Abstract
Actinobacillus actinomycetemcomitans, a pathogen associated with aggressive periodontitis, resists phagocytic killing by polymorphonuclear leukocytes (PMNs). It is susceptible to ciprofloxacin, which PMNs actively accumulate. This study tested the hypothesis that ciprofloxacin-loaded PMNs are more effective at killing A. actinomycetemcomitans than control PMNs. Isolated human PMNs were loaded by brief incubation with 0.5 μg of ciprofloxacin/ml. Opsonized bacteria (ATCC 43718) were incubated at 37°C with control and ciprofloxacin-loaded PMNs and in the presence and absence of 0.5 μg of ciprofloxacin/ml. When assayed at bacteria-to-PMN ratios of 30:1 and 90:1, ciprofloxacin-loaded PMNs killed significantly more bacteria and achieved significantly shorter half times for killing than control PMNs (P < 0.05; Tukey's test). At ratios of 3:1 and 10:1, these differences were not significant.
In the periodontium and elsewhere, polymorphonuclear leukocytes (PMNs) serve as the primary host defense against bacterial infections. PMN deployment is a complex process that involves adherence to endothelial cells, transmigration into tissues, and chemotaxis toward the invading microorganisms. Once they have migrated to an infection site, PMNs phagocytose bacteria and attempt to kill them with reactive oxygen metabolites and microbicidal proteins (reviewed in references 16 and 24). Although PMNs are highly effective in defending against bacterial infections, some pathogens are difficult to kill. For example, Actinobacillus actinomycetemcomitans, a pathogen that has been implicated in aggressive periodontitis, adult periodontitis, and refractory periodontitis (9, 34), resists phagocytic killing by PMNs (10, 11, 33). Its virulence factors include a leukotoxin that kills PMNs and macrophages (32, 34). In patients with aggressive periodontitis, the defense against A. actinomycetemcomitans may be further compromised by a defect in PMN chemotaxis (12, 26). It is unclear whether this defect is inherited or induced by pro-inflammatory cytokines in the serum (1, 27). In addition, PMN oxidative killing mechanisms are not completely effective under the anaerobic conditions that prevail in the diseased periodontium (16).
Antimicrobial agents can help control bacterial infections when the host defense is impaired or overwhelmed. Ciprofloxacin and other fluoroquinolones are especially useful in this situation because they accumulate and remain active inside PMNs. The fluoroquinolones inhibit bacterial DNA topoisomerase II and produce bactericidal effects against a broad spectrum of bacteria. They are highly active against most aerobic and facultative gram-negative bacteria and possess good activity against gram-positive bacteria (22). Ciprofloxacin inhibits most strains of A. actinomycetemcomitans at an MIC of approximately 0.01 μg/ml (17, 18) and is often used in combination with metronidazole to treat mixed periodontal infections (18, 23). This combination does not inhibit gram-positive facultative bacteria and facilitates recolonization of the pocket by facultative streptococci of low pathogenic potential (9). PMNs and macrophages take up ciprofloxacin so efficiently that intracellular levels of the agent can exceed plasma levels severalfold (6, 8, 20). At least two different transport systems play a role in the accumulation of fluoroquinolones by PMNs. One is a low-affinity system that operates continuously and can be competitively inhibited by adenine, while the other is an inducible high-affinity system that can be competitively inhibited by a variety of cationic amino acids (31). With their ability to rapidly infiltrate an infection site in great numbers, PMNs have the potential to enhance resolution of an infection by increasing the local concentration of ciprofloxacin at these sites (13).
In this study, we tested the hypothesis that ciprofloxacin enhances phagocytic killing of A. actinomycetemcomitans by PMNs. Human PMNs were isolated from peripheral blood collected from healthy donors by Ficoll-Hypaque density gradient centrifugation and dextran sedimentation (4). Residual erythrocytes were eliminated by hypotonic lysis. Afterwards, PMNs were washed three times with Ca2+/Mg2+-free phosphate-buffered saline and resuspended in Hanks' balanced salt solution (HBSS). Cells isolated in this manner are generally >99% PMNs (based on cytospin preparations stained with Wright-Giemsa) and >99% viable (based on trypan blue exclusion). PMNs were loaded with 0.5 μg of ciprofloxacin/ml for 15 min at 37°C. Control PMNs were subjected to a similar incubation without the agent. Intracellular concentrations were calculated from measurements of intracellular ciprofloxacin content (31) and intracellular water space (conducted with [3H]-labeled water from NEN Life Science Products as previously described) (8).
