Abstract
The emergence of multidrug-resistant microbes has serious implications for managing infection and sepsis and has stimulated efforts to develop alternative treatments, such as antimicrobial peptides. The objective of this study was to test a designer peptide, novispirin G10, against multidrug-resistant microorganisms. By two-stage radial diffusion assays, its activity against such organisms compared favorably with that of standard antibiotics and other antimicrobial peptides. It killed bacteria very rapidly, was nonhemolytic, and was relatively noncytotoxic. The peptide induced an immediate, massive efflux of potassium from Pseudomonas aeruginosa, suggesting that it altered the permeability of its inner membrane. The presence of human serum reduced but did not eliminate its activity. We tested the in vivo activity of novispirin G10 in rats with an infected, partial-thickness burn that covered 20% of their total body surface area. The burned area was seeded with 106 CFU of a Silvadene-resistant P. aeruginosa strain, and 24 h later a single treatment with 0, 1, 3, or 6 mg of synthetic novispirin G10 (n = 16 at each concentration) per kg was given intradermally. Significant bacterial killing (P < 0.0001) was evident within 4 h in each peptide group compared to controls receiving vehicle. Antimicrobial peptides such as novispirin G10 may provide a useful alternative or adjunct to standard antibiotic agents in treating burns or other wound infections.
Despite contemporary practices of wound management and supportive care, wound infections and sepsis remain important causes of morbidity and mortality in surgical patients (2, 25), especially when caused by multidrug-resistant bacteria (4, 13, 15). Even though the emergence of multidrug-resistant bacteria is promoted by antibiotic use, antibiotics remain a mainstay of treatment for wound infections, typically in association with adequate debridement and drainage procedures. For wound infections, the current standard of care involves using systemic antibiotics or topical antimicrobial agents, such as silver sulfadiazine, mafenide acetate, and gentamicin sulfate, all initially introduced in the 1960s (18, 36, 50). These products have various limitations, including a limited ability to penetrate partial- and full-thickness burns, limited efficacy against both gram-positive and gram-negative bacteria, and potential toxicity to host cells. Recent reports that mortality remains significantly higher in patients who receive inadequate antimicrobial therapy (29) support the need for novel strategies to prevent and treat wound infections (4, 13, 14, 27).
Antimicrobial peptides have been isolated from diverse organisms, including plants, insects, bacteria, and vertebrates (34), and constitute an important component of the mammalian innate immune response. Some of these peptides are induced at epithelial surfaces in response to invading organisms (17, 20, 28). Many antimicrobial peptides kill microorganisms by causing membrane permeabilization, although not necessarily as their sole mode of action (19). Some antimicrobial peptides also direct chemotaxis, promote wound healing, and contribute to adaptive immunity by mobilizing memory T cells and immature dendritic cells (17, 56).
Several classes of mammalian peptide antibiotics have been ascribed pivotal roles in innate immunity (9). Among these are various cysteine-rich peptides such as defensins and protegrins (23, 33, 37) and the more structurally diverse cathelicidins (53, 57, 58). Produced as precursors, they require proteolytic processing to liberate the mature functional antimicrobial peptide. Cathelicidins contain a conserved N-terminal cathelin domain and a structurally diverse C-terminal domain that carries the peptide's antimicrobial activity. Rabbit CAP18 was the first cathelicidin precursor described, and its mature peptide has broad-spectrum antimicrobial activity (32). Cathelicidins have since been identified in many other species, including hCAP18/LL37 in humans (1), protegrins in swine (31), CRAMP in mice (21, 45), and SMAP29 in sheep (6, 46).
Possible limitations to the therapeutic use of naturally occurring antimicrobial peptides in humans include their cytotoxic potential and factors related to the cost of large-scale production. Efforts to overcome these potential obstacles led to the development of a novel α-helical antimicrobial peptide, ovispirin-1, that was modeled loosely on the N-terminal 18 residues of the sheep cathelicidin SMAP29 (12, 43, 55). While it was nearly as effective as the parent molecule of 29 residues against gram-negative and gram-positive organisms (11, 42), we noted that ovispirin-1 exhibited considerable in vitro toxicity towards several human epithelial cell lines.
Minor structural modifications of ovispirin-1 led to the novispirin peptides described in this report. The primary objectives of this study were (i) to ascertain the in vitro antimicrobial activity of novispirin G10 against multidrug-resistant microorganisms, (ii) to compare its cytotoxicity with that of ovispirin-1, and (iii) to examine its in vivo effectiveness in an infected burn wound model.
MATERIALS AND METHODS
Peptide.
Novispirin G10 and the other antimicrobial peptides used in this study were prepared by solid-phase synthesis and purified by reverse-phase high-pressure liquid chromatography (RP-HPLC). For comparison, we used ovispirin-1, both as a model antimicrobial peptide and as the starting point for novispirin design (12, 55). We also used protegrin PG-1, a β-sheet peptide that resembles IB-367 (iseganan), which is currently in phase III human clinical trials (8), and PGG, a model antimicrobial peptide whose primary sequence represents a consensus of the N-terminal domain of many other α-helical peptides (52). The primary sequences of all of these peptides are shown in Table 1.
TABLE 1.
Primary sequence of the antimicrobial peptides used in this studya
| Peptide | Residue at position:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Novispirins | ||||||||||||||||||
| G10 | K | N | L | R | R | I | I | R | K | G | I | H | I | I | K | K | Y | G |
| T10 | K | N | L | R | R | I | I | R | K | T | I | H | I | I | K | K | Y | G |
| G7 | K | N | L | R | R | I | G | R | K | I | I | H | I | I | K | K | Y | G |
| T7 | K | N | L | R | R | I | T | R | K | I | I | H | I | I | K | K | Y | G |
| Ovispirin | K | N | L | R | R | I | I | R | K | I | I | H | I | I | K | K | Y | G |
| PGG | G | L | L | R | R | L | R | K | K | I | G | E | I | F | K | K | Y | G |
| Protegrin-1 | R | G | G | R | L | C | Y | C | R | R | R | F | C | V | C | V | G | R∗ |
Bold letters indicate the residues that distinguish these novispirins from ovispirin. The asterisk denotes C-terminal amidation.
The peptides (≥98% pure) were dissolved in 0.01% acetic acid and used for all in vitro and in vivo studies. Potential endotoxin contamination was monitored with the chromogenic Limulus amoebocyte lysate assay (BioWhittaker, Walkersville, Md.) using Escherichia coli endotoxin (supplied with the kit) as the standard. Endotoxin levels for the peptides were negligible.
