Abstract
Oxacillin-resistant Staphylococcus aureus is rapidly killed by the endopeptidase lysostaphin, and the addition of β-lactam antibiotics provides synergistic killing. We investigated the possibility that β-lactams given in combination with lysostaphin would improve the activity of lysostaphin against oxacillin-resistant Staphylococcus epidermidis (ORSE), which is normally less susceptible to lysostaphin. Checkerboard synergy testing was performed for lysostaphin given in combination with oxacillin against 10 ORSE isolates for which the lysostaphin MICs were ≥ 8 μg/ml. The fractional inhibitory concentration index ranged from 0.0234 to 0.2656, indicating synergy, which was confirmed in growth curve experiments. In the rabbit model of experimental aortic valve endocarditis using an ORSE strain, the combination of lysostaphin and nafcillin was as effective as vancomycin alone and significantly better than lysostaphin or nafcillin alone. We conclude that β-lactam antibiotics given in combination with lysostaphin are synergistic against many strains of ORSE.
Oxacillin-resistant Staphylococcus epidermidis (ORSE) is increasingly implicated as a cause of hospital-acquired infections, especially nosocomial bacteremia (23). Most such infections are also resistant to multiple other antibiotics, a finding that has led to a search for alternative treatment agents (1). Lysostaphin, a peptidase produced by Staphylococcus simulans, has recently been studied as a potential therapeutic agent for use against Staphylococcus aureus (4, 7, 20).
Earlier studies in animal models of oxacillin-susceptible S. aureus-induced infection indicated that lysostaphin was an effective treatment agent against intraperitoneal infection and renal abscess models in mice and against experimental aortic valve endocarditis in dogs caused by a penicillinase-producing S. aureus strain (3, 10, 14, 15, 16, 19, 24-27, 32). Though there was evidence of efficacy, the impurity of lysostaphin preparations and wide availability of alternative antistaphylococcal antibiotics halted further development of lysostaphin as a therapeutic agent. With the development of a recombinant lysostaphin that is more than 90% pure, it has been possible to study the efficacy of lysostaphin in an experimental S. aureus endocarditis model. In the treatment of experimental aortic valve endocarditis caused by oxacillin-resistant S. aureus (ORSA) in the rabbit, lysostaphin demonstrated a bactericidal effect, with significant reductions of 8.5 log10 CFU/g in the mean aortic valve vegetation counts compared to those found in untreated controls (7).
Although lysostaphin appeared to be an effective treatment of serious experimental S. aureus infections, high-level resistance to lysostaphin developed following exposure to low subinhibitory levels of the compound (4). The development of resistance among S. aureus isolates, mediated by alterations in the crossbridge of the muropeptide, was suppressed by the concomitant administration of β-lactams with antistaphylococcal activity (4, 28). In addition to suppressing the development of lysostaphin resistance, the combinations of β-lactams given with lysostaphin were synergistic against S. aureus, as demonstrated by microdilution checkerboard testing, growth curve experiments, and an in vivo model of experimental aortic valve endocarditis caused by ORSA (4). Nafcillin given in combination with low-dose lysostaphin resulted in a mean reduction of 7.53 log10 CFU/g in aortic valve vegetation counts, whereas either agent given alone was ineffective (4). As ORSE is less susceptible to lysostaphin (22), we wanted to test the hypothesis that the combination of β-lactams and lysostaphin would improve the activity of lysostaphin. In this report, we characterize the in vitro susceptibilities of ORSE to combinations of lysostaphin and β-lactam antibiotics by the use of growth curve and checkerboard testing. Additionally, the effectiveness of combinations of lysostaphin and nafcillin was tested in a rabbit model of endocarditis caused by ORSE.
Forty-one ORSE isolates, a collection of diverse clinical strains recovered from patients with central venous catheter infections, were examined. MICs were determined by the broth microdilution method using cation-adjusted Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) and a standard inoculum of 105 CFU/ml to determine the level of susceptibility of the isolates to lysostaphin. MIC determinations were performed in the presence of 0.1% bovine serum albumin (Sigma) to prevent the adsorption of lysostaphin to polystyrene microtiter wells as described previously (20). The activity of antimicrobials in combination was assessed by the checkerboard microdilution method using microtiter trays, as previously described (4, 6, 11). Combinations of lysostaphin and oxacillin were tested at concentrations of 0.015 to 64 μg/ml and 0.125 to 512 μg/ml, respectively. Microtiter plate contents were incubated at 37°C and read at 24 and 48 h. The FIC (fractional inhibitory concentration) index was calculated by adding the FICs read at 48 h (MIC of drug A in combination with drug B/MIC of drug A alone) of lysostaphin and oxacillin. A FIC index that was > 4.0 was antagonistic, a FIC index that fell between 0.5 and 4.0 was additive or indifferent, and a FIC index that was < 0.5 was indicative of synergy. Checkerboard test results represented the average of duplicate testing for each isolate.