Pure cultures of A. actinomycetemcomitans strain Y4 (ATCC 43718) were grown in brain heart infusion broth at 37°C in humidified air with 10% CO2. Bacteria were harvested from broth cultures, washed, and opsonized for 30 min at 37°C in HBSS containing 20% pooled human serum (Sigma Chemical Company, St. Louis, Mo.). The phagocytic killing assay was initiated by adding opsonized, prewarmed A. actinomycetemcomitans suspensions to polypropylene microcentrifuge tubes containing one of the following solutions: 20% human serum (in HBSS), 0.5 μg of ciprofloxacin/ml in 20% human serum, control PMNs in 20% human serum, or ciprofloxacin-loaded PMNs in 20% human serum containing 0.5 μg of ciprofloxacin/ml. Ciprofloxacin was maintained in the medium of the loaded PMNs throughout the assay, since its removal promotes efflux of the agent (13). The incubation tubes were slowly rotated end over end for 120 min at 37°C to facilitate phagocytosis. At the beginning of the incubation and every 30 min thereafter, aliquots were removed and diluted in sterile water to lyse the PMNs. After further dilution, the samples were spread on brain heart infusion broth agar plates and incubated for 48 h at 37°C in 5% CO2. Surviving A. actinomycetemcomitans colonies were counted to assess bacterial killing. Assays were conducted at several bacteria-to-PMN ratios (3:1, 10:1, 30:1, and 90:1). Half times for killing were calculated as described by van Furth et al. (28).
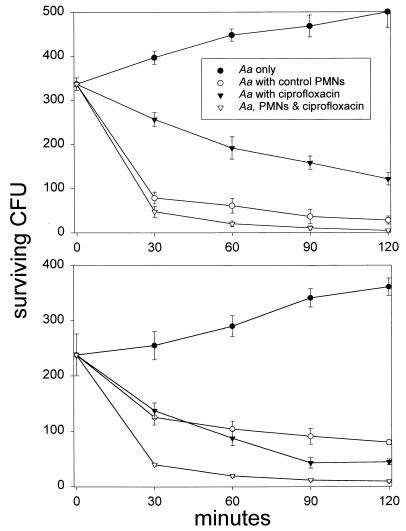
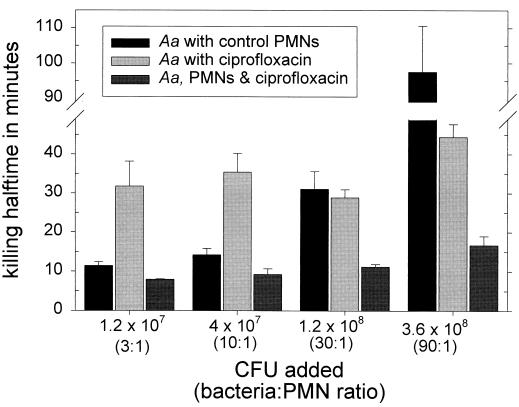
When PMNs were preincubated with 0.5 μg of ciprofloxacin/ml for 15 min prior to the addition of bacteria, the intracellular concentration of this agent reached 3.3 ± 0.7 μg/ml (approximately 330-fold higher than the MIC). Under these conditions, bacterial killing was enhanced. The magnitude of this effect was dependent on the ratio of bacteria to PMNs. At a ratio of 10 bacteria per PMN, control and ciprofloxacin-loaded PMNs rapidly killed A. actinomycetemcomitans, while ciprofloxacin alone produced a more gradual pattern of inhibition (Fig. 1, upper panel). PMNs loaded with ciprofloxacin killed significantly more bacteria than ciprofloxacin alone at every time point between 30 and 120 min (P < 0.05; Tukey's test) but did not kill significantly more bacteria than control PMNs (P > 0.05; Tukey's test). When the bacteria-to-PMN ratio was increased to 30:1 (Fig. 1, lower panel), ciprofloxacin-loaded PMNs killed significantly more bacteria than control PMNs at every time point between 30 and 120 min and significantly more bacteria than ciprofloxacin alone at 30 and 60 min (P < 0.05; Tukey's test). The half times for bacterial killing were consistent with these findings (Fig. 2). Using CFU additions that yielded bacteria-to-PMN ratios of 3:1 or 10:1, the half times for killing by control and ciprofloxacin-loaded PMNs were similar but were significantly shorter than with ciprofloxacin alone (P < 0.05). At a ratio of 30 bacteria per PMN, the half time for killing by ciprofloxacin-loaded PMNs was significantly shorter than that by control PMNs or ciprofloxacin alone (P < 0.05). At a ratio of 90:1, the half time for killing by ciprofloxacin alone or ciprofloxacin-loaded PMNs was significantly shorter than that by control PMNs (P < 0.05).