Bacteria.
Bacterial isolates from human burn patients were kindly provided by Carl Pierson, Department of Pathology, University of Michigan. All burn strains were analyzed with API test strips (BioMerieux, Hazelwood, Mo.) to confirm identity, and aliquots were stored frozen in 50% skim milk at −80°C. Sensitivities to systemic and topical antibiotic agents (i.e., silver sulfadiazine, mafenide acetate, and 0.5% silver nitrate) were performed by the Trauma-Burn Clinical Laboratory of the University of Michigan. Bacteria were grown overnight in Trypticase soy broth (Becton Dickinson, Franklin Lakes, N.J.) at 275 rpm and 37°C. An aliquot of the resulting stationary-phase cultures was then transferred to 20 ml of Trypticase soy broth and incubated at 37°C for 2.5 h to reach log phase. This subculture was transferred to a 50-ml conical polystyrene tube and centrifuged for 10 min at 4°C at 880 × g. The bacterial pellet was washed once with chilled phosphate-buffered saline, pH 7.4, and resuspended in 5 ml of the same cold buffer. One milliliter was removed to measure its optical density at 620 nm (OD620). The bacterial concentration (CFU per milliliter) was calculated from the formula OD620 × 2.5 × 108.
Bacterial growth inhibition assay.
To monitor growth inhibition in vitro, a radial diffusion assay was performed as previously described (48). Briefly, the underlay agar consisted of 1% agarose (A-6013; Sigma Chemical, St. Louis, Mo.) and 0.3 mg Trypticase soy broth powder in 10 mM sodium phosphate with 100 mM NaCl (normal-salt medium) or 175 to 200 mM NaCl (high-salt medium), pH 7.4. Retention of activity against P. aeruginosa in high-salt media is important because skin surfaces may have high salt concentrations locally.
To study the impact of divalent cations (1 mM Mg2+ or 1 mM Ca2+) on the bactericidal activity of novispirin G10, radial diffusion assays were performed as previously described except that the underlay gels were buffered with either 10 mM HEPES or 5 mM HEPES plus 5 mM citrate. The overlay agar consisted of 6% (wt/vol) Trypticase soy broth and 1% agarose in phosphate-buffered saline for all assays. Bacteria (approximately 5 × 106 CFU) were mixed with 10 ml of underlay gel (43°C) and immediately poured into square petri dishes (9 by 9 cm). A series of 3-mm wells were punched after the agarose solidified. After appropriate serial dilutions were done, 5 μl of novispirin G10, protegrin-1, vancomycin (Abbott Labs, Chicago, Ill.), gentamicin, ciprofloxacin, and fluconazole (Sigma-Aldrich, St. Louis, Mo.) were added to the designated wells. Plates were incubated at 37°C for 3 h.
The layer containing bacteria was covered with a 10-ml overlay of the nutrient-rich agar. After 18 h of incubation at 37°C, the plates were stained with 0.001% Coomassie blue for 10 h. The clear zones (bacterial growth inhibition) around the punched wells indicated antibacterial activity. The diameters of the clear zones were converted into units by subtracting the well diameter and multiplying the difference by 10. Results were plotted using a semilog scale and correlation coefficients and x intercepts obtained from linear regression analysis. The minimum effective concentration (MEC) corresponded to the x intercept value. All assays were performed in triplicate and repeated at least once.
Cytotoxic and hemolytic activity.
A tetrazolium reduction assay (Boehringer-Mannheim, Indianapolis, Ind.) was used to study cytotoxicity. ME-180 human cervical epithelial cells (ATCC HTB-33) and A549 human lung epithelial cells (ATCC CCL-185) were grown to confluency in RPMI medium (ME-180) or Ham's F12K medium with 2 mM l-glutamine (A549), both containing 10% fetal bovine serum and 50 μg/ml gentamicin. The cells were harvested with trypsin-EDTA and washed with the respective medium. After their concentration and viability (trypan blue exclusion) were determined, they were diluted to 5 × 104 cells/ml. Aliquots (100 μl) were dispersed into 96-well tissue plates (Corning Life Sciences, Acton, Mass.) and incubated for 5 h at 37°C in room air with 5% CO2. Then serially diluted peptides were added, and after 20 h of additional incubation, 10 μl of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-dophenyl tetrazolium bromide) solution was added. Four hours later, 100 μl of solubilization buffer was added and left overnight to dissolve the MTT-formozon crystals formed. The following day, MTT reduction was determined by optical density measurements at 600 and 650 nm, using a Spectramax 250 spectrophotometer (Molecular Devices, Sunnyvale, Calif.). Hemolytic activity was tested by incubating various concentrations of peptide with a suspension (2.5%, vol/vol) of washed human, murine, sheep or bovine red cells in phosphate-buffered saline. After 30 min at 37°C, the tubes were centrifuged, and the optical density was measured at 540 nm. The percentage of total hemoglobin released to the supernatant was calculated in the conventional manner.
Kinetics of antimicrobial activity.
The Silvadene-resistant P. aeruginosa strain 2 used in our in vivo study was exposed to various concentrations of novispirin G10 in medium containing 10 mM sodium phosphate buffer (pH 7.4), 100 mM NaCl, and 0.3 mg of Trypticase soy broth powder/ml. Quantitative bacterial counts were performed after various time points to monitor bactericidal activity. Kinetic experiments were also performed with P. aeruginosa strain 2 (which was used in the in vivo experiments) and with Escherichia coli ML-35p, a reporter strain that had been constructed by transforming E. coli ML-35 (ATCC 43827) with pBP-322 to introduce a plasmid-encoded periplasmic β-galactosidase, as previously described (33). Measurements of potassium efflux were done with an ion-selective electrode, as described elsewhere (39).
Animal model.
The research protocol described below was approved by the University of Michigan Animal Review Board for the Care and Use of Animals. This study was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animal use. It also adhered to the principles in the “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health.
