Abstract
Moxifloxacin (MXF) is a new 8-methoxyquinolone with potent activity against Mycobacterium tuberculosis and a half-life of 9 to 12 h in humans. Previous in vivo studies using daily doses of 100 mg/kg of body weight have demonstrated bactericidal activity comparable to that of isoniazid (INH) in a murine model of tuberculosis (TB). Recent pharmacokinetic data suggest that MXF may have been underadministered in these studies and that a 400-mg/kg dose in mice better approximates the area under the concentration-time curve obtained in humans after a 400-mg oral dose. Therefore, the bactericidal activity of MXF in doses up to 400 mg/kg given daily or weekly for 28 days was assessed in mice infected intravenously with 5 × 106 CFU of M. tuberculosis. INH was used as a positive control. After 3 days of daily therapy, the CFU counts from splenic homogenates for mice treated with MXF in doses of 100 to 400 mg/kg/day were lower than those from pretreatment controls. No significant differences in CFU counts were seen when mice receiving INH or MXF at 50 mg/kg/day were compared to pretreatment controls. After 28 days of therapy, dose-dependent reductions in CFU counts in splenic homogenates were seen for daily MXF therapy. The maximum bactericidal effect was seen with daily doses of 400 mg/kg, which resulted in a reduction in CFU counts of 1 log10 greater than that with INH treatment, although the difference was not statistically significant. CFU counts from lung homogenates after 28 days of therapy were significantly lower in all treatment groups than in untreated controls. The weekly administration of MXF in doses ranging from 50 to 400 mg/kg resulted in no significant bactericidal activity. Mice receiving daily MXF doses of 200 and 400 mg/kg/day failed to gain weight and appeared ill after 28 days of therapy, findings suggestive of drug toxicity. In conclusion, MXF has dose-dependent bactericidal activity against M. tuberculosis in the mouse when given in doses up to 400 mg/kg, where its pharmacokinetic profile better approximates that of standard human dosages. Combination regimens which take advantage of the enhanced pharmacodynamic profile of MXF at these doses have the potential to shorten the course of antituberculous therapy or allow more intermittent (i.e., once-weekly) therapy and should be evaluated in the mouse model of TB.
Tuberculosis (TB) continues to be a leading infectious cause of death in the world. Despite the efforts made to apply directly observed therapy (DOT) worldwide, the control of TB remains elusive in areas where poor public health infrastructure and limited access to antituberculous agents make DOT difficult and costly to administer. Inadequate treatment may lead to multidrug-resistant TB, further compounding the situation. A significant need has arisen for new antimycobacterial agents that, first, make DOT more convenient or less expensive to administer and, second, improve the treatment of multidrug-resistant TB (8, 9). The former goal requires the development of agents with potent bactericidal and/or sterilizing activity which allows shortening of the treatment duration or agents with improved pharmacokinetics which allow lengthening of the dosing interval. The latter goal requires new agents with mechanisms of action which differ from those of the present first-line agents. A potent new fluoroquinolone with a favorable pharmacokinetic profile may help achieve both of these goals.
Moxifloxacin (MXF) is a new 8-methoxyquinolone with promising antimycobacterial activity whose role in the chemotherapy of TB has yet to be defined. The MIC of MXF is 0.5 μg/ml for Mycobacterium tuberculosis, suggesting potent in vitro activity (4, 16). In vivo, MXF has demonstrated dose-dependent bactericidal activity against M. tuberculosis that is greater than that of other potent fluoroquinolones such as sparfloxacin and comparable to that of isoniazid (INH) when given in daily doses of 50 to 100 mg/kg of body weight in a mouse model of TB (4, 7).