FIG. 1.
Effect of ciprofloxacin on the killing of A. actinomycetemcomitans. A. actinomycetemcomitans was added to tubes containing 20% human serum (Aa only), control PMNs in 20% serum (Aa with control PMNs), 0.5 μg of ciprofloxacin/ml in 20% serum (Aa with ciprofloxacin), or ciprofloxacin-loaded PMNs in 20% serum containing 0.5 μg of ciprofloxacin/ml (Aa, PMNs & ciprofloxacin). The suspensions were rotated at 37°C for 2 h, and aliquots were removed every 30 min and diluted for assessment of bacterial killing. Data are presented as the means ± standard errors of the means (SEM) of three separate experiments performed with PMNs obtained from different donors. The experiments represented in the upper panel utilized bacteria-to-PMN ratios of 10:1. PMNs loaded with ciprofloxacin killed significantly more bacteria than ciprofloxacin alone at 30, 60, 90, and 120 min (P < 0.05; Tukey's test), but failed to kill significantly more bacteria than control PMNs (P > 0.05). The experiments whose results are presented in the lower panel were conducted with 30 bacteria per PMN. Under these conditions, PMNs loaded with ciprofloxacin killed significantly more bacteria than control PMNs at every time point between 30 and 120 min. Moreover, ciprofloxacin-loaded PMNs killed more bacteria than ciprofloxacin alone at 30 and 60 min (P < 0.05; Tukey's test).
FIG. 2.
Half times for killing of A. actinomycetemcomitans by PMNs and ciprofloxacin. In these experiments, the indicated CFU were added to tubes containing 4 × 106 PMNs or 0.5 μg of ciprofloxacin/ml. Half times for bacterial killing were calculated from the numbers of CFU recovered after 30 min. The data are presented as the means ± SEM of three separate experiments. Within each group of bars, significant treatment effects were observed (P < 0.03; repeated measurement by analysis of variance). At lower ratios of bacteria to PMNs (in a solution containing 1.2 × 107 or 4 × 107 CFU), the half time for killing by control or ciprofloxacin-loaded PMNs was significantly shorter than by ciprofloxacin alone (P < 0.05; Tukey's test). At a ratio of 30:1, the half time for killing by ciprofloxacin-loaded PMNs was significantly shorter than that produced by control PMNs or ciprofloxacin alone (P < 0.05). At a ratio of 90:1, the half time for killing by ciprofloxacin alone or by ciprofloxacin-loaded PMNs was significantly shorter than that produced by control PMNs (P < 0.05).
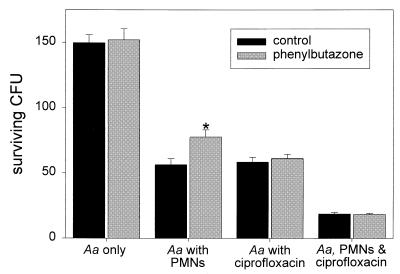
Treatment of PMNs with phenylbutazone inhibits their respiratory burst and impairs their oxygen-dependent bacterial killing mechanisms. Incubation of A. actinomycetemcomitans with phenylbutazone (0.2 mg/ml) did not affect bacterial survival (P > 0.42; paired t test) (Fig. 3). Similarly, phenylbutazone had no significant effect on bacterial killing by ciprofloxacin alone (P > 0.11). However, PMNs treated with phenylbutazone killed significantly fewer bacteria than untreated control PMNs (P = 0.015). This difference in killing between control and phenylbutazone-treated PMNs was essentially eliminated when the PMNs were loaded with ciprofloxacin (P > 0.8).
FIG. 3.
The effect of ciprofloxacin on killing by PMNs with an impaired respiratory burst. Experimental conditions were similar to those described for Fig. 1 (lower panel), except that phenylbutazone (0.2 mg/ml final concentration) was added to subaliquots of bacteria alone, bacteria plus PMNs, bacteria plus ciprofloxacin, and bacteria plus ciprofloxacin-loaded PMNs. The data are presented as the means ± SEM of CFU recovered after 30 min. The asterisk denotes a significant difference in killing between control and phenylbutazone-treated PMNs (P = 0.015; paired t test).
