Abstract
The essential oil of Melaleuca alternifolia (tea tree) has broad-spectrum antimicrobial activity. The mechanisms of action of tea tree oil and three of its components, 1,8-cineole, terpinen-4-ol, and α-terpineol, against Staphylococcus aureus ATCC 9144 were investigated. Treatment with these agents at their MICs and two times their MICs, particularly treatment with terpinen-4-ol and α-terpineol, reduced the viability of S. aureus. None of the agents caused lysis, as determined by measurement of the optical density at 620 nm, although cells became disproportionately sensitive to subsequent autolysis. Loss of 260-nm-absorbing material occurred after treatment with concentrations equivalent to the MIC, particularly after treatment with 1,8-cineole and α-terpineol. S. aureus organisms treated with tea tree oil or its components at the MIC or two times the MIC showed a significant loss of tolerance to NaCl. When the agents were tested at one-half the MIC, only 1,8-cineole significantly reduced the tolerance of S. aureus to NaCl. Electron microscopy of terpinen-4-ol-treated cells showed the formation of mesosomes and the loss of cytoplasmic contents. The predisposition to lysis, the loss of 260-nm-absorbing material, the loss of tolerance to NaCl, and the altered morphology seen by electron microscopy all suggest that tea tree oil and its components compromise the cytoplasmic membrane.
The essential oil derived by steam distillation from the leaves of Melaleuca alternifolia is also known as tea tree oil (TTO) or Melaleuca oil. TTO is well characterized and contains approximately 100 terpenes and their related alcohols (6). The physical and chemical properties of commercial TTO are regulated by an international standard (23). TTO has antibacterial (8, 17), antifungal (18, 19), antiviral (4), and anti-inflammatory (5) properties in vitro, suggesting that it may have a role in the treatment of cutaneous infection. Clinical trials have demonstrated that TTO may be efficacious in the treatment of acne (2) and oral candidiasis (24) and in the decolonization of methicillin-resistant Staphylococcus aureus carriers (7). Although the in vitro antimicrobial activity and in vivo efficacy of TTO have been reported, less is known about its mechanism of action. Since this will have implications for its spectrum of activity, selective toxicity, and the development of resistance, we examined the mechanism of action of TTO and its components against S. aureus.
MATERIALS AND METHODS
TTO and components.
TTO (batch 971) was kindly provided by Australian Plantations Pty. Ltd., Wyrallah, New South Wales, Australia. The levels of the components, determined by gas chromatographic analysis according to the international standard (23), were as follows: 41.5% terpinen-4-ol, 21.2% γ-terpinene, 10.2% α-terpinene, 3.5% terpinolene, 2.9% α-terpineol, 2.5% α-pinene, 2.1% 1,8-cineole, 1.5% ρ-cymene, 1% aromadendrene, 1% δ-cadinene, 0.9% ledene, 0.9% limonene, 0.6% globulol, 0.4% sabinene, and 0.3% viridiflorol. Terpinen-4-ol and α-terpineol (Aldrich Chemical Co. Inc., Milwaukee, Wis.) were 97 and 98% pure, respectively. 1,8-Cineole (Sigma Chemical Co., St. Louis, Mo.) was at least 99% pure. Terpinen-4-ol and α-terpineol were chosen on the basis of their antimicrobial activities (10). 1,8-Cineole was selected since it has some antimicrobial activity and its presence in TTO has been contentious, due mainly to its erroneous reputation as a skin irritant (10).
Bacterium.
S. aureus ATCC 9144 was used in all experiments and was obtained from the culture collection of the Department of Microbiology, The University of Western Australia, Crawley, Western Australia, Australia.
Inoculum effect.
The MICs and minimal bactericidal concentrations (MBCs) of TTO for S. aureus were determined by a previously described broth microdilution method (8), except that the test medium was Mueller-Hinton broth (Oxoid Ltd., Basingstoke, United Kingdom), the Tween 80 concentration was reduced to 0.001% (vol/vol), and several inoculum concentrations were used. Final inoculum concentrations were 5 × 103, 5 × 105, 5 × 107, and 5 × 109 CFU/ml.
Preparation of bacterial suspensions.
Unless stated otherwise, suspensions of organisms in the stationary phase of growth were prepared by inoculating two colonies of S. aureus from overnight cultures on blood agar into 400 ml of Mueller-Hinton broth, which was incubated at 37°C for 18 h with shaking. After incubation, the bacteria were separated from the growth medium by centrifugation at 10,000 × g for 12 min at 4°C, washed twice with phosphate-buffered saline (PBS; pH 7.4), and resuspended in PBS supplemented with 0.001% Tween 80 (Sigma) (PBS-T). Suspensions were adjusted so that the optical density at 620 nm (OD620) of a 1-in-100 dilution was ∼0.3, which was ∼3 × 1010 CFU/ml.
General experimental conditions.