MXF has a favorable pharmacokinetic profile in humans, with 86 to 92% oral bioavailability, a maximum concentration in serum of approximately 4 μg/ml, a half-life in serum of 9 to 12 h, and good penetration into the intracellular space (1, 6, 11-13, 15). Given its potent antimycobacterial activity, the pharmacodynamic profile may enable once-weekly use of MXF in combination with other agents with similar half-lives during the intensive and/or continuation phases of TB treatment. In fact, the addition of once-weekly MXF (100 mg/kg) was recently shown to significantly enhance the sterilizing activity of once-weekly INH and rifapentine (RFP) and to prevent the selection of INH- and RFP-resistant mutants (5). If significant bactericidal activity could be demonstrated with intermittent dosing, MXF or another fluoroquinolone with similar antituberculous activity may be able to replace or to supplement INH in both phases of treatment, since the latter agent is considered to have only weak sterilizing activity (2).
At the time previous studies with MXF were designed, the 50- and 100-mg/kg doses in mice were felt to be equivalent to 200- and 400-mg doses, respectively, in human adults. Recent pharmacokinetic data now suggest that MXF may have been underdosed in these experiments. A 400-mg (5.7 mg/kg for a 70-kg subject) oral dose in humans results in an area under the concentration-time curve (AUC24) of 30 to 40 μg · h/ml (13, 15). A 100-mg/kg oral dose of MXF in mice, on the other hand, results in an AUC24 of only 9.5 μg·h/ml (7), whereas a 1,000-mg/kg oral dose produces an AUC of 80.5 μg · h/ml (H. M. Siefert, unpublished data). Taking into account this degree of linearity in the pharmacokinetics of MXF in the mouse, a dose of 400 mg/kg in the mouse should more closely approximate the AUC24 of 30 to 40 μg · h/ml produced by the 400-mg dose approved for human use in community-acquired pneumonia and acute exacerbations of chronic bronchitis. Since fluoroquinolones exhibit concentration-dependent killing (10), a higher value for the AUC achieved by increasing the dose from 100 to 400 mg/kg would be expected to confer even greater bactericidal activity in the mouse. It is therefore critical that the optimal MXF dose be determined before further experiments involving combination therapy are carried out.
The present experiment was designed to determine whether the bactericidal activity of MXF in the mouse model was further enhanced by an increase in the daily dose up to 400 mg/kg. An additional aim of the study was to determine if once-weekly doses up to 400 mg/kg have significant bactericidal activity against M. tuberculosis and justify using increased MXF doses when evaluating once-weekly combination regimens.
MATERIALS AND METHODS
Antimicrobial agents.
MXF was kindly provided by Bayer (West Haven, Conn.). INH was purchased from Sigma (St. Louis, Mo.).
M. tuberculosis strain.
The CDC1551 strain of M. tuberculosis was cultivated at 37°C in roller bottles in 7H9 broth (Difco Laboratories, Detroit, Mich.) supplemented with 10% oleic acid-albumin-dextrose-catalase, 0.05% Tween 80, and 0.1% glycerol. For animal infection, liquid cultures were declumped by brief bath sonication and settling and then diluted in 7H9-albumin-dextrose-catalase broth. Estimated titers measured by hemacytometer counts were later confirmed by plating dilutions for colony counts. The latter were used as the definitive inoculum titers.
Animal model.
Inbred, female BALB/c mice (4 to 6 weeks of age; average weight, 17 g) were purchased from Charles River Laboratories (Wilmington, Mass.); housed in a pathogen-free, biosafety level 3 environment; and allowed to acclimate to their new environment for at least 4 days prior to infection. Food and water were provided ad libitum. On day −1, infection was produced by intravenous tail vein injection of 0.1 ml from a suspension estimated to contain 107 organisms. Following infection, mice were randomly divided into groups (10 mice per group). The following day (day 0), 20 mice were sacrificed to establish baseline CFU counts in the spleen. Treatment was also initiated on day 0. In the daily treatment groups, INH (25 mg/kg) or MXF (50, 100, 200, or 400 mg/kg) was given by esophageal cannula (gavage) six times weekly in a volume of 0.2 ml. In the weekly treatment groups, MXF (50, 100, 200, or 400 mg/kg) was given in similar fashion once weekly. On day 1, five mice each from the INH and weekly MXF groups were sacrificed to assess the bactericidal effect of a single pulse dose. Spleen CFU counts were determined by homogenizing each organ aseptically in 5 ml of phosphate-buffered saline with 0.05% Tween 80 by using the Stomacher 80 lab system (Seward, London, United Kingdom). The homogenates were plated in serial dilutions in duplicate on Middlebrook 7H10-oleic acid-albumin-dextrose-catalase-0.5% glycerol agar plates (Difco). CFU counts were recorded after incubation at 37°C in a 5% CO2 incubator for 4 weeks. On day 3, five mice each from the INH and daily MXF groups were sacrificed to determine the bactericidal activity of three daily doses. Spleen CFU counts were performed as described above. On day 28, the remaining mice were weighed and sacrificed. Spleen and lung CFU counts were performed as described above, except that lung CFU counts were performed on single lungs. The companion lung was retained for histopathology.