A. actinomycetemcomitans is one of the most common isolates from the subgingival flora of patients with aggressive forms of periodontitis (25). Since A. actinomycetemcomitans is capable of invading the gingival soft tissue (21), systemic antibiotics can be useful in eliminating this pathogen. Patients with aggressive periodontitis frequently express a PMN chemotactic defect that impairs their ability to control subgingival A. actinomycetemcomitans levels (12, 26). PMNs play a critical protective role in the host defense against pathogens associated with periodontal diseases (14, 16). If it were possible to augment PMN bactericidal activity against A. actinomycetemcomitans with an antimicrobial agent, this would enhance the effectiveness of periodontal therapy. Ciprofloxacin exhibits unique properties that make it useful in this regard. The agent distributes to the periodontium at concentrations that are fivefold higher than serum levels (5) and is highly active against A. actinomycetemcomitans and a broad spectrum of other bacteria (17, 18, 22). In addition, it is actively accumulated inside PMNs (6, 8). PMN uptake of ciprofloxacin is mediated by a low-affinity system that appears to operate continuously (31). When activated, PMNs also express a second, higher-affinity transport system. This transport system is acquired during granulocytic maturation and facilitates avid accumulation of ciprofloxacin within the cell (3).
The results of the study presented here suggest that ciprofloxacin may enhance the phagocytic killing of A. actinomycetemcomitans in an inoculum-dependent manner. At bacteria-to-PMN ratios of 10:1 or less, PMNs are capable of rapidly killing most of the available A. actinomycetemcomitans (15). Under these conditions, significantly more bacteria were killed when they were incubated with ciprofloxacin-loaded PMNs than with a comparable level of ciprofloxacin alone, but there was not a pronounced difference in killing between ciprofloxacin-loaded PMNs and control PMNs. At ratios of 30:1 or higher, it is more difficult for PMNs to cope with the massive bacterial challenge. In this situation, ciprofloxacin-loaded PMNs killed significantly more bacteria than control PMNs and the half times for phagocytic killing were significantly shorter. We speculate that antimicrobial effects produced by high levels of ciprofloxacin inside loaded PMNs supplemented their capacity for phagocytic killing. However, lower concentrations of ciprofloxacin were present in the assay medium in our experiments, so it is possible that some of the observed effects of ciprofloxacin were produced at an extracellular site of action.
Ciprofloxacin is not the only antimicrobial agent that has a propensity to concentrate inside PMNs. Clindamycin accumulates in an active form and potentiates PMN killing of certain microorganisms (7, 35). However, a previous study demonstrated that clindamycin has little effect on PMN bactericidal activity against A. actinomycetemcomitans or Eikenella corrodens (another organism associated with periodontitis) (2). At bacteria-to-PMN ratios of up to 50:1, there was no evidence that clindamycin enhances the bactericidal capacity of PMNs for these organisms. In contrast to the activity of ciprofloxacin, most strains of A. actinomycetemcomitans and E. corrodens are resistant to clindamycin (30). The agent's propensity for intracellular accumulation does not appear to overcome its poor activity against these pathogens.
While PMNs can utilize nonoxidative mechanisms to kill A. actinomycetemcomitans, their oxidative killing mechanisms also play an important role (15). Treatment with phenylbutazone impairs the PMN respiratory burst and oxidative killing. In the present study, PMNs treated with phenylbutazone killed significantly fewer bacteria than control PMNs. When the PMNs were loaded with ciprofloxacin, however, control and phenylbutazone-treated PMNs exhibited similar bactericidal activities against A. actinomycetemcomitans. With respect to this organism, accumulation of ciprofloxacin could potentially help PMNs overcome impairment of their oxygen-dependent killing mechanisms, which occurs when PMNs infiltrate hypoxic periodontitis sites or express oxidase system defects (as in chronic granulomatous disease). With certain other organisms, there appears to be a greater interaction between fluoroquinolones and oxidative killing by PMNs. As an example, previous studies suggest a synergistic interaction between ciprofloxacin and the oxygen-dependent antimicrobial mechanisms of PMNs against Staphylococcus aureus (19, 29).