Adult male pathogen-free rats weighing 175 to 200 g (Harlan Sprague-Dawley, Indianapolis, Ind.) were used in all experiments. Rats were housed in standard cages at the University Laboratory Animals Facility and allowed to acclimate to their surroundings for 7 days prior to the experiment. They were kept on a 12-h light cycle and provided ad libitum with rat chow and water throughout the study. Ketamine hydrochloride (100 mg/kg; Fort Dodge Laboratories, Fort Dodge, Iowa) and xylazine (13 mg/kg; Bayer Corporation, Shawnee Mission, Kans.) were administered intraperitoneally for anesthesia. During the study, all animals were singly housed and all received 0.3 mg/kg buprenorphine (Buprenex; Reckitt & Coleman Pharmaceuticals Inc., Richmond, Va.) intraperitoneally twice daily for postburn pain control.
The skin over the lumbosacral, dorsal flank, and back regions was thoroughly clipped using a 35-W model 5-55E electrical clipper (Oyster-Golden A-S, Head no. 80, blade size 40). It was then treated with a depilatory cream (Nair; Carter Wallace, New York, N.Y.), resulting in thorough and uniform removal of fur. Twenty-four hours later, the rats were anesthetized and placed in an insulated, custom-made mold, which exposed two areas, each 25 cm2, on the right and left lateral flanks. Together, these areas approximated 20% of the total body surface area. The burn surface area was determined for each rat using Meeh's formula: body surface area (cm2) = 9.46 × [animal weight (g)]2/3 (24). We induced a scald injury as previously described (49, 54). The protective animal/mold assembly was immersed in 60°C water (shaker bath, model 2568; Forma Scientific, Marietta, Ohio) for 20 s to ensure that only the area of skin protruding through the mold contacted the scalding water.
Immediately after scalding, the animals were removed from the mold, quickly dried, and resuscitated with 0.9% saline (0.1 ml/g of body weight) subcutaneously. To establish the skin infection, 106 CFU of P. aeruginosa 2 (Table 2) were applied topically on sterile gauze to both areas. Occlusive dressings (Tegaderm; 3M Health Care, St. Paul, Minn.) were applied to prevent cross contamination and to ensure optimal growth conditions for the applied bacteria. After 48 h, the rats were randomized into three groups. Group 1 received 1, 3, or 6 mg/kg of synthetic novispirin G10 (n = 16 at each concentration). For rats weighing 200 g, these doses corresponded to 0.2, 0.6, and 1.2 mg of peptide, respectively. Group 2 rats were carrier controls that received 0.01% acetic acid (n = 16). Group 3 consisted of noninjected burned controls (n = 8).
TABLE 2.
Summary of the systemic and topical sensitivities of bacterial isolates obtained from infected human burn woundsa
| Organism | Strain no. | Susceptibility
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic Agents
|
Topical agents
|
|||||||||||
| AMP | CEF | DOX | AMK | AZT | CFT | IMI | TOB | SSD | SMA | AgN | ||
| Staphylococcus aureus | 1 | R | R | S | S | S | S | |||||
| Staphylococcus epidermidis | 1 | R | R | S | S | R | S | S | R | S | ||
| Enterococcus faecalis | 1 | S | S | S | R | R | ||||||
| Pseudomonas aeruginosa | 1 | R | R | S | S | S | S | S | R | R | S | |
| 2 | R | R | R | R | R | R | R | R | ||||
| 3 | S | S | S | S | S | S | S | S | ||||
| Acinetobacter baumanii | 1 | R | R | S | ||||||||
The breakpoints used for the MICs for the clinical isolates were determined by the microbiology department of the University of Michigan, referring to the NCCLS standards. Inhibition zones of less than 5 mm in the radial diffusion assay were considered resistant for silver sulphadiazine, mafenide acetate, and silver nitrate. S, sensitive; R, resistant. AMP, ampicillin; CEF, cefazolin; DOX, doxycycline; AMK, amikacin; AZT, aztreonam; CFT, ceftazidime; IMI, imipenem; TOB, tobramycin; SSD, silver sulphadiazine; MA, mafenide acetate; AgN, 0.5% silver nitrate. Note that P. aeruginosa 2 was found to be resistant to all antibiotics tested (not all tested antibiotics are shown).
Treatment was applied by one intradermal injection of 500 μl with a 30-gauge needle to both marked center portions (4 cm2 each) of the burned and infected area. Following the treatment, burned wounds were immediately covered by occlusive dressings, as mentioned above, to ensure retention of the drug at the site. After 4 h, treated areas of the infected wound tissues were harvested aseptically, weighed, homogenized, serially diluted, and plated in triplicate on Trypticase soy agar with 5% sheep blood and Pseudomonas isolation agar (both from Becton Dickinson). Bacterial plates were then incubated for 18 h, and the CFU were counted in a blinded fashion. Results are expressed as CFU per gram of infected skin tissue. In addition, 5-μm-thick paraffin tissue sections of skin were stained (hematoxylin/eosin) to evaluate the morphology of the burn wound after treatment with novispirin G10 or vehicle control or the no-treatment control.
Peripheral blood analysis.
A total of 20 μl of EDTA-anticoagulated blood was obtained by clipping the distal aspect of the tail before injury and then again before sacrifice. The EDTA-anticoagulated blood samples were used to obtain a complete blood count and differential with a Hemavet Mascot multispecies hematology system counter 1500R (CDC Technologies, Inc., Oxford, Conn.).
Statistical analysis.
Data were analyzed using analysis of variance (ANOVA) and Student's t test when the data had a normal distribution (StatView; Abacus Concepts/SAS Institute, Cary, N.C.). Results were considered to be significant at P < 0.05.
RESULTS
Antimicrobial activity.
Organisms isolated from infected human burn wounds provided our initial test panel. Their susceptibility to conventional systemic and topical antimicrobial agents is shown in Table 2. Because P. aeruginosa strain 2 was resistant to amikacin, aztreonam, ceftazidime, imipenem, and tobramycin and also to all three topical agents tested, it was selected for further study and for use in the burn model.
Table 3 summarizes data obtained from two-stage radial diffusion assays that tested sensitivity to three conventional antibiotics (vancomycin, gentamicin, and ciprofloxacin) and two antimicrobial peptides, novispirin G10 and protegrin PG-1. In addition to being resistant to the eight conventional antibiotics shown in Table 2, P. aeruginosa strain 2 was also resistant to gentamicin and ciprofloxacin. However, it was highly susceptible to both novispirin G10 and protegrin PG-1. novispirin G10 was somewhat less effective than PG-1 against Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus faecalis and slightly more effective against Pseudomonas aeruginosa and Acinetobacter baumanii.
TABLE 3.