Histopathology.
Single lung specimens were fixed with formaldehyde (10%), embedded in paraffin, and sectioned for staining. Hematoxylin and eosin and acid-fast (Ziehl-Neelsen carbol-fuchsin method) stains were performed on the sections.
Statistical analysis.
Multiple comparisons among pairs of group means were performed by Bonferroni's method (3) for all data presented. Because six groups were compared, differences were considered significant at the level of 0.0033 (i.e., 0.05/[(6 × 5)/2]).
RESULTS
Titer of infectious inoculum.
The titer of the broth culture used for infection was determined to be 5 × 107 CFU/ml. Each mouse therefore received approximately 5 × 106 organisms with the intravenous injection of 0.1 ml.
Survival rate.
No mortality related to TB infection or adverse drug reaction occurred during the course of the study. Two mice from the INH-treated group died within the first week from asphyxiation due to inadvertent instillation of drug solution into the trachea during gavage.
Enumeration of CFU in the spleen.
Limited multiplication of bacilli occurred in untreated mice over days 0 to 3. One day after initiation of treatment (day 1), the mean CFU counts in spleen homogenates of mice receiving a single pulse dose of INH or MXF were no different from those for pretreatment and untreated control mice (Table 1). After 3 days of daily therapy (day 3), the CFU counts in splenic homogenates for mice treated with MXF in doses of 100 to 400 mg/kg were significantly lower than those in pretreatment controls, although there were no significant differences among MXF doses of 100, 200, or 400 mg/kg. The CFU counts in mice receiving INH or MXF at 50 mg/kg were not significantly different from those in pretreatment controls (Table 1). On completion of therapy (day 28), the CFU counts for mice treated with a daily regimen of INH or with MXF at doses of 100 to 400 mg/kg/day were significantly lower than those for pretreatment controls (Table 1). Moreover, daily MXF regimens reduced the CFU counts in a strongly and statistically significant dose-dependent manner, with the maximum effect seen in the 400-mg/kg group. For mice treated with this dose, the CFU counts after 28 days were 1 log10 lower than those for mice in the INH group, although the difference did not achieve statistical significance. CFU counts for mice treated with weekly MXF monotherapy were higher than those for pretreatment controls and similar to those for untreated controls at 28 days. No dose-dependent effect could be demonstrated. Mean log CFU counts were 5.75 (±0.19), 5.38 (±0.17), 5.47 (±0.17), and 5.20 (±0.14) for mice treated with four weekly doses of 50, 100, 200, and 400 mg/kg, respectively, versus 3.91 (±0.55) for pretreatment controls and 5.57 (±0.16) for untreated controls after 28 days.
TABLE 1.
CFU counts in the spleens of mice infected with M. tuberculosis and treated daily with increasing doses of MXF
| Drug regimen (mg/kg of body wt) | Mean CFU count (log10) ± SD at the following day of therapya:
|
|||
|---|---|---|---|---|
| 0 | 1 | 3 | 28 | |
| Untreated control | 3.91 ± 0.55 | 3.76 ± 0.16 | 4.30 ± 0.32 | 5.57 ± 0.16 |
| INH (25) | 3.66 ± 0.10 | 3.46 ± 0.27 | 1.37 ± 0.96** | |
| MXF (50) | 3.92 ± 0.25 | 3.22 ± 0.71 | 4.5 ± 0.22 | |
| MXF (100) | 3.42 ± 0.22 | 2.94 ± 0.08* | 2.52 ± 0.19 | |
| MXF (200) | 3.67 ± 0.016 | 3.18 ± 0.30* | 2.05 ± 0.25 | |
| MXF (400) | 3.61 ± 0.07 | 2.94 ± 0.27* | 0.37 ± 0.83** | |
*, P < 0.05 compared with pretreatment value. **, P > 0.05.