Experiments were conducted at room temperature (22°C). The concentrations of TTO and its components used for treatment were derived from MICs previously determined by broth microdilution methods and ranged from one-half to two times the MICs. The MICs of TTO, terpinen-4-ol, and α-terpineol for S. aureus were 0.25%; and the MIC of 1,8-cineole was 0.5% (9, 10). Stock concentrations of TTO or its components were prepared in PBS-T at 10-fold the desired final concentration (in percent [vol/vol]) and were added to bacterial suspensions at a ratio of 1:9. PBS-T was added to the control suspensions. No inactivating agents have been established for TTO or its components. In the absence of an inoculum effect, dilution was used to arrest treatment and reduce carryover. The minimum dilution used was 1 in 10. All serial dilutions were done in PBS-T. All experiments except time-kill assays and inoculum effect assays were conducted four to six times; time-kill assays were done in triplicate, and inoculum effect assays were done in duplicate. Viable counts were plated in duplicate by using a spiral plater (Don Whitley Scientific Ltd., Shipley, United Kingdom) and nutrient agar (NA) (Oxoid) in all experiments with the exception of those investigating an inoculum effect. After incubation at 37°C for 24 to 72 h, the colonies were counted manually with the aid of a counting template (Don Whitley Scientific). The detection threshold was 103 CFU/ml. For inoculum effect assays, 10 μl of serial 10-fold dilutions of inocula was plated onto blood agar in quadruplicate, the plates were incubated, and the resulting colonies were counted. In assays for bacterial killing, bacteriolysis, and loss of 260-nm-absorbing material, samples taken 5 min before treatment addition were adjusted to account for dilution and used to determine the concentrations and ODs at 0 min.
Bacterial killing assays.
The activities of TTO and its components against S. aureus were evaluated by measuring the reduction in the numbers of CFU per milliliter over 2 h. Bacterial suspensions (5 ml) were prepared as described above. After a pretreatment sample (0.5 ml) was taken, TTO or one of its components was added to yield final concentrations of one-half, one, and two times the respective MICs. The suspensions were mixed for 20 s with a vortex mixer; and a sample (0.5 ml) was removed at 30 s, serially diluted, and plated onto NA. Additional samples were taken at 30, 60, and 120 min. When rapid killing occurred, samples were also taken at 3, 6, 9, 12, and 15 min. Samples were diluted, plated onto NA, and incubated overnight. The mean number of survivors was determined.
Bacteriolysis.
Suspensions of S. aureus were prepared as described above. After retrieval of the pretreatment sample, the agents were added so that the suspensions contained TTO or one of its components at concentrations equivalent to the MIC and two times the MIC. The suspensions were mixed for 20 s with a vortex mixer, a sample was removed at 30 s and diluted 1 in 100, and the OD620 was measured. Additional samples were taken at 30, 60, 90, and 120 min; and the ODs were measured immediately. Corresponding dilutions of test agents were used as blanks. Preliminary work indicated that the ODs of undiluted samples and samples diluted 1 in 10 were inconsistent (data not shown). The 1-in-100 dilution reduced the level of carryover of TTO or its components to levels which contributed negligibly to the absorbance. The results were expressed as a ratio of the OD620 at each time interval versus the OD620 at 0 min (in percent).
In two separate experiments, after the initial OD620 measurement, the samples were retained and the OD620 was remeasured 6.5 or 23 h later. The OD620s of the control suspensions diminished over these time intervals; the OD620s of the samples obtained at 0 min were measured again 6.5 h later and were 91.5% of the original OD620, while the OD620s measured 23 h later were 84.4% of the original OD620. Since there was an inherent progressive reduction in the OD620 over time, when the OD620s of the samples were measured 6.5 or 23 h later, their OD620s were compared to the OD620s of the 0-min samples, which were also remeasured 6.5 or 23 h later.
Loss of 260-nm-absorbing material.
Suspensions of bacteria were prepared as described above. A pretreatment sample was taken, diluted 1 in 100, and filtered through a 0.2-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.). The treatment agents were added at final concentrations equivalent to their MICs. Additional samples were removed after 30 and 60 min, diluted, and filtered as described above. Filtrates of the appropriate dilution of each agent were prepared, and 200-μl volumes were used to blank the wells of a UV-transparent 96-well tray (Molecular Devices, Sunnyvale, Calif.) at 260 nm. The blanks were discarded, the wells were loaded with the corresponding test filtrates, and the OD260s were determined. The OD620s of four to eight samples obtained at each time point were measured. The OD260 at each time point was expressed as a proportion of the initial OD260. Mean ratios for each treatment agent and time were calculated and compared to the means for the corresponding untreated samples by using the two-tailed Student t test (P < 0.05).
Loss of salt tolerance.