Radial diffusion assay results comparing novispirin G10 and protegrin-1 with commonly used antimicrobial agentsd
| Organism | Strain no. | MEC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| Novispirin G10 | Protegrin-1 | Vancomycin | Gentamicin | Ciprofloxacin | ||
| S. aureus | 1 | 4.2 ± 0.31 | 2.85 ± 0.51 | 3.36 ± 0.57 | ND | ND |
| S. epidermidis | 1 | 2.8 ± 0.34 | 1.32 ± 0.08a | 2.17 ± 0.45 | ND | ND |
| E. faecalis | 1 | 4.3 ± 0.18a | 1.37 ± 0.18a | 0.79 ± 0.09 | ND | ND |
| P. aeruginosa | 1 | 1.2 ± 0.1b,c | 1.58 ± 0.17b,c | ND | 0.32 ± 0.21 | 0.39 ± 0.01 |
| 2 | 0.4 ± 0.08b,c | 0.062 ± 0.11b,c | ND | >128 | >128 | |
| 3 | 0.7 ± 0.09c | 1.56 ± 0.06c | ND | ND | 0.46 ± 0.02 | |
| A. baumanii | 1 | 2.5 ± 0.7b | 4.54 ± 0.16b | ND | >128 | ND |
P < 0.05 comparing G10 and PG-1 versus vancomycin.
P < 0.05 comparing G10 and PG-1 versus gentamicin.
P < 0.05 comparing G10 and PG-1 versus ciprofloxacin. Data are from three individual experiments; each condition was performed in quadruplicate.
All clinical isolates from human burn wounds showed significant sensitivity to novispirin G10. Note that P. aeruginosa 2, which was resistant to all systemic and topical antibiotics tested, was exceptionally sensitive to novispirin G10 and PG-1. ND, not determined. MEC > 128 means that the tested bacteria are not susceptible to the drug tested. Roman letters indicate significance.
Whereas zones of inhibition became visible around G10 or PG-1 (5 μg/ml) within 2 h of adding the overlay gels, at least 6 h of incubation was required before similar zones were evident around wells containing 4 μg/ml of gentamicin or 16 μg/ml of vancomycin (data not shown). The MEC of ciprofloxacin for P. aeruginosa strains 1 and 3 was lower than the MECs of G10 and PG-1. However, whereas the P. aeruginosa strain was resistant to 128 μg/ml of ciprofloxacin or gentamicin, it was exceptionally sensitive to G10 and PG-1 (P < 0.001).
The activity of novispirin G10 against P. aeruginosa strain 2 was slightly decreased when the radial diffusion assays were done with underlay gels that contained 60% RPMI 1640 and 40% phosphate-buffered saline, and its activity was further diminished when the underlay gels also contained up to 20% human serum (Table 4). Ovispirin-1, PGG, and the other novispirins also showed reduced potency as the concentration of serum increased. In contrast, the activity of protegrin PG-1 was affected relatively little by the presence of serum.
TABLE 4.
Effect of normal human seruma
| Peptide | MEC for P. aeruginosa strain 2 (μg/ml)
|
|||
|---|---|---|---|---|
| 0% NHS | 2.5% NHS | 10% NHS | 20% NHS | |
| Ovispirin | 4.7 | 14.5 | 28.4 | 145 |
| G7 novispirin | 7.4 | 8.3 | 26.9 | 116 |
| G10 novispirin | 3.1 | 5.2 | 20.3 | 46.9 |
| T7 novispirin | 3.2 | 7.2 | 28.4 | 30.9 |
| T10 novispirin | 3.1 | 2.7 | 9.6 | 25.0 |
| PG-1 (protegrin-1) | 1.1 | 0.9 | 3.2 | 6.1 |
| PGG | 4.2 | 6.7 | 21.2 | 52.1 |
The underlay gels in these radial diffusion experiments contained 60% RPMI 1640 1% agarose, 0 to 20% normal human serum (NHS), and 20 to 40% phosphate-buffered saline.
Bactericidal kinetics.
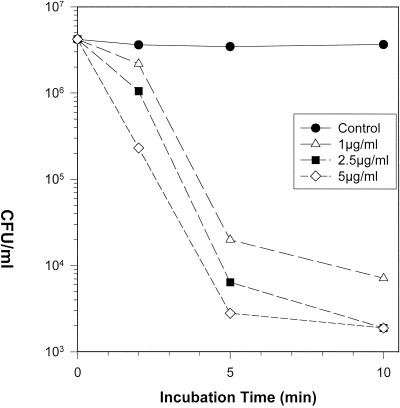
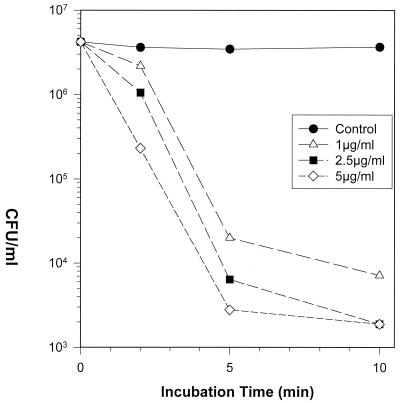
Novispirin G10-mediated bactericidal activity occurred rapidly and in a concentration-dependent manner. Figure 1 shows an experiment with P. aeruginosa strain 2, the organism later tested in the in vivo model. In vitro exposure to 1 μg/ml of novispirin G10 reduced the colony count by >2 log10 (>99%) at 5 min and by almost 3 log10 at 10 min. Higher concentrations (2.5 and 5 μg/ml) of novispirin G10 reduced the colony count by approximately 3 log10 at 5 min and below the limit of detectability at 10 min. G10 and exposure to 5 μg/ml reduced the colony count by >3 log10 (>99.9%) in 5 min. Similar rapid killing occurred when Escherichia coli ML-35p or the multiresistant Pseudomonas aeruginosa strain MR3007 was exposed to novispirin G10 (data not shown).
FIG. 1.
Microbicidal kinetics. P. aeruginosa strain 2 was exposed to the indicated concentrations of novispirin G10 in a medium composed of 100 mM NaCl, 10 mM sodium phosphate buffer (pH 7.4), and 0.3 mg of Trypticase soy broth powder/ml. Aliquots were removed after 5 and 10 min, diluted, spread on Trypticase soy agar plates, and incubated overnight to allow colony development.