Enumeration of CFU in the lung.
CFU counts from lung homogenates obtained on day 28 were significantly lower for mice receiving daily INH or any dose of MXF than for untreated controls (data not shown). A dose-dependent effect was again seen for daily MXF regimens, although the effect did not achieve statistical significance.
Lung histopathology.
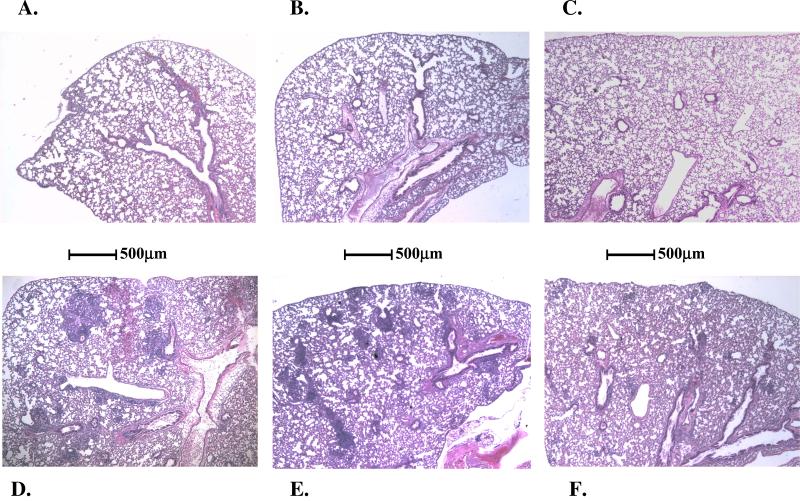
As expected, on day 28, all lungs from untreated mice were noted on gross inspection to have multiple 1- to 2-mm nodules visible on the lung surface. On histologic examination, widespread evidence of lesions and numerous acid-fast bacilli were observed (Fig. 1). In contrast, the lungs of mice treated with daily doses of MXF (even at the lowest dose of 50 mg/kg) had no evidence of lesion formation and were similar to those in normal uninfected mice. Mice treated with weekly MXF regimens demonstrated intermediate histopathologic changes, with lesions that were smaller and fewer in number than those in untreated mice. The size and number of lesions decreased with increasing weekly doses of MXF.
FIG. 1.
Photomicrographs of mouse lungs removed on day 28 (hematoxylin and eosin stain; magnification, ×36). (A) Uninfected and untreated; (B) infected and treated daily with 25 mg of INH/kg; (C) infected and treated daily with 400 mg of MXF/kg; (D) infected and untreated; (E) infected and treated weekly with 200 mg of MXF/kg; (F) infected and treated weekly with 400 mg of MXF/kg.
Evaluation of drug toxicity.
Mice receiving daily MXF doses of 200 and 400 mg/kg failed to gain weight over the 4 weeks of the study. This finding was associated with diminished activity and unkempt fur. Food intake over this period was not assessed. The failure to gain weight was likely not due to M. tuberculosis infection, since infected control mice and mice treated with INH or lower doses of MXF gained weight during the same period. At necropsy, no gross anatomical changes were noted in internal structures for mice treated with higher daily doses of MXF compared to INH-treated mice. Examination of histopathologic specimens from liver, heart, and kidney also failed to reveal microscopic differences between the groups.