Growth curve assays were performed in 50 ml of cation-adjusted Mueller-Hinton broth inoculated with the test organisms at a starting concentration of 5 × 105 CFU/ml as described previously (4). Test organisms included CTS 41 (lysostaphin MIC, 16 μg/ml; oxacillin MIC, >32 μg/ml) and Butler 920 (lysostaphin MIC, 1 μg/ml; oxacillin MIC, >32 μg/ml). Synergy was defined as suppression of growth at 24 h in the presence of both antibiotics.
The rabbit model of aortic valve endocarditis, as previously described (21), was used to evaluate the antibiotic treatment regimens. Seventy-two hours after transcarotid placement of a polyethylene catheter across the aortic valve, 1 ml of an overnight culture containing approximately 109 CFU of the test organism (Butler 920; ORSE)/ml was injected intravenously through the marginal ear vein of the rabbits. Butler 920 has previously been used in the rabbit model of endocarditis (2). After 24 h, blood cultures were obtained and the rabbits were randomly assigned to one of the following treatment groups: (i) lysostaphin, 1 mg/kg of body weight intravenously (i.v.) twice a day (BID), (ii) nafcillin, 200 mg/kg intramuscularly (i.m.) BID, (iii) lysostaphin, 1 mg/kg intravenously BID; and nafcillin, 200 mg/kg i.m. BID, (iv) vancomycin, 30 mg/kg i.v. BID, or (v) no treatment (control group). After 3 days of antibiotic treatment, surviving rabbits were sacrificed with intravenous pentobarbital and the heart and kidneys were aseptically harvested from each rabbit for quantitative counts. Tissue homogenates were also plated onto Mueller-Hinton agar containing lysostaphin (16 μg/ml) in order to screen for resistant subpopulations. Isolates recovered from aortic valve tissue homogenates also underwent antimicrobial susceptibility testing by the microdilution method to check for small changes in susceptibility to lysostaphin.
For the final analysis, animals that fulfilled the following criteria were included: (i) positive blood culture at 24 h, (ii) survival for at least 24 h of antibiotic treatment; (iii) proper placement of the catheter across the aortic valve at necropsy with macroscopic evidence of aortic valve endocarditis (visible vegetations); and (iv) aortic valve vegetation and kidney tissue yielding pure cultures of the test organism.
The mean number of bacteria per gram of vegetation and kidney tissue in all treatment groups was compared by the analysis of variance. The Student-Newman-Keuls test was used to adjust for multiple comparisons. A P of <0.05 was considered statistically significant.
Forty-one ORSE isolates underwent testing of antimicrobial susceptibility to lysostaphin. Lysostaphin MICs ranged from 0.125 to >64 μg/ml, with a MIC at which 50% of the isolates tested are inhibited (MIC50) of 4 μg/ml and a MIC at which 90% of the isolates tested are inhibited of 64 μg/ml. For comparison, previous work has indicated that typical lysostaphin MICs for S. aureus range from 0.007 to 0.125 μg/ml (7).
In order to test the hypothesis that combinations of β-lactam antibiotics given with lysostaphin could improve its activity, we performed broth microdilution checkerboard testing with lysostaphin and oxacillin against 10 ORSE isolates with reduced susceptibilities to lysostaphin (MICs ≥ 8 μg/ml). The combination of lysostaphin and oxacillin was synergistic in all of the strains tested. The FIC index ranged from 0.0234 to 0.2656. This was seen in strains with low- as well as high-level resistance to oxacillin (MICs, 1 to 512 μg/ml). Synergism was also seen in checkerboard testing for a number of β-lactams given in combination with lysostaphin, including cefazolin, ceftazadime, ceftriaxone, and imipenem (data not shown).
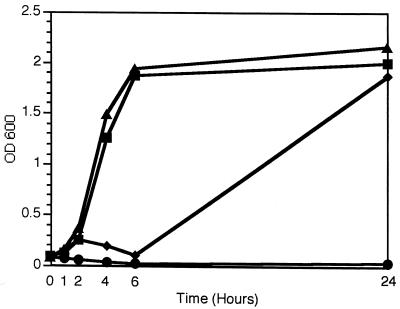
The synergistic activity of lysostaphin in combination with β-lactams was confirmed in growth curve experiments of the test organism Butler 920, shown in Fig. 1. The combination of nafcillin and lysostaphin was more effective in inhibiting growth than was either drug alone. Similar results were seen with the highly lysostaphin-resistant isolate CTS 41 (lysostaphin MIC, 16 μg/ml; data not shown).