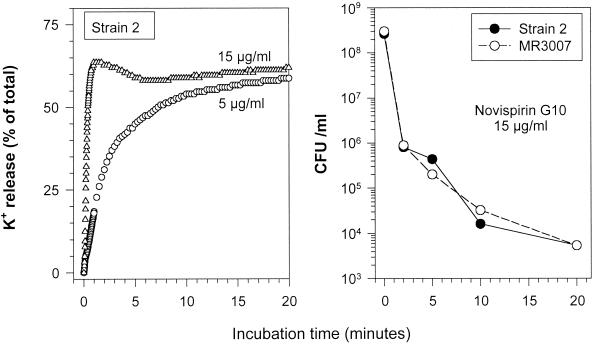
To assess the ability of novispirin G10 to permeabilize the inner membrane of P. aeruginosa strain 2, we measured both potassium efflux and colony counts after exposing the peptide to 5 or 15 μg/ml of novispirin G10. Figure 2 shows that a massive efflux of potassium occurred within seconds of adding the peptide and that virtually all of the organisms were nonviable within 2 min.
FIG. 2.
Induction of potassium efflux. (Left panel) Potassium efflux from stationary-phase P. aeruginosa strain 2 exposed to 5 or 15 μg of novispirin G10 per ml was monitored with an ion-selective potassium electrode. (Right panel) Colony counts were done under the same conditions after exposing two P. aeruginosa strains (strains 2 and MR 3007) to 15 μg of novispirin G10 per ml.
Cytotoxic and hemolytic properties.
To pursue the goal of developing antimicrobial peptides with reduced cytotoxicity, we tested their effects on two cell lines, ME-180 cervical epithelial cells and A549 pulmonary epithelial cells (Fig. 3). Their cytotoxicity to these target cells is indicated by their 50% effective concentration (EC50) values, which signify the peptide concentration that caused a 50% reduction of MTT-tetrazolium reduction relative to unexposed controls. The EC50 values for ovispirin-1 (20 μg/ml), PGG (33 μg/ml), and protegrin PG-1 (22.4 μg/ml) were similar. The novispirins were 2- to 7-fold less cytotoxic for ME-180 cells, having EC50 values of 59 μg/ml (novispirin G7), 84 μg/ml (novispirin T10), 126 μg/ml (novispirin T7), and 163 μg/ml (novispirin G10)
FIG. 3.
Cytotoxic properties. The lowest cytotoxic effect was seen with novispirin G10 compared to the other antimicrobial peptides tested in the ME-180 cervical epithelial or A549 pulmonary epithelial cell line.
Judging from their EC50 values, ovispirin-1 (14 μg/ml), PGG (30 μg/ml), protegrin PG-1 (22.4 μg/ml), and novispirin T7 (25.8 μg/ml) were about equally cytotoxic for A-549 pulmonary cells and novispirin G7 (47.3 μg/ml) was slightly less so. novispirin T10 (EC50, 150 μg/ml) and novispirin G10 (EC50, 163 μg/ml) were considerably less cytotoxic for A-549 cells.
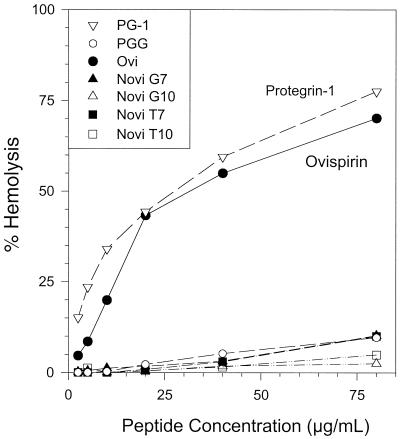
We tested hemolytic activity by exposing 2.5% suspensions of washed red blood cells to serial twofold peptide dilutions for 30 min at 37°C. Low concentrations of ovispirin-1 and protegrin PG-1 were quite hemolytic for human red blood cells, whereas the novispirins were essentially nonhemolytic at 80 μg/ml, the highest concentration we tested (Fig. 4). Although we obtained similar results with murine red blood cells (data not shown), none of these peptides, including ovispirin-1 and protegrin PG-1, lysed sheep or bovine red blood cells appreciably, even at 80 μg/ml (data not shown). Thus, based largely on its much reduced cytotoxicity for human epithelial cells, we selected novispirin G10 for the in vivo experiments.
FIG. 4.
Hemolytic properties. A suspension of washed human cells (2.8%, vol/vol) was incubated with various concentrations of PG-1, PGG, ovispirin, or novispirin (G7, G10, T7, and T10) for 30 min at 37°C and then centrifuged. The percentage of total hemoglobin released to the supernatant was measured.
In vivo activity of novispirin G10.
Macroscopic evaluation of burned skin immediately after the scald injury revealed notable paleness. A few minutes later a reddish pink coloration appeared, giving way to progressive pallor over the next 1 to 2 h. Edema reached a peak after approximately 3 to 4 h and then slowly regressed, disappearing completely during the ensuing 24 h. Tissues treated with novispirin G10 or its vehicle control did not differ in macrosopic appearance from the tissues of nontreated controls. No clear histological evidence of cytotoxicity was observed in any of the tissue samples regardless of treatment group.
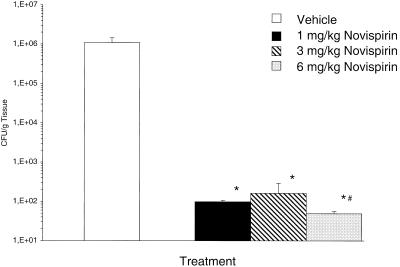
For the in vivo study, we used P. aeruginosa strain 2, which was globally resistant to all the systemic and topical antibiotics routinely tested in our hospital laboratory (Table 2). In Figure 5 the results of tissue bacterial counts in response to increasing doses of novispirin G10 are depicted. The bacterial counts per gram of tissue were significantly lower for the novispirin G10-treated groups (1, 3, and 6 mg/kg) compared to carrier control-treated animals (P < 0.0001). No difference was seen between doses of 1 and 3 mg/kg; however bacterial counts were significantly lower with 6 mg/kg compared to the 1-mg/kg dose (P = 0.017). Testing of the skin homogenates in radial diffusion assays 4 h after administration failed to demonstrate antimicrobial activity. In previously conducted studies 24 h posttreatment, we demonstrated that this model ensures bacterial killing using a different compound called protegrin-1 (49).
FIG. 5.