The ability of S. aureus cells treated with TTO or its components to grow on NA supplemented with NaCl (Merck Pty. Ltd., Kilsyth, Victoria, Australia) was investigated. In preliminary experiments, untreated suspensions of S. aureus were plated onto NA and NA containing NaCl at 5 to 100 g/liter (NA-NaCl) and incubated, and the resulting colonies were counted. Concentrations of NaCl that modestly compromised the colony-forming abilities of untreated organisms were selected. These were 50 and 75 g/liter. Suspensions of bacteria were prepared as described above and treated with TTO and 1,8-cineole at one-half, one, and two times their MICs and with terpinen-4-ol and α-terpineol at one-half and one time their MICs. After 30 min, samples were removed, serially diluted, and inoculated onto NA and the selective medium NA-NaCl. After incubation, the numbers of CFU per milliliter on each NA-NaCl plate were compared to those on the NA plate, and the result was expressed as a percentage. The mean proportions of survivors from treated suspensions were compared to the corresponding means for the untreated controls by the two-tailed Student t test (P < 0.05).
Electron microscopy of terpinen-4-ol-treated bacteria.
Suspensions of S. aureus in the stationary phase of growth were prepared by inoculating and incubating 30 ml of heart infusion broth (HIB; Gibco Diagnostics, Madison, Wis.). Organisms were harvested by centrifugation at 1,500 × g for 10 min, and the pellet was resuspended in HIB supplemented with Tween 80 (0.5% [vol/vol]) (HIB-T). Suspensions of S. aureus were treated with 0.3% terpinen-4-ol for 10 min. Controls in HIB-T stood for 10 min. After centrifugation at 1,500 × g for 5 min, the pellets were fixed overnight in 2.5% glutaraldehyde in 0.1 M cacodylate buffer at room temperature. Fixed microbial pellets were processed in graded alcohols, propylene oxide, and araldite and cured for 48 h at 60°C. Ultrathin sections were stained with uranyl acetate and lead citrate and were examined with a Philips 410 transmission electron microscope at an accelerating voltage of 80 kV.
RESULTS
Inoculum effect.
Inoculum concentrations over the range 103 to 109 CFU/ml did not significantly alter the MICs or MBCs of TTO. At an inoculum of 5 × 103 CFU/ml, the MIC was 0.12% and the MBC was 0.25%. At inocula of 5 × 105 and 5 × 107 CFU/ml, the MICs were both 0.25% and the MBCs were both 0.5%. At an inoculum of 5 × 109 CFU/ml, the MIC was 0.5% and the MBC was 1%.
Bacterial killing assays.
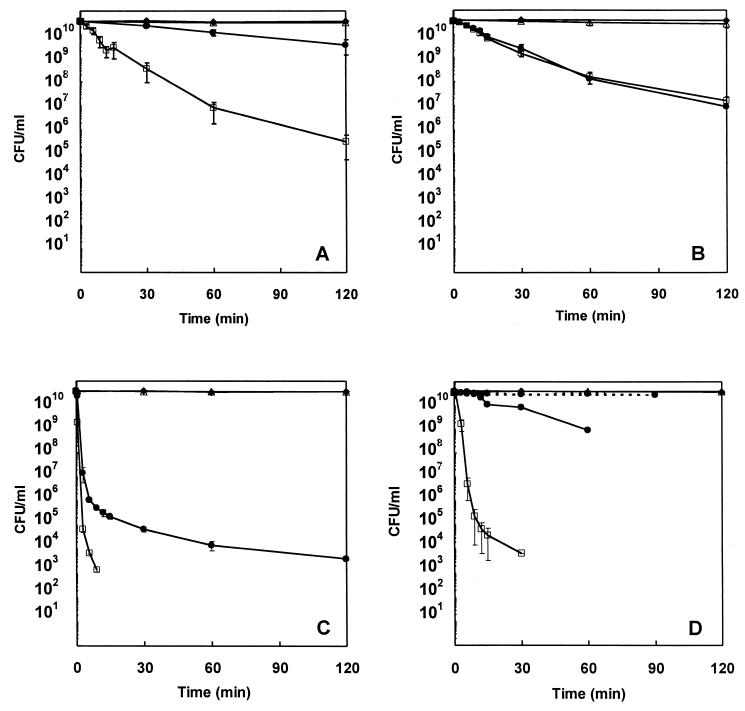
Treatment of S. aureus with TTO at two times the MIC reduced the viability of S. aureus by ∼5 log10 over 120 min, while treatment with TTO at the MIC resulted in an ∼1 log10 reduction (Fig. 1A). Treatment with 1,8-cineole at the MIC or two times the MIC resulted in greater than 3 log10 reductions over 120 min (Fig. 1B). Terpinen-4-ol or α-terpineol at one-half times the MIC had little effect on the viability of S. aureus. In contrast, treatment with either of these components at two times the MIC effected reductions of ∼7.5 and ∼6.5 log10 in 9 and 30 min, respectively (Fig. 1C and D). Treatment with terpinen-4-ol at the MIC consistently reduced the viability by 6.5 log10 over 120 min, while treatment with α-terpineol at the MIC gave inconsistent results. Representative results are shown in Fig. 1D.