FIG. 1.
Influence of the combination of lysostaphin and nafcillin on the growth of ORSE Butler 920. Growth curves were completed over 24 h in the presence of no antibiotics (▴); lysostaphin, 0.5 μg/ml (▪); nafcillin, 2 μg/ml (♦); or a combination of lysostaphin, 0.5 μg/ml; and nafcillin, 2 μg/ml (•). OD 600, optical density at 600 nm.
Results of treatment of experimental aortic valve endocarditis caused by the ORSE clinical strain Butler 920 are shown in Table 1. Results are similar to those of in vitro experiments. Peak concentrations of lysostaphin in serum in rabbits treated with the 1-mg/kg dose have been determined to range between 0.434 and 1 μg/ml. Concentrations in serum of lysostaphin were unaffected by the coadministration of nafcillin (data not shown). Peak concentrations in serum of nafcillin were expected to be approximately 50 μg/ml, and peak vancomycin concentrations (69 μg/ml) have been determined previously in this model (5). The combination of lysostaphin and nafcillin was as effective as vancomycin alone and was better than lysostaphin alone in the rabbit experimental endocarditis model. With lysostaphin or nafcillin given as a single agent, a modest reduction in the mean aortic valve vegetation counts compared to a control value of 1.25 or 0.79 log10 CFU/g, respectively, was seen. With vancomycin given as a single agent, a significant reduction (P < 0.05) in the mean aortic valve vegetation count, 6.17 log10 CFU/g, was achieved. The rabbits treated with the combination of nafcillin and lysostaphin had a significant reduction in mean log10 vegetation counts (5.32 log10 CFU/g) compared to rabbits treated with lysostaphin, nafcillin, or no antibiotics, and values were similar to those seen with vancomycin. Mean bacterial counts of kidney tissue were similar in all groups.
TABLE 1.
Outcome of 3-day treatment of experimental ORSE (Butler 920) aortic valve endocarditis with lysostaphin and nafcillin
| Treatment regimen | No. of samples sterile at site
|
Mean log10 CFU/g ± SD of tissue
|
||
|---|---|---|---|---|
| Aortic valve | Kidney | Aortic valve | Kidney | |
| Controls | 0/8 | 1/7 | 9.39 ± 0.49 | 2.99 ± 1.19 |
| Vancomycin | 2/9 | 4/7 | 3.22 ± 2.81a | 2.47 ± 1.93 |
| Lysostaphin (1 mg/kg BID) | 0/7 | 2/7 | 8.14 ± 1.02 | 4.11 ± 2.84 |
| Nafcillin (200 mg/kg i.m. BID) | 0/5 | 3/4 | 8.60 ± 3.36 | 3.25 ± 4.50 |
| Lysostaphin (1 mg/kg BID) + nafcillin (200 mg/kg i.m. BID) | 1/8 | 5/8 | 4.07 ± 2.28a | 2.54 ± 2.48 |
P < 0.5 versus lysostaphin alone, nafcillin alone, and controls (Student-Newman-Keuls test).
In previous experiments in the rabbit model of endocarditis due to ORSA treated with identical doses of lysostaphin (1 mg/kg i.v. BID) we found lysostaphin-resistant mutants among vegetation material (4). While the parent isolates were oxacillin resistant, lysostaphin-resistant mutants became oxacillin susceptible. We screened bacterial colonies from isolated vegetation material for susceptibility to lysostaphin and oxacillin. All colonies from rabbits treated with lysostaphin alone or the combination of lysostaphin and nafcillin were oxacillin resistant. In addition susceptibility to lyostaphin as measured in broth microdilution testing was unchanged in bacterial colonies isolated from vegetation material in comparison to the parent isolate Butler 920. There was no evidence of bacterial colonies with high-level lysostaphin resistance among rabbbits treated with either lysostaphin alone or lysostaphin given in combination with nafcillin.
We have previously demonstrated the effectiveness of lysostaphin for treatment of experimental aortic valve endocarditis due to S. aureus. In addition it was demonstrated that combinations of β-lactams and lysostaphin are synergistic and suppressed the formation of lysostaphin-resistant mutants among S. aureus (4). In the present study, we also show that combinations of β-lactams and lysostaphin are synergistic against ORSE. This was demonstrated in checkerboard testing, growth curve experiments, and the rabbit model of experimental aortic valve endocarditis. In the treatment of experimental endocarditis caused by an ORSE strain (Butler 920) for which the lysostaphin MIC was 1 μg/ml, the combination of lysostaphin and nafcillin was as effective a treatment as vancomycin alone. Although the ORSE strain tested in this model was more susceptible to lysostaphin than several other tested strains, the results were still unexpected.