Bacterial clearance in an infected burn wound model. This graph summarizes the bacterial counts for P. aeruginosa in the burn wound 4 h after intradermal injection (500 μl/animal) into the burn wound of either 1, 3, or 6 mg of novispirin G10 (n = 16 for each concentration) or vehicle control (n = 16) per kg. ∗, P < 0.05, novispirin G10 versus vehicle control; #, P < 0.05, novispirin G10 at 1 mg/kg versus 6 mg/kg.
Peripheral blood analysis.
The numbers of white blood cells (P = 0.009) and neutrophils (P = 0.02) were significantly increased in the novispirin G10-treated group compared to the vehicle control group. Significantly higher counts were seen for eosinophils in the novispirin group compared to normal controls (P = 0.02); the same trend was seen comparing novispirin with vehicle, although this was not statistically significant.
No statistically significant differences were seen for lymphocytes, monocytes, hemoglobin, hematocrit, red blood cell count, or platelet count after treatment with novispirin G10 versus vehicle control and normal controls.
DISCUSSION
As a barrier and immune organ, the skin plays a key role in protecting the body from a hostile environment (3, 10). The low incidence of infection at normal epithelial surfaces reflects the presence of innate, broad-spectrum antimicrobial defense mechanisms. Compromise of these mechanisms may facilitate bacterial infections, which delay or even reverse the wound healing process. This is exemplified by burn wounds, in which bacterial counts of less than 106 per g of tissue are crucial for successful skin grafting (5). The exact factors and mechanisms placing burn wounds at higher risk for infection are unknown. However, it is generally thought that injury compromises the immune system locally, allowing bacterial growth, superinfection, and sepsis to ensue where bacteria otherwise would have been controlled by functional tissues (49).
It appears that the array of antimicrobial peptides in the innate immune response play an important role in the protective barrier function of the epithelia. For example, antimicrobial peptide expression in keratinocytes is strongly induced on contact with bacteria or pro-inflammatory cytokines (44). In localized insults, such as thermal injury, it has been demonstrated that the confined expression of antimicrobial peptides is reduced, presumably rendering the site of injury more vulnerable to bacterial colonization (38, 40).
The emergence of virulent, antibiotic-resistant strains of bacteria has created a pressing need for alternative therapies for infection (30). In light of this, the field of antimicrobial peptide research is promising. Many of these peptides demonstrate extremely broad-spectrum antimicrobial activity, including gram-positive and gram-negative bacteria and fungi (22, 34, 45). In addition, they achieve bacterial killing much more rapidly than any commercially available antibiotic (47). Recently, a new family of synthetic, α-helical antimicrobial peptides called ovispirins was described (12, 43, 55). Although some of these peptides largely retained the antimicrobial activity of naturally occurring peptides, they manifested appreciable cytotoxicity. The novispirin peptides described in this report are variants of ovispirin 1 that displayed more favorable toxic/therapeutic ratios in vitro. Novispirin G10, which manifested the least cytotoxicity, was chosen for our in vivo study.
Novispirin G10 was as effective in the infected burn model as protegrin-1 (49), a naturally occurring antimicrobial peptide with superior antimicrobial properties but greater intrinsic cytotoxicity. Our in vitro studies were largely carried out using a quantitative and highly sensitive two-layer radial diffusion assay. In these studies, novispirin G10 and PG-1 showed similar activity against gram-negative bacteria, but novispirin was slightly less potent against gram-positive bacteria.
Addition of calcium and magnesium inhibited the antimicrobial potency of novispirin G10, an effect that could be reversed by adding citrate, a chelator of these cations (data not shown). Since the normal concentrations of magnesium and calcium in rat wound fluid are 0.8 to 2 mM, whereas those in RPMI 1640 are about 0.42 mM, the peptide is unlikely to be as effective in vivo, as indicated by the MECs shown in Table 4 (26). However, presumably because of the peptide's rapid effect and considerable potency, it was highly effective in reducing the number of bacteria in the wound. Divalent cations also reduce the antimicrobial potency of other antimicrobial peptides, including lactoferricin (7), LL-37 (16), α-defensins (35), and β-defensins (51). The mechanism of this effect has not been precisely defined.
In studies examining the cytotoxic and hemolytic properties of the peptides, novispirin G10 demonstrated minimal damage to host cells, in contrast to PG-1 and ovispirin, which demonstrated considerable cytotoxic effects. It is significant that even at the higher drug concentrations required to attain the growth-inhibitory concentrations in the presence of magnesium and calcium in vivo, injury to host cells from novispirin G10 is still negligible and definitely within an acceptable range. Recent studies with other naturally occurring peptides such as defensins (HNP and NP-2) and pig antimicrobial peptides from leukocytes (PR-39, prophenin PF-2, and protegrin PG-2) have also recently demonstrated time- and concentration-dependent cytotoxicity (41).
Unexpected was the species-specific cytolytic effect on blood cells in this study. This suggests that “designer” amphipathic peptides with antimicrobial features demonstrate specificity for host cells depending on their conformational structure. Several properties of cathelicidin-derived peptides make them attractive candidates for possible drug development. Novispirin and protegrins have extremely broad-spectrum antimicrobial activity and, unlike many α- and β-defensins, retain activity in the presence of serum proteins and also at physiologic or even elevated salt concentrations, a distinct advantage for potential therapeutic uses.
That novispirin G10 is devoid of disulfide bridges or unusual residues should allow facile and less costly chemical synthesis. Finally, the rapidity of killing by novispirin G10 should provide an advantage for topical applications, allowing bacterial killing before the peptide is mechanically cleared or otherwise inactivated, e.g., by local proteases. To evaluate the effectiveness of novispirin G10 in vivo, we used an animal burn wound infection model as previously described (49) with a P. aeruginosa strain that was resistant to all conventional topical antibiotics. Our studies revealed a rapid decrease in bacterial counts of up to 3 log10 units after intradermal injection of novispirin G10. We have shown previously that topical application was less effective in bacterial clearance than intradermal injection, probably because antimicrobial peptides are positively charged and traverse the basal layer of the epidermis insufficiently well to eradicate invasive bacteria (49).
The in vitro and in vivo findings in this study do not preclude the occurrence of cytotoxic responses when other cell types are exposed to novispirin G10, but our data suggest that this peptide does not share the cytolytic proclivities of PG-1 and ovispirin.