FIG. 1.
Time-kill curves of S. aureus ATCC 9144 in control suspensions (♦) and after treatment with TTO (A), 1,8-cineole (B), terpinen-4-ol (C), or α-terpineol (D) at one-half the MICs (▵), the MICs (•), and two times the MICs (□). The MICs of TTO, terpinen-4-ol, and α-terpineol were 0.25% (vol/vol); and that of 1,8-cineole was 0.5%. The organisms were suspended in PBS-T. Each symbol indicates the means ± SEs for at least three replicates. The lower detection threshold was 103 CFU/ml.
Bacteriolysis.
The mean OD620 of untreated S. aureus suspensions after 120 min was 96.9% (n = 9; standard error [SE] = 1.3%) of the original. Table 1 shows the OD620s of suspensions treated with TTO or its components. Treatment with TTO or 1,8-cineole at the MIC or two times the MIC had no effect (OD620 range, 95 to 97% of the original). Treatment with terpinen-4-ol or α-terpineol reduced the OD620 slightly. Table 1 also shows the results obtained when the OD620s of dilutions of S. aureus suspensions was remeasured either 6.5 or 23 h after the initial OD620s were measured. Modest reductions in the OD620s of the TTO-treated suspensions were noted when they were measured again 6.5 h later. In contrast, large reductions were noted in the OD620s of the suspensions treated with the components of TTO, particularly those left for 23 h, in which only ∼30% of the original OD620s remained. Notably, the OD620s of the suspensions treated with terpinen-4-ol or α-terpineol at two times the MIC for 30 s were reduced to 44.9 and 67.4%, respectively, when they were read again 23 h later (data not shown).
TABLE 1.
Proportion of initial OD620 of suspensions of S. aureus remaining after treatment with TTO or its components for 120 min
| Treatment agent and concn (fold MIC)a | OD620 at 120 min/OD620 at 0 min (%) measuredb:
|
||
|---|---|---|---|
| Immediately | After 6.5 h | After 23 h | |
| Control | 96.9 ± 1.3c | 101.9 | 98.9 |
| TTO | |||
| One time | 95.1 ± 3.1 | 89.6 | NDd |
| Two times | 96.8 ± 1.4 | 85.5 | ND |
| 1,8-Cineole | |||
| One time | 94.8 ± 0.6 | ND | 40.8 |
| Two times | 95.7 ± 1.0 | ND | 33.3 |
| Terpinen-4-ol | |||
| One time | 91.0 ± 3.9 | 75.6 | 27.2 |
| Two times | 91.6 ± 1.3 | 53.4 | 28.5 |
| α-Terpineol | |||
| One time | 91.3 ± 1.5 | 78.9 | 28.4 |
| Two times | 89.4 ± 1.6 | 55.8 | 29.5 |
The MICs of TTO, terpinen-4-ol, and α-terpineol were 0.25% (vol/vol); and that of 1,8-cineole was 0.5%.
In two separate experiments, after the initial immediate measurement, the samples were retained and the OD620s were remeasured 6.5 or 23 h later.
Values are means ± SEs.
ND, not done.
Loss of 260-nm-absorbing material.
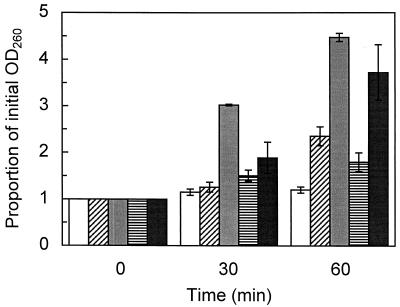
The OD260s of filtrates from control suspensions were not significantly different after 30 min but were significantly different (P = 0.020) after 60 min (Fig. 2). Significant increases in the OD260s occurred after 60 min of treatment with TTO (P = 0.019), 1,8-cineole (P = 4.3 × 10−4), terpinen-4-ol (P = 0.038), or α-terpineol (P = 0.005) at the MIC. Only 1,8-cineole treatment resulted in significant (P = 1.4 × 10−7) increases after 30 min.
FIG. 2.
Appearance of 260-nm-absorbing material in the filtrates of S. aureus control suspensions (white bars) and after treatment with the MICs of TTO (0.25%; bars with diagonal stripes), 1,8-cineole (0.5%; grey bars), terpinen-4-ol (0.25%; bars with horizontal stripes), or α-terpineol (0.25%; black bars). The organisms were suspended in PBS-T. The means ± SEs for at least three replicates are illustrated.
Loss of salt tolerance.