Resistance to lysostaphin among S. aureus is mediated by changes in the muropeptide crossbridge. Mutations of the femA gene, which controls the addition of the second and third glycines of the forming crossbridge, result in the formation of a new crossbridge structure composed of a single glycine (9, 12, 13, 17, 18, 28). Strains with this monoglycine crossbridge are lysostaphin resistant but also become hypersusceptible to β-lactam antibiotics. This mechanism of resistance gives partial explanation to the observed synergism among S. aureus strains treated with combinations of lysostaphin and β-lactams (4).
Resistance to lysostaphin among coagulase-negative staphylococci is mediated by a distinctly different alteration of the muropeptide crossbridge. Increased incorporation of amino acids other than glycine (predominantly serine and alanine) accounts for the decreased susceptibility of many coagulase-negative staphylococci to lysostaphin (8, 12, 22, 30). This is best exemplified by the organism that produces lysostaphin, S. simulans. The lysostaphin immunity factor (lif ) gene found in S. simulans mediates an increased incorporation of serine into the third and fifth positions of the crossbridge, thereby protecting itself from the lytic action of lysostaphin (12, 29, 31). lif and epr, a gene nearly identical to lif found in Staphylococcus capitis, have significant homology to femA and femB and act together with these Fem proteins to increase the incorporation of serine into the crossbridge (8, 12, 30).
The observed synergism between lysostaphin and β-lactams for ORSE is unexplained. Exposure to β-lactams may produce changes in the muropeptide crossbridge that increase susceptibility to lysostaphin. This could be due to an alteration in glycine content or change in the degree of cross-linking in coagulase-negative staphylococci following exposure to β-lactam antibiotics. Interestingly, our study suggests that mutations in femA seen among lysostaphin-resistant S. aureus mutants are not seen in coagulase-negative staphylococci exposed to low levels of lysostaphin. In the rabbit model of endocarditis due to ORSE Butler 920, we were unable to document the presence of any oxacillin-susceptible mutants following treatment with low-dose lysostaphin indicative of mutations in the femA gene. Identical low doses of lysostaphin reliably produced lysostaphin-resistant, oxacillin-susceptible mutants among vegetation material in rabbits infected with an ORSA strain. Further studies to examine the cell wall structure and amino acid composition of muropeptide ORSE strains following exposure to β-lactams and lysostaphin are under way.
Acknowledgments
We thank Katrina Williams, Geri Hale-Cooper, and Liz Hanners for their continued technical expertise.
This work was supported in part by NIH STTR Grant R-41HL60334.
REFERENCES
- 1.Archer, G. L., and M. W. Climo. 1994. Antimicrobial susceptibility of coagulase-negative staphylococci. Antimicrob. Agents Chemother. 38:2231-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, A. J., J. L. Johnston, and G. L. Archer. 1986. Imipenem therapy of experimental Staphylococcus epidermidis endocarditis. Antimicrob. Agents Chemother. 29:748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramley, A. J., and R. Foster. 1990. Effects of lysostaphin on Staphylococcus aureus infections of the mouse mammary gland. Res. Vet. Sci. 49:120-121. [PubMed] [Google Scholar]
- 4.Climo, M. W., K. Ehlert, and G. L. Archer. 2001. Mechanism and suppression of lysostaphin resistance among oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Climo, M. W., S. M. Markowitz, D. S. Williams, C. G. Hale-Cooper, and G. L. Archer. 1997. Comparison of the in-vitro and in-vivo efficacy of FK037, vancomcyin, imipenem and nafcillin against staphylococcal species. J. Antimicrob. Chemother. 40:59-66. [DOI] [PubMed] [Google Scholar]
- 6.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and β-lactams are dynergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeHart, H. P., H. E. Heath, L. S. Heath, P. A. LeBlanc, and G. L. Sloan. 1995. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 61:1475-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonge, B. L. M., T. Sidow, Y.-S. Chang, H. Labischinski, B. Berger-Bachi, D. A. Gage, and A. Tomasz. 1993. Altered muropeptide composition in Staphylococcus aureus strains with an inactivated femA locus. J. Bacteriol. 175:2779-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon, R. E., J. S. Goodman, and M. G. Koenig. 1968. Lysostaphin: an enzymatic approach to staphylococcal disease. III. Combined lysostaphin-methicillin therapy of established staphylococcal abscesses in mice. Yale J. Biol. Med. 41:62-68. [PMC free article] [PubMed] [Google Scholar]