DISCUSSION
In the present study, several important observations were made. First, MXF demonstrated significant bactericidal activity in the mouse model. In fact, MXF doses between 100 and 400 mg/kg/day produced significant reductions in CFU counts from spleen homogenates after only 3 days of therapy whereas INH at 25 mg/kg and MXF at 50 mg/kg did not. Although a dose-dependent effect on bactericidal activity after 3 days of therapy with increasing doses of MXF above 100 mg/kg could not be demonstrated, it is possible that such a dose-dependent effect was obscured by the small sample sizes. That MXF at the increased doses used in this study had a 3-day bactericidal activity exceeding that of INH is significant, as INH is generally considered to have the greatest early bactericidal activity of the present first-line antituberculous agents (2).
MXF also demonstrated significant bactericidal activity, which was strongly dose dependent, when CFU counts were performed after 28 days of therapy. The CFU counts in spleen homogenates from mice receiving 400 mg/kg were 4 log10 and 2 log10 lower than those from mice receiving 50 and 100 mg/kg, respectively. If, as suggested by recent pharmacokinetic studies (7, 11, 13-15), the 400-mg/kg dose in mice more accurately represents human pharmacokinetics after a 400-mg dose of MXF, this additional bactericidal activity may hold promise for shortening the course of therapy for tuberculosis.
While regimens employing daily doses of MXF exhibited exceptional bactericidal activity against M. tuberculosis in our mouse model, regimens using weekly MXF doses up to 400 mg/kg failed to demonstrate significant bactericidal activity. Such a finding is in apparent contrast with that of Lounis et al., who recently demonstrated the additive sterilizing effect of once-weekly dosing of MXF given in combination with once-weekly INH and RFP (5). However, when considering drug efficacy during the continuation phase of therapy, the sterilizing activity is more important than the bactericidal activity, and the sterilizing activity of MXF in the study of Lounis et al. might have been potentiated by its combination with other active agents. If that was the case, increasing the maximum concentration in serum with larger doses of MXF, a drug which exhibits concentration-dependent killing (10), may improve the antimycobacterial activity without increasing the toxicity. This is not pure speculation, because weekly monotherapy with higher doses of MXF in this study was not completely without antimycobacterial activity, as the size and number of lesions in the lungs of mice receiving 200 to 400 mg weekly were reduced compared to those for untreated mice. If the sterilizing activity of the once-weekly INH-RFP-MXF combination could be augmented with a higher dose of MXF (e.g., 400 mg/kg) and/or RFP (e.g., 15 mg/kg), the level of efficacy of this once-weekly regimen could reach equivalency with that of the standard regimen of daily INH, rifampin, and pyrazinamide for 2 months, followed by daily INH and rifampin for 4 months.
Lastly, MXF appeared to demonstrate an untoward side effect profile in mice that was also dose dependent. The 200- and 400-mg/kg daily doses of MXF were associated with failure to gain weight, decreased activity, and unkempt fur after 4 weeks of therapy. Given the evidence of effective and dose-dependent antituberculosis activity at these doses, the findings suggest drug toxicity rather than failure to control the infection. These findings are also similar to those from repeated-dose toxicity studies in rats and monkeys which described dose-related reductions in weight gain associated with abnormally elevated serum aminotransferase levels after treatment with MXF at 100 and 500 mg/kg for courses ranging from 4 to 13 weeks of duration (14). While no histopathologic evidence of organ-specific damage was demonstrated in these intermediate-term trials, long-term trials with 6-month administration of similar doses did produce histopathologic evidence of hepatocellular damage in rats in a dose-dependent fashion (14). Repeated-dose toxicity studies have not been performed with mice as far as we are aware. However, preclinical studies have shown MXF to have an oral 50% lethal dose (LD50) of 435 to 758 mg/kg for mice, while the corresponding values were 1,320 mg/kg for rats and 1,500 mg/kg for cynomolgus monkeys (14). Despite the substantial difference in the oral LD50 between mice and rats, the intravenous LD50 was 105 to 130 mg/kg for mice and 112 to 146 mg/kg for rats, suggesting that the lethal doses were related to the peak blood levels (14). Assuming linear pharmacokinetics, the peak serum level expected for a 400-mg/kg oral dose in the mouse should be as high as 30 μg/ml (7), or up to 10 times the value obtained in humans after a 400-mg dose (5.7 mg/kg in a 70-kg human) administered either orally or in an infusion over 1 h (12, 15). The greater clearance of MXF in the mouse necessitates higher doses to achieve an AUC similar to that achieved in humans but results in substantially higher peak serum concentrations. If the toxicity of MXF is related to peak serum levels, the toxicity is very likely to be specific to the mouse and should not be considered predictive of toxicity in humans.