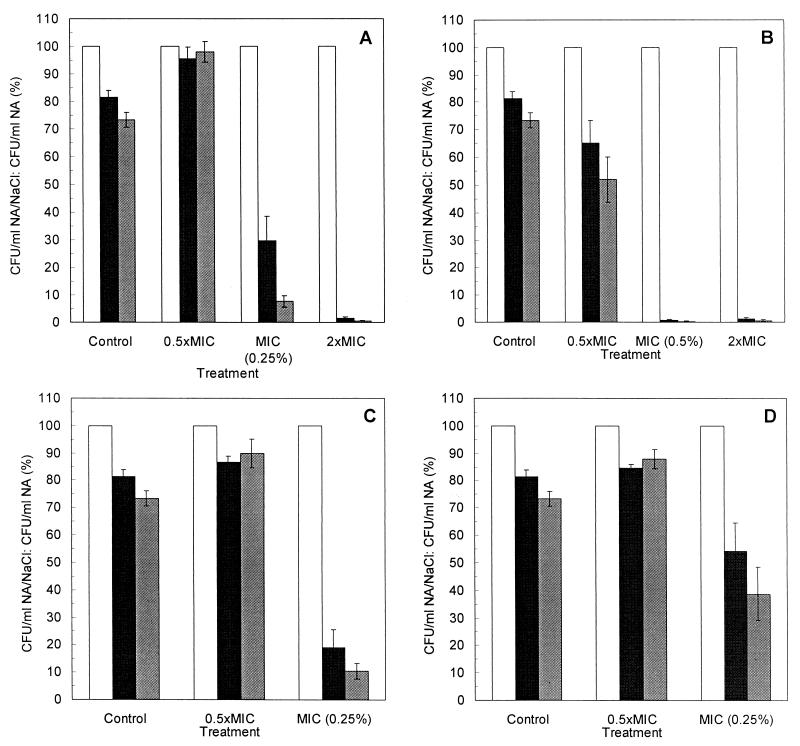
The results of tests of salt tolerance loss are shown in Fig. 3A to D. The addition of NaCl to NA reduced the colony-forming ability of untreated S. aureus cells to 81.4% (n = 22; SE = 2.5%) with NaCl at 50 g/liter and 73.3% (n = 22; SE = 2.8%) with NaCl at 75 g/liter. Treatment with TTO or 1,8-cineole at two times their MICs significantly reduced the ability of survivors to form colonies on NA-NaCl (with NaCl at 50 or 75 g/liter), with only 1.5% or less of the survivors able to form colonies. 1,8-Cineole at the MIC had a similar effect, with 0.7% or less of the survivors able to form colonies on NA-NaCl. Treatment with TTO and terpinen-4-ol at their MICs also reduced the ability of survivors to form colonies on NA-NaCl, but to a lesser extent (<30%). By treatment with α-terpineol at the MIC, the proportion of survivors able to form colonies on NA-NaCl was not significantly reduced when NaCl was used at 50 g/liter (P = 0.072) but was significantly reduced when NaCl was used at 75 g/liter (P = 0.032). Of the four treatments at one-half the MIC, only 1,8-cineole significantly reduced the colony-forming ability of the survivors, and this was only when the higher salt concentration was used (P = 0.030).
FIG. 3.
Proportion of S. aureus cells able to form colonies on NA (white bars), NA supplemented with 50 g of NaCl per liter (black bars), and NA supplemented with 75 g of NaCl per liter (grey bars) after 30 min of treatment with TTO (A), 1,8-cineole (B), terpinen-4-ol (C), or α-terpineol (D) at one-half the MICs, the MICs, and two times the MICs. The MICs of TTO, terpinen-4-ol, and α-terpineol were 0.25% (vol/vol); and that of 1,8-cineole was 0.5%. The organisms were suspended in PBS-T. The means ± SEs for at least four replicates are illustrated.
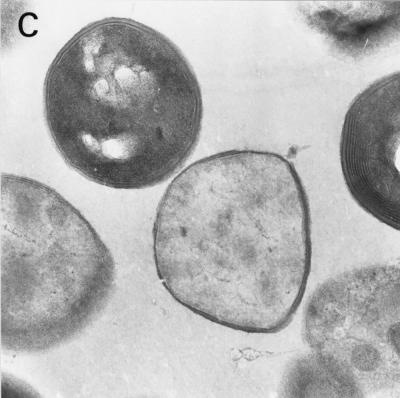
Electron microscopy of terpinen-4-ol-treated bacteria.