Overall, these studies demonstrate that novispirin G10 is highly effective in vitro and in vivo against clinically relevant isolates from human burn wounds, making it a potential candidate for local treatment of infected burn wounds. The impressive potency of novispirin coupled with its simple (i.e., α-helical coil) molecular structure may also make it an excellent candidate for development of gene delivery-based therapies. Such a drug delivery system could allow local production of this powerful antimicrobial peptide within the wound, exactly where it would be most beneficial. It will also be important to determine whether this peptide synergizes with conventional antibiotics or other endogenous antimicrobials. Furthermore, insight into mechanisms of endogenous antimicrobial peptide upregulation may allow the development of compounds that elicit epithelial defense reactions by stimulating locally increased synthesis of endogenous antimicrobial peptides. Antimicrobial peptides represent promising candidates for further investigation and development of effective topical therapeutics.
Acknowledgments
We thank William R. Forsyth for his contributions to the design of ovispirin.
This work was supported in part by National Institutes of Health grants GM60205, GM54911, HL03803-01, DK53296, and AI 43934.
REFERENCES
- 1.Agerberth, B., H. Gunne, J. Odeberg, P. Kogner, H. G. Boman, and G. H. Gudmundsson. 1995. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 92:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekseev, A. A., V. P. Iakovlev, and V. D. Fedorov. 1999. Infection in burn patients: the problems of pathogenesis, prevention and treatment. Khirurgiia 6:4-9. [PubMed] [Google Scholar]
- 3.Allgower, M., G. A. Schoenenberger, and B. G. Sparkes. 1995. Burning the largest immune organ. Burns 21:S7-S47. [DOI] [PubMed] [Google Scholar]
- 4.Ayats, J., X. Corbella, C. Ardanuy, M. A. Dominguez, A. Ricart, J. Ariza, R. Martin, and J. Linares. 1997. Epidemiological significance of cutaneous, pharyngeal, and digestive tract colonization by multiresistant Acinetobacter baumannii in ICU patients. J. Hosp. Infect. 37:287-295. [DOI] [PubMed] [Google Scholar]
- 5.Bacchetta, C. A., W. Magee, G. Rodeheaver, M. T. Edgerton, and R. F. Edlich. 1975. Biology of infections of split thickness skin grafts. Am. J. Surg. 130:63-67. [DOI] [PubMed] [Google Scholar]
- 6.Bagella, L., M. Scocchi, and M. Zanetti. 1995. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 376:225-228. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy, W. R., H. Wakabayashi, M. Takase, K. Kawase, S. Shimamura, and M. Tomita. 1993. Role of cell-binding in the antibacterial mechanism of lactoferricin B. J. Appl. Bacteriol. 75:478-484. [PubMed] [Google Scholar]
- 8.Bellm, L., R. I. Lehrer, and T. Ganz. 2000. Protegrins: new antibiotics of mammalian origin. Expert Opin. Investig. Drugs 9:1731-1742. [DOI] [PubMed] [Google Scholar]
- 9.Boman, H. G. 1998. Gene-encoded peptide antibiotics and the concept of innate immunity: an update review. Scand. J. Immunol. 48:15-25. [DOI] [PubMed] [Google Scholar]
- 10.Bos, J. D., and M. L. Kapsenberg. 1993. The skin immune system: progress in cutaneous biology. Immunol. Today 14:75-78. [DOI] [PubMed] [Google Scholar]
- 11.Brogden, K., V. Kalfa, P. McCray, and B. Tack. 1999. Activities of cathelicidin-derived peptides against ovine respiratory pathogens and Pseudomonas aeruginosa. Pulmonology 19:320. [Google Scholar]
- 12.Brogden, K. A., V. C. Kalfa, M. R. Ackermann, D. E. Palmquist, P. B. McCray, and B. F. Tack. 2001. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens in vitro and in an ovine model of pulmonary infection. Antimicrob. Agents Chemother. 45:331-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buttery, J. P., S. J. Alabaster, R. G. Heine, S. M. Scott, R. A. Crutchfield, A. Bigham, S. N. Tabrizi, and S. M. Garland. 1998. Multiresistant Pseudomonas aeruginosa outbreak in a pediatric oncology ward related to bath toys. Pediatr. Infect. Dis. J. 17:509-513. (Erratum, 18:9, 1999.) [DOI] [PubMed] [Google Scholar]
- 14.Carbon, C. 1999. Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 44(Suppl. A):31-36. [DOI] [PubMed] [Google Scholar]
- 15.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127-1132. [DOI] [PubMed] [Google Scholar]
- 16.Cho, Y., J. S. Turner, N. N. Dinh, and R. I. Lehrer. 1998. Activity of protegrins against yeast-phase Candida albicans. Infect. Immun. 66:2486-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehlbaum, P., M. Rao, M. Zasloff, and G. M. Anderson. 2000. An essential amino acid induces epithelial beta-defensin expression. Proc. Natl. Acad. Sci. USA 97:12723-12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox, C. L., Jr., B. W. Rappole, and W. Stanford. 1969. Control of pseudomonas infection in burns by silver sulfadiazine. Surg. Gynecol. Obstet. 128:1021-1026. [PubMed] [Google Scholar]
- 19.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo, R. L., and K. M. Huttner. 1998. Antimicrobial peptides: an emerging concept in cutaneous biology. J. Investig. Dermatol. 111:739-743. [DOI] [PubMed] [Google Scholar]
- 21.Gallo, R. L., K. J. Kim, M. Bernfield, C. A. Kozak, M. Zanetti, L. Merluzzi, and R. Gennaro. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272:13088-13093. [DOI] [PubMed] [Google Scholar]
- 22.Ganz, T., and R. I. Lehrer. 1999. Antibiotic peptides from higher eukaryotes: biology and applications. Mol. Med. Today 5:292-297. [DOI] [PubMed] [Google Scholar]
- 23.Ganz, T., and R. I. Lehrer. 1995. Defensins. Pharmacol. Ther. 66:191-205. [DOI] [PubMed] [Google Scholar]