In conclusion, the present study demonstrates that increasing doses of MXF up to 200 to 400 mg/kg/day have enhanced bactericidal activity against M. tuberculosis that is at least comparable and perhaps superior to that of INH in a mouse model of active TB infection. Although once-weekly doses up to 400 mg/kg had no demonstrable bactericidal effect, studies using MXF in combination with other agents with similar pharmacokinetics are warranted to better evaluate its potential in once-weekly therapeutic regimens. In addition, the pharmacokinetics and the toxicity of high doses of MXF in uninfected mice deserve further characterization.
Acknowledgments
This work was supported by a supplement to NIH grant AI43846.
REFERENCES
- 1.Ballow, C., J. Lettieri, V. Agarwal, P. Liu, H. Stass, and J. T. Sullivan. 1999. Absolute bioavailability of moxifloxacin. Clin. Ther. 21:513-522. [DOI] [PubMed] [Google Scholar]
- 2.Brindle, R., J. Odhiambo, and D. Mitchison. 2001. Serial counts of Mycobacterium tuberculosis in sputum as surrogate markers of the sterilizing activity of rifampicin and pyrazinamide in treating pulmonary tuberculosis. BMC Pulm. Med. 1:2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfrey, K. 1985. Statistics in practice. Comparing the means of several groups. N. Engl. J. Med. 313:1450-1456. [DOI] [PubMed] [Google Scholar]
- 4.Ji, B., N. Lounis, C. Maslo, C. Truffot-Pernot, P. Bonnafous, and J. Grosset. 1998. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lounis, N., A. Bentoucha, C. Truffot-Pernot, B. Ji, R. J. O'Brien, A. Vernon, G. Roscigno, and J. Grosset. 2001. Effectiveness of once-weekly rifapentine and moxifloxacin regimens against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 45:3482-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubasch, A., I. Keller, K. Borner, P. Koeppe, and H. Lode. 2000. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob. Agents Chemother. 44:2600-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazaki, E., M. Miyazaki, J. M. Chen, R. E. Chaisson, and W. R. Bishai. 1999. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 43:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien, R. J., and P. P. Nunn. 2001. The need for new drugs against tuberculosis. Obstacles, opportunities, and next steps. Am. J. Respir. Crit. Care Med. 163:1055-1058. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien, R. J., and A. A. Vernon. 1998. New tuberculosis drug development. How can we do better? Am. J. Respir. Crit. Care Med. 157:1705-1707. [DOI] [PubMed] [Google Scholar]
- 10.Peloquin, C. A. 1997. Using therapeutic drug monitoring to dose the antimycobacterial drugs. Clin. Chest Med. 18:79-87. [DOI] [PubMed] [Google Scholar]
- 11.Stass, H., A. Dalhoff, D. Kubitza, and U. Schuhly. 1998. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 42:2060-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stass, H., and D. Kubitza. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 43(Suppl. B):83-90. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan, J. T., M. Woodruff, J. Lettieri, V. Agarwal, G. J. Krol, P. T. Leese, S. Watson, and A. H. Heller. 1999. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 43:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Keutz, E., and G. Schluter. 1999. Preclinical safety evaluation of moxifloxacin, a novel fluoroquinolone. J. Antimicrob. Chemother. 43(Suppl. B):91-100. [DOI] [PubMed] [Google Scholar]
- 15.Wise, R., J. M. Andrews, G. Marshall, and G. Hartman. 1999. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob. Agents Chemother. 43:1508-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodcock, J. M., J. M. Andrews, F. J. Boswell, N. P. Brenwald, and R. Wise. 1997. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob. Agents Chemother. 41:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]