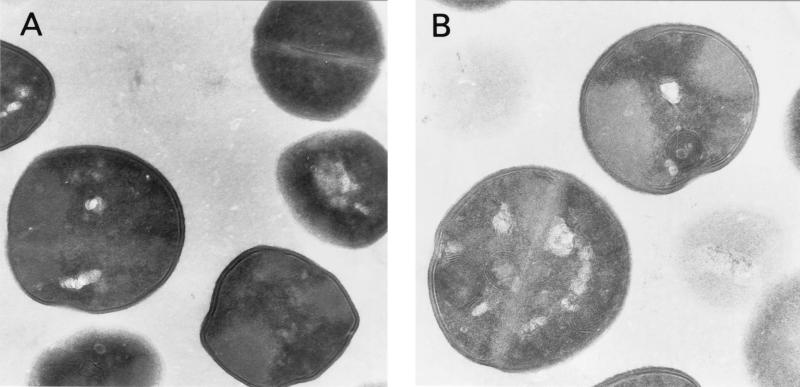
Terpinen-4-ol-treated S. aureus cells contained multilamellar, mesosomelike structures (Fig. 4B and C) that were not seen in untreated cells (Fig. 4A). In addition, the contents of some treated cells appeared depleted and amorphous (Fig. 4C).
FIG. 4.
Electron micrographs of S. aureus cells stained with uranyl acetate after no treatment (A) and after treatment with 0.3% terpinen-4-ol for 10 min (B and C). Magnifications: ×11,500 (A) and ×14,200 (B and C).
DISCUSSION
Interactions with the hydrophobic structures of bacteria play a key role in the antimicrobial actions of hydrocarbons (32). Consequently, assumptions regarding the mechanism of action of TTO have been based on the nature of its components. In the present investigation, S. aureus cells in the stationary phase of growth were killed by TTO and its components. Organisms in this growth phase are generally less sensitive to injury than those in the exponential phase (11), and this has been shown for Escherichia coli treated with TTO (16). Since antimicrobial agents that affect cellular synthetic processes often have little effect on organisms in the stationary phase of growth (15, 28), these results suggest that the principal target of TTO is not a macromolecular synthetic process.
Some antimicrobial agents cause gross membrane damage and provoke whole-cell lysis (14, 28); and this has been reported previously for essential oils from oregano, rosewood, and thyme (20). However, the major components of these oils, including carvacrol, citronellol, geraniol, and thymol (38), are not found in TTO. The failure of TTO or its components to lyse S. aureus cells suggests that their primary mechanism of action is not gross cell wall damage. Treatment-induced release of membrane-bound cell wall autolytic enzymes will induce lysis eventually (15), and this may explain the delayed lysis of S. aureus seen when suspensions were reexamined after several hours; S. aureus cells treated for as little as 30 s were disproportionately sensitive to lysis. While the activation of autolytic enzymes may have been responsible for this effect, the lysis may also have been due to weakening of the cell wall and the subsequent rupture of the cytoplasmic membrane due to osmotic pressure (rather than a specific action on the cytoplasmic membrane).
Marked leakage of cytoplasmic material is considered indicative of gross and irreversible damage to the cytoplasmic membrane (21). Many antimicrobial compounds that act on the bacterial cytoplasmic membrane induce the loss of 260-nm-absorbing material, including chlorhexidine (21), hexachlorophene (25), phenethyl alcohol (34), tetracyclines, polymyxin (12), α-pinene (1), and lemongrass oil (27). S. aureus suspensions treated with TTO or its components lost significant 260-nm-absorbing material, suggesting that nucleic acids were lost through a damaged cytoplasmic membrane.
Sublethal injury of microbial cell membranes may alter their permeability and affect the membrane's ability to osmoregulate the cell adequately or to exclude toxic materials (15). Consequently, the loss of tolerance to salts or other potentially toxic compounds may be exploited to reveal membrane damage (22) in sublethally injured bacteria. Treatment of S. aureus with TTO or its components significantly reduced the ability of the survivors to form colonies on media containing NaCl. This effect was most marked with 1,8-cineole, which was also the only compound with which treatment with one-half the MIC resulted in a significant loss of salt tolerance. These results correlate well with the OD260 results since, in each case, treatment with TTO or its components at the MICs induced the loss of salt tolerance and 260-nm-absorbing material. Notably, treatment with TTO, terpinen-4-ol, or α-terpineol at one-half the MIC had no effect on salt tolerance or actually increased it. Treatment with these agents at one-half their MICs may have killed the most susceptible cells, leaving the more robust and, possibly, more salt-tolerant cells. However, of the three treatments with which an increase in salt tolerance was noted, only treatment with TTO at one-half the MIC for 30 min reduced the viability of S. aureus. Alternatively, the treatments may have induced the expression of stress tolerance mechanisms.
Electron microscopy of terpinen-4-ol-treated cells corroborated the inability of terpinen-4-ol to lyse S. aureus and suggested membrane damage by the appearance of mesosomes and a loss of cytoplasmic material. These lesions have been reported previously after treatment with antimicrobial agents including vancomycin (29), phenethyl alcohol (11), defensins (30), and betane (3). Similar cytoplasmic losses, as well as bleb formation, were reported for E. coli treated with lemongrass oil (26), and both effects were noted when E. coli was treated with TTO (16).