- 11.Domaracki, B. E., A. Evans, K. E. Preston, H. Fraimov, and R. A. Venezia. 1998. Increased oxacillin activity associated with glycopeptides in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 17:143-150. [DOI] [PubMed] [Google Scholar]
- 12.Ehlert, K., M. Tschierske, C. Mori, W. Schröder, and B. Berger-Bächi. 2000. Site-specific serine incorporation by Lif and Epr into positions 3 and 5 of the staphylococcal peptidoglycan interpeptide bridge. J. Bacteriol. 182:2635-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlert, K., W. Schroder, and H. Labischinski. 1997. Specificities of FemA and FemB for different glycine residues: FemB cannot substitute for FemA in staphylococcal peptidoglycan pentaglycine side chain formation. J. Bacteriol. 179:7573-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg, L. M., J. M. DeFranco, C. Wantanakunakorn, and M. Hamburger. 1968. Studies in experimental staphylococcal endocarditis in dogs. VI. Treatment with lysostaphin. Antimicrob. Agents Chemother. 1967:45-53. [PubMed] [Google Scholar]
- 15.Harrison, E. F., and C. B. Cropp. 1967. Therapeutic activity of lysostaphin in experimental staphylococcal infections. Can. J. Microbiol. 13:93-97. [Google Scholar]
- 16.Harrison, E. F., and W. A. Zygmunt. 1967. Lysostaphin in experimental renal infections. J. Bacteriol. 93:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopp, U., M. Roos, J. Wencke, and H. Labischinski. 1996. Staphylococcal peptidoglycan interpeptide bridge biosynthesis: a novel antistaphylococcal target? Microb. Drug Resist. 2:29-41. [DOI] [PubMed] [Google Scholar]
- 18.Maidhof, H., B. Reinicke, P. Blümel, B. Berger-Bächi, and H. Labischinski. 1991. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J. Bacteriol. 173:3506-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldham, E. R., and M. J. Daley. 1991. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J. Dairy Sci. 74:4175-4182. [DOI] [PubMed] [Google Scholar]
- 20.Patron, R. L., M. W. Climo, B. P. Goldstein, and G. L. Archer. 1999. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 43:1754-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a poly-ethylene catheter in the left side of the heart. Yale J. Biol. Med 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson, J. M., J. K. Hardman, and G. L. Sloan. 1979. Relationship between lysostaphin endopeptidase production and cell wall composition in Staphylococcus staphylolyticus. J. Bacteriol. 137:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaberg, D. R., D. H. Culver, and R. P. Gaines. 1991. Major trends in microbial etiology of nosocomial infection. Am. J. Med. 91(Suppl. 3B):725-735. [DOI] [PubMed] [Google Scholar]
- 24.Schaffner, W., M. A. Melly, and M. G. Koenig. 1967. Lysostaphin therapy in enzymatic approach to staphylococcal disease. II. In vivo studies. Yale J. Biol. Med. 39:230-244. [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffner, W., M. A. Melly, J. H. Hash, and M. G. Koenig. 1967. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J. Biol. Med. 39:215-229. [PMC free article] [PubMed] [Google Scholar]
- 26.Schuhardt, V. T., and C. A. Schindler. 1964. Lysostaphin therapy in mice infected with Staphylococcus aureus. J. Bacteriol. 88:815-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stark, F. R., C. Thornsvard, E. P. Flannery, and M. S. Artenstein. 1974. Systemic lysostaphin in man. Apparent antimicrobial activity in a neutropenic patient. N. Engl. J. Med. 291:239-240. [DOI] [PubMed] [Google Scholar]
- 28.Strandén, A. M., K. Ehlert, H. Labischinski, and B. Berger-Bächi. 1997. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 179:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss, A., G. Thumm, and F. Gotz. 1998. Influence of Lif, the lysostaphin immunity factor, on acceptors of surface proteins and cell wall sorting efficiency in Staphylococcal carnosus. J. Bacteriol. 180:4960-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugai, M., T. Fujiwara, K. Ohta, H. Komatsuzawa, M. Ohara, and H. Suginaka. 1997. epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus. J. Bacteriol. 179:4311-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschierske, M., K. Ehlert, A. M. Stranden, and B. Berger-Bachi. 1977. Lif, the lysostaphin immunity factor, complements FemB in staphylococcal peptidoglycan interpeptide bridge formation. FEMS Microbiol. Lett. 153:261-264. [DOI] [PubMed] [Google Scholar]
- 32.Zygmunt, W., and P. A. Tavormina. 1972. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog. Drug Res. 16:309-333. [DOI] [PubMed] [Google Scholar]