- 24.Gilpin, D. A. 1996. Calculation of a new Meeh constant and experimental determination of burn size. Burns 22:607-611. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield, E., and A. T. McManus. 1997. Infectious complications: prevention and strategies for their control. Nurs. Clin. N. Am. 32:297-309. [PubMed] [Google Scholar]
- 26.Grzesiak, J. J., and M. D. Pierschbacher. 1995. Changes in the concentrations of extracellular Mg++ and Ca++ down-regulate E-cadherin and up-regulate alpha 2 beta 1 integrin function, activating keratinocyte migration on type I collagen. J. Investig. Dermatol. 104:768-774. [DOI] [PubMed] [Google Scholar]
- 27.Hanberger, H., J. A. Garcia-Rodriguez, M. Gobernado, H. Goossens, L. E. Nilsson, and M. J. Struelens. 1999. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU Study Groups. JAMA 281:67-71. [DOI] [PubMed] [Google Scholar]
- 28.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 30.Karam, G. H., and J. E. Heffner. 2000. Emerging issues in antibiotic resistance in blood-borne infections. Am. J. Respir. Crit. Care Med. 162:1610-1616. [DOI] [PubMed] [Google Scholar]
- 31.Kokryakov, V. N., S. S. Harwig, E. A. Panyutich, A. A. Shevchenko, G. M. Aleshina, O. V. Shamova, H. A. Korneva, and R. I. Lehrer. 1993. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 327:231-236. [DOI] [PubMed] [Google Scholar]
- 32.Larrick, J. W., J. G. Morgan, I. Palings, M. Hirata, and M. H. Yen. 1991. Complementary DNA sequence of rabbit CAP18—a unique lipopolysaccharide binding protein. Biochem. Biophys. Res. Commun. 179:170-175. [DOI] [PubMed] [Google Scholar]
- 33.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 34.Lehrer, R. I., and T. Ganz. 1996. Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann. N. Y. Acad. Sci. 797:228-239. [DOI] [PubMed] [Google Scholar]
- 35.Lehrer, R. I., T. Ganz, M. E. Selsted, B. M. Babior, and J. T. Curnutte. 1988. Neutrophils and host defense. Ann. Intern. Med. 109:127-142. [DOI] [PubMed] [Google Scholar]
- 36.Lindberg, R. B., J. A. Moncrief, W. E. Switzer, S. E. Order, and W. Mills, Jr. 1965. The successful control of burn wound sepsis. J. Trauma 5:601-616. [DOI] [PubMed] [Google Scholar]
- 37.Martin, E., T. Ganz, and R. I. Lehrer. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128-136. [DOI] [PubMed] [Google Scholar]
- 38.Milner, S. M., and M. R. Ortega. 1999. Reduced antimicrobial peptide expression in human burn wounds. Burns 25:411-413. [DOI] [PubMed] [Google Scholar]
- 39.Orlov, D. L., T. Nguyen, and R. I. Lehrer. 2002. Potassium release, a useful tool for studying antimicrobial peptides. J. Microbiol. Methods 49:325-328. [DOI] [PubMed]
- 40.Ortega, M. R., T. Ganz, and S. M. Milner. 2000. Human beta defensin is absent in burn blister fluid. Burns 26:724-726. [DOI] [PubMed] [Google Scholar]
- 41.Pleskach, V. A., G. M. Aleshina, I. V. Artsybasheva, O. V. Shamova, I. V. Kozhukharova, T. A. Goilo, and V. N. Kokriakov. 2000. Cytotoxic and mitogenic effect of antimicrobial peptides from neutrophils on cultured cells. Tsitologiia 42:228-234. [PubMed] [Google Scholar]
- 42.Saiman, L., Z. Liu, T. Starner, P. McCray, and B. Tack. 1999. Drug resistant organisms from CF patients are inhibited by cathelicidin peptides. Pulmonology 19:320. [Google Scholar]
- 43.Saiman, L., S. Tabibi, T. D. Starner, P. San Gabriel, P. L. Winokur, H. P. Jia, P. B. McCray, and B. F. Tack. 2001. Cathelicidin peptides inhibit multiply antibiotic-resistant pathogens from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroder, J. M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 45.Shin, S. Y., S. W. Kang, D. G. Lee, S. H. Eom, W. K. Song, and J. I. Kim. 2000. CRAMP analogues having potent antibiotic activity against bacterial, fungal, and tumor cells without hemolytic activity. Biochem. Biophys. Res. Commun. 275:904-909. [DOI] [PubMed] [Google Scholar]
- 46.Skerlavaj, B., M. Benincasa, A. Risso, M. Zanetti, and R. Gennaro. 1999. SMAP-29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 463:58-62. [DOI] [PubMed] [Google Scholar]
- 47.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinberg, D. A., and R. I. Lehrer. 1997. Designer assays for antimicrobial peptides. Disputing the “one-size-fits-all” theory. Methods Mol. Biol. 78:169-186. [DOI] [PubMed] [Google Scholar]
- 49.Steinstraesser, L., M. Föhn, R. Klein, A. Aminlari, D. Remick, G. Su, and S. Wang. 2001. Feasibility of biolistic gene therapy in burns. Shock 15:272-277. [DOI] [PubMed] [Google Scholar]
- 50.Stone, H. H. 1966. Review of pseudomonas sepsis in thermal burns: verdoglobin determination and gentamicin therapy. Ann. Surg. 163:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomita, T., S. Hitomi, T. Nagase, H. Matsui, T. Matsuse, S. Kimura, and Y. Ouchi. 2000. Effect of ions on antibacterial activity of human beta defensin 2. Microbiol. Immunol. 44:749-754. [DOI] [PubMed] [Google Scholar]
- 52.Tossi, A., C. Tarantino, and D. Romeo. 1997. Design of synthetic antimicrobial peptides based on sequence analogy and amphipathicity. Eur. J. Biochem. 250:549-558. [DOI] [PubMed] [Google Scholar]
- 53.Travis, S. M., N. N. Anderson, W. R. Forsyth, C. Espiritu, B. D. Conway, E. P. Greenberg, P. B. McCray, Jr., R. I. Lehrer, M. J. Welsh, and B. F. Tack. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker, H. L., and A. D. Mason, Jr. 1968. A standard animal burn. J. Trauma 8:1049-1051. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi, S., D. Huster, A. Waring, R. I. Lehrer, W. Kearney, B. F. Tack, and M. Hong. 2001. Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophys. J. 81:2203-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
- 57.Zanetti, M., R. Gennaro, and D. Romeo. 1997. The cathelicidin family of antimicrobial peptide precursors: a component of the oxygen-independent defense mechanisms of neutrophils. Ann. N. Y. Acad. Sci. 832:147-162. [DOI] [PubMed] [Google Scholar]
- 58.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]