The original premise was that TTO and/or its components act on microbial membranes. The loss of 260-nm-absorbing material, increased susceptibility to NaCl, the formation of mesosomes, and the loss of cytoplasmic material suggest that the cytoplasmic membrane is compromised by treatment with TTO and its components. Additional support for this idea comes from work showing that TTO treatment of E. coli or S. aureus induces the loss of potassium ions, inhibits respiration, and promotes the uptake of propidium iodide (13). In other work done with essential oils and their components, α-pinene inhibited respiratory activity in yeast mitochondria (1) and β-pinene and limonene had similar effects in intact yeast cells and isolated mitochondria (35). Collectively, these observations suggest that further work could examine the effect of treatment on microbial energy transduction, in particular, the transmembrane proton motive force.
When the mechanisms of action of lipophilic biocides have been examined, effects on the cytoplasmic membrane and/or the enzymes embedded in it have been demonstrated (1, 3, 31, 33, 35-37). The data on TTO and its components support these observations. However, the possibility remains that sites of action other than the cytoplasmic membrane exist. Furthermore, it is possible that other oil components, not examined thus far, may contribute to the antimicrobial activity of the oil by other mechanisms. Given the heterogeneous composition of TTO and the antimicrobial activities of many of its components, it seems unlikely that there is only one mechanism of action or that only one component is responsible for the antimicrobial action. Further work is required to understand fully the mechanisms involved.
Acknowledgments
This work was supported by a grant (grant UWA-24A) from the Rural Industries Research & Development Corporation, Canberra, Australian Capital Territory, Australia.
REFERENCES
- 1.Andrews, R. E., L. W. Parks, and K. D. Spence. 1980. Some effects of Douglas fir terpenes on certain microorganisms. Appl. Environ. Microbiol. 40:301-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassett, I. B., D. L. Pannowitz, and R. S. C. Barnetson. 1990. A comparative study of tea tree oil versus benzoylperoxide in the treatment of acne. Med. J. Aust. 153:455-458. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge, E. G., I. Boyd, I. Dew, M. Haswell, and C. W. G. Lowe. 1991. Electron and light microscopy of damaged bacteria, p. 135-153. In S. P. Denyer and W. B. Hugo (ed.), Mechanisms of action of chemical biocides: their study and exploitation. Blackwell Scientific Publications, Oxford, United Kingdom.
- 4.Bishop, C. D. 1995. Antiviral activity of the essential oil of Melaleuca alternifolia (Maiden and Betche) Cheel (tea tree) against tobacco mosaic virus. J. Essent. Oil Res. 7:641-644. [Google Scholar]
- 5.Brand, C., A. Ferrante, R. H. Prager, T. V. Riley, C. F. Carson, J. J. Finlay-Jones, and P. H. Hart. 2001. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 50:213-219. [DOI] [PubMed] [Google Scholar]
- 6.Brophy, J. J., N. W. Davies, I. A. Southwell, I. A. Stiff, and L. R. Williams. 1989. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J. Agric. Food Chem. 37:1330-1335. [Google Scholar]
- 7.Caelli, M., J. Porteous, C. F. Carson, R. Heller, and T. V. Riley. 2000. Tea tree oil as an alternative topical decolonisation agent for methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 46:236-237. [DOI] [PubMed] [Google Scholar]
- 8.Carson, C. F., B. D. Cookson, H. D. Farrelly, and T. V. Riley. 1995. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J. Antimicrob. Chemother. 35:421-424. [DOI] [PubMed] [Google Scholar]
- 9.Carson, C. F., K. A. Hammer, and T. V. Riley. 1995. Broth micro-dilution method for determining the susceptibility of Escherichia coli and Staphylococcus aureus to the essential oil of Melaleuca alternifolia (tea tree oil). Microbios 82:181-185. [PubMed] [Google Scholar]
- 10.Carson, C. F., and T. V. Riley. 1995. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 78:264-269. [DOI] [PubMed] [Google Scholar]
- 11.Corre, J., J. J. Lucchini, G. M. Mercier, and A. Cremieux. 1990. Antibacterial activity of phenethyl alcohol and resulting membrane alterations. Res. Microbiol. 141:483-497. [DOI] [PubMed] [Google Scholar]
- 12.Corry, J. E. L., H. Van Doorne, and D. A. A. Mossel. 1977. Recovery and revival of microbial cells, especially those from environments containing antibiotics, p. 174-196. In M. Woodbine (ed.), Antibiotics in agriculture. Proceedings of the 25th Easter School, University of Nottingham. Butterworths, London, United Kingdom.
- 13.Cox, S. D., C. M. Mann, J. L. Markham, H. C. Bell, J. E. Gustafson, J. R. Warmington, and S. G. Wyllie. 2000. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88:170-175. [DOI] [PubMed] [Google Scholar]
- 14.Denyer, S. P., and W. B. Hugo. 1991. Biocide-induced damage to the bacterial cytoplasmic membrane, p. 171-187. In S. P. Denyer and W. B. Hugo (ed.), Mechanisms of action of chemical biocides: their study and exploitation. Blackwell Scientific Publications, Oxford, United Kingdom.
