0.01%, the sensitivity of the assay is comparable to that of the WHO test. Particularly in relation to the isotopic assay, which requires a minimum level of parasitemia of 0.2 to 0.5%, the new assay was considerably more sensitive, an especially important factor for field application.
The assay may easily be adapted to the requirements of individual laboratories to further simplify drug sensitivity testing. It may, for example, be used with predosed plates, such as those provided by WHO for the schizont maturation assay, avoiding possible quality control issues associated with self-dosing of the plates and the necessity of obtaining antimalarial drugs (26). Other simple modifications comprise adjustments in levels of parasitemia or incubation times.
In addition to its potential value both as a tool for drug resistance surveillance and as a screen for new antimalarials, a number of other uses for the assay are under consideration. Antimalarials may be tested in a dilution matrix or in various combinations to evaluate potential pharmacodynamic interactions. In pharmacokinetic studies it may be used in bioassays to measure antimalarial activity in blood specimens obtained after the administration of antimalarial drugs. Another possible application is the testing of the inhibitory activities of specimens obtained in the course of vaccine trials.
In conclusion, the HRP2 drug susceptibility assay was found to be very sensitive, fast and simple to establish, highly reproducible, and easy to perform. It can be used for a wide range of applications, from epidemiological studies to the screening of new drugs, and may have the potential to replace traditional in vitro techniques.
FIG. 2.
Activities of mefloquine (MEF; R2 = 0.9990), quinine (QNN; R2 = 0.9892), chloroquine (CHL; R2 = 0.9891), and artesunate (ARS; R2 = 0.9970) against all P. falciparum strains tested (n = 20) by a modified WHO schizont maturation (morphology) assay.
Acknowledgments
This work was supported by the U.S. Department of Defense Global Emerging Infection Surveillance Program.
REFERENCES
- 1.Basco, L. K., F. Marquet, M. M. Makler, and J. Le Bras. 1995. Plasmodium falciparum and Plasmodium vivax: lactate dehydrogenase activity and its application for in vitro drug susceptibility assay. Exp. Parasitol. 80:260-271. [DOI] [PubMed] [Google Scholar]
- 2.Basco, L. K., and J. Le Bras. 1993. In vitro activity of artemisinin derivatives against African isolates and clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 49:301-307. [DOI] [PubMed] [Google Scholar]
- 3.Beadle, C., G. W. Long, W. R. Weiss, P. D. McElroy, S. M. Maret, A. J. Oloo, and S. L. Hoffman. 1994. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 343:564-568. [DOI] [PubMed] [Google Scholar]
- 4.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed]
- 5.Cerutti, N., A. Marin, E. R. Massa, and D. Savoia. 1999. Immunological investigation of malaria and new perspectives in paleopathological studies. Boll. Soc. Ital. Biol. Sper. 75:17-20. [PubMed] [Google Scholar]
- 6.Desakorn, V., K. Silamut, B. Angus, D. Sahassananda, K. Chotivanich, P. Suntharasamai, J. Simpson, and N. J. White. 1997. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans. R. Soc. Trop. Med. Hyg. 91:479-483. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins, R. E., C. J. Canfield, D. M. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druilhe, P., A. Moreno, C. Blanc, P. H. Brasseur, and P. Jacquier. 2001. A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 64:233-241. [DOI] [PubMed] [Google Scholar]
- 9.Howard, R. J., S. Uni, M. Aikawa, S. B. Aley, J. H. Leech, A. M. Lew, T. E. Wellems, J. Rener, and D. W. Taylor. 1986. Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 103:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 11.Lynn, A., S. Chandra, P. Malhotra, and V. S. Chauhan. 1999. Heme binding and polymerization by Plasmodium falciparum histidine rich protein II: influence of pH on activity and conformation. FEBS Lett. 459:267-271. [DOI] [PubMed] [Google Scholar]
- 12.Makler, M. T., J. M. Ries, J. A. Williams, J. E. Bancroft, R. C. Piper, B. L. Gibbins, and D. J. Hinrichs. 1993. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 48:739-741. [DOI] [PubMed] [Google Scholar]
- 13.Mayxay, M., S. Pukittayakamee, K. Chotivanich, S. Looareesuwan, and N. J. White. 2001. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:179-182. [DOI] [PubMed] [Google Scholar]
- 14.Moreno, A., P. Brasseur, N. Cuzin-Ouattara, C. Blanc, and P. Druilhe. 2001. Evaluation under field conditions of the colourimetric DELI-microtest for the assessment of Plasmodium falciparum drug resistance. Trans. R. Soc. Trop. Med. Hyg. 95:100-103. [DOI] [PubMed] [Google Scholar]
- 15.Noedl, H., W. H. Wernsdorfer, S. Krudsood, P. Wilairatana, P. Viriyavejakul, H. Kollaritsch, G. Wiedermann, and S. Looareesuwan. 2001. In vivo-in vitro model for the assessment of clinically relevant antimalarial cross-resistance. Am. J. Trop. Med. Hyg. 65:696-699. [DOI] [PubMed] [Google Scholar]
- 16.Pandey, A. V., H. Bisht, V. K. Babbarwal, J. Srivastava, K. C. Pandey, and V. S. Chauhan. 2001. Mechanism of malarial haem detoxification inhibition by chloroquine. Biochem. J. 355:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papalexis, V., M. Siomos, N. Campanale, X. Guo, G. Kocak, M. Foley, and L. Tilley. 2001. Histidine-rich protein 2 of the malaria parasite, Plasmodium falciparum, is involved in detoxification of the by-products of haemoglobin degradation. Mol. Biochem. Parasitol. 115:77-86. [DOI] [PubMed] [Google Scholar]
- 18.Reed, M. B., K. J. Saliba, S. R. Caruana, K. Kirk, and A. F. Cowman. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906-909. [DOI] [PubMed] [Google Scholar]
- 19.Riekmann, K. H., L. H. Sax, G. H. Camp, and J. E. Mrema. 1978. Drug sensitivity of Plasmodium falciparum. An in vitro micro technique. Lancet i:22-23. [DOI] [PubMed]
- 20.Rock, E. P., K. Marsh, A. J. Saul, T. E. Wellems, D. W. Taylor, W. L. Maloy, and R. J. Howard. 1987. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 95:209-227. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, D. J., I. Y. Gluzman, and D. E. Goldberg. 1996. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 271:219-222. [DOI] [PubMed] [Google Scholar]
- 22.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 190:792-794. [DOI] [PubMed] [Google Scholar]
- 23.Webster, H. K., E. F. Boudreau, K. Pavanand, K. Yongvanitchit, and L. W. Pang. 1985. Antimalarial drug susceptibility testing of Plasmodium falciparum in Thailand using a microdilution radioisotope method. Am. J. Trop. Med. Hyg. 34:228-235. [DOI] [PubMed] [Google Scholar]
- 24.Wernsdorfer, W. H. 1980. Field evaluation of drug resistance in malaria. In vitro micro-test. Acta Trop. 37:222-227. [PubMed] [Google Scholar]
- 25.Wongsrichanalai, C., T. Wimonwattrawatee, P. Sookto, A. Laoboonchai, D. G. Heppner, D. E. Kyle, and W. H. Wernsdorfer. 1999. In vitro sensitivity of Plasmodium falciparum to artesunate in Thailand. Bull. W. H. O. 77:392-398. [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. 1990. In vitro micro-test (Mark II) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, sulfadoxine/pyrimethamine and amodiaquine. WHO document MAP/87.2, rev. 1. World Health Organization, Geneva, Switzerland.