- 15.Gilbert, P. 1984. The revival of microorganisms sublethally injured by chemical inhibitors, p. 175-197. In M. H. E. Andrews and A. D. Russell (ed.), The revival of injured microbes. Academic Press, London, United Kingdom.
- 16.Gustafson, J. E., Y. C. Liew, S. Chew, J. Markham, H. C. Bell, S. G. Wyllie, and J. R. Warmington. 1998. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 26:194-198. [DOI] [PubMed] [Google Scholar]
- 17.Hammer, K. A., C. F. Carson, and T. V. Riley. 1996. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil). Am. J. Infect. Control 24:186-189. [DOI] [PubMed] [Google Scholar]
- 18.Hammer, K. A., C. F. Carson, and T. V. Riley. 1997. In vitro susceptibility of Malassezia furfur to the essential oil of Melaleuca alternifolia. J. Med. Vet. Mycol. 35:375-377. [PubMed] [Google Scholar]
- 19.Hammer, K. A., C. F. Carson, and T. V. Riley. 1998. In vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J. Antimicrob. Chemother. 42:591-595. [DOI] [PubMed] [Google Scholar]
- 20.Horne, D. S., M. Holm, C. Oberg, S. Chao, and D. G. Young. 2001. Antimicrobial effects of essential oils on Streptococcus pneumoniae. J. Essent. Oil Res. 13:387-392. [Google Scholar]
- 21.Hugo, W. B., and R. Longworth. 1964. Some aspects of the mode of action of chlorhexidine. J. Pharm. Pharmacol. 16:655-662. [DOI] [PubMed] [Google Scholar]
- 22.Iandolo, J. J., and Z. J. Ordal. 1966. Repair of thermal injury of Staphylococcus aureus. J. Bacteriol. 91:134-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Organisation for Standardisation. 1996. Essential oils—oil of Melaleuca, terpinen-4-ol type (tea tree oil). ISO-4730. International Organisation for Standardisation, Geneva, Switzerland.
- 24.Jandourek, A., J. K. Vaishampayan, and J. A. Vazquez. 1998. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS 12:1033-1037. [PubMed] [Google Scholar]
- 25.Joswick, H. L., T. R. Corner, J. N. Silvernale, and P. Gerhardt. 1971. Antimicrobial actions of hexachlorophene: release of cytoplasmic materials. J. Bacteriol. 108:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunlana, E. O., S. Höglund, G. Onawunmi, and O. Sköld. 1987. Effects of lemongrass oil on the morphological characteristics and peptidoglycan synthesis of Escherichia coli cells. Microbios 50:43-59. [PubMed] [Google Scholar]
- 27.Onawunmi, G. O., and E. O. Ogunlana. 1985. Effects of lemon grass oil on the cells and spheroplasts of Escherichia coli NCTC 9001. Microbios Lett. 28:63-68. [Google Scholar]
- 28.Russell, A. D., A. Morris, and M. C. Allwood. 1973. Methods for assessing damage to bacteria induced by chemical and physical agents. Methods Microbiol. 8:95-182. [Google Scholar]
- 29.Sanyal, D., and D. Greenwood. 1993. An electron microscope study of glycopeptide antibiotic-resistant strains of Staphylococcus epidermidis. J. Med. Microbiol. 39:204-210. [DOI] [PubMed] [Google Scholar]
- 30.Shimoda, M., K. Ohki, Y. Shimamoto, and O. Kohashi. 1995. Morphology of defensin-treated Staphylococcus aureus. Infect. Immun. 63:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1994. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269:8022-8028. [PubMed] [Google Scholar]
- 32.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikkema, J., B. Poolman, W. N. Konings, and J. A. M. de Bont. 1992. Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J. Bacteriol. 174:2986-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver, S., and L. Wendt. 1967. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J. Bacteriol. 93:560-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uribe, S., J. Ramirez, and A. Peña. 1985. Effects of β-pinene on yeast membrane functions. J. Bacteriol. 161:1195-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uribe, S., P. Rangel, G. Espínola, and G. Aguirre. 1990. Effects of cyclohexane, an industrial solvent, on the yeast Saccharomyces cerevisiae and on isolated yeast mitochondria. Appl. Environ. Microbiol. 56:2114-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams, D. E., L. J. Swango, G. R. Wilt, and S. D. Worley. 1991. Effect of organic N-halamines on selected membrane functions in intact Staphylococcus aureus cells. Appl. Environ. Microbiol. 57:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Windholz, M., S. Budavari, R. F. Blumetti, and E. S. Otterbein (ed.). 1983. The Merck index, 10th ed. Merck & Co., Inc., Rahway, N.J.