In conclusion, ciprofloxacin appears to enhance PMN bactericidal activity against A. actinomycetemcomitans Y4, a leukotoxic strain that is capable of resisting phagocytic killing. This effect is most significant when PMNs are challenged by a high-concentration inoculum of bacteria, an overwhelming circumstance that is commonly associated with the acute phase of an infection. In this situation, PMNs loaded with ciprofloxacin and maintained in the presence of a therapeutic level of the agent kill A. actinomycetemcomitans more rapidly than control PMNs or comparable levels of ciprofloxacin alone. This effect may be dependent on an interaction between the antimicrobial effects of ciprofloxacin and nonoxidative PMN bactericidal mechanisms, since ciprofloxacin can enhance bacterial killing in PMNs with an impaired respiratory burst. It should be noted that the ability of A. actinomycetemcomitans to produce leukotoxin and resist killing differs among strains and may depend on environmental factors. Laboratory strains of this organism appear to be more susceptible to killing by PMNs than fresh isolates (10). This suggests that ciprofloxacin could have a greater impact on the killing of A. actinomycetemcomitans in vivo than it did in the present laboratory study. This could potentially make ciprofloxacin very useful for treating periodontal A. actinomycetemcomitans infections. Further studies are needed to determine whether ciprofloxacin significantly enhances the elimination of A. actinomycetemcomitans from individuals with aggressive or chronic forms of periodontitis.
Acknowledgments
This work was supported by U.S. Public Health Service grant DE-12601 from the National Institute for Dental and Craniofacial Research.
REFERENCES
- 1.Agarwall, S., J. P. Huang, N. Piescso, J. B. Suzuki, A. E. Richelli, and L. P. Johns. 1996. Altered neutrophil function in localized juvenile periodontitis: intrinsic or induced? J. Periodontol. 67:337-344. [DOI] [PubMed] [Google Scholar]
- 2.Baker, P. J., and M. E. Wilson. 1988. Effect of clindamycin on neutrophil killing of gram-negative periodontal bacteria. Antimicrob. Agents Chemother. 32:1521-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bounds, S. J., R. Nakkula, and J. D. Walters. 2000. Fluoroquinoline transport by human monocytes: characterization and comparison to other cells of myeloid lineage. Antimicrob. Agents Chemother. 44:2609-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human peripheral blood. Scand. J. Clin. Lab. Investig. 21:77-89. [PubMed] [Google Scholar]
- 5.Conway, T. B., F. M. Beck, and J. D. Walters. 2000. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J. Periodontol. 71:1448-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Easmon, C. S. F., and J. P. Crane. 1985. Uptake of ciprofloxacin by human neutrophils. J. Antimicrob. Chemother. 16:67-73. [DOI] [PubMed] [Google Scholar]
- 7.Faden, J., J. J. Hong, and P. L. Ogra. 1985. In vitro effects of clindamycin on neutrophil function. J. Antimicrob. Chemother. 16:649-657. [DOI] [PubMed] [Google Scholar]
- 8.Garraffo, R., D. Jambou, R. M. Chichmanian, S. Ravoire, and P. Lapalus. 1991. In vitro and in vivo ciprofloxacin pharmacokinetics in human neutrophils. Antimicrob. Agents Chemother. 35:2215-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haffajee, A. D., and S. S. Socransky. 1994. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000 5:78-111. [DOI] [PubMed] [Google Scholar]
- 10.Holm, A., S. Kalfas, and S. E. Holm. 1993. Killing of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus by human polymorphonuclear leukocytes in serum and saliva. Oral Microbiol. Immunol. 8:134-140. [DOI] [PubMed] [Google Scholar]
- 11.Kalmar, J. R., R. R. Arnold, and T. E. Van Dyke. 1987. Direct interaction of Actinobacillus actinomycetemcomitans with normal and defective (LJP) neutrophils. J. Periodontal Res. 22:179-181. [DOI] [PubMed] [Google Scholar]
- 12.Lavine, W. S., E. G. Maderazo, J. Stolman, P. A. Ward, R. B. Cogan, I. Greenblatt, and P. B. Robertson. 1979. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressive periodontitis. J. Periodontol. 14:10-19. [DOI] [PubMed] [Google Scholar]
- 13.Mandell, G. L., and E. Coleman. 2001. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 45:1794-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, D. R., I. B. Lamster, and A. I. Chasens. 1984. Role of the polymorphonuclear leukocyte in periodontal health and disease. J. Clin. Periodontol. 11:1-15. [DOI] [PubMed] [Google Scholar]
- 15.Miyasaki, K. T., M. E. Wilson, A. J. Brunetti, and R. J. Genco. 1986. Oxidative and nonoxidative killing of Actinobacillus actinomycetemcomitans by human neutrophils. Infect. Immun. 53:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyasaki, K. T. 1991. The neutrophil: mechanisms of controlling periodontal bacteria. J. Periodontol. 62:761-774. [DOI] [PubMed] [Google Scholar]
- 17.Pajukanta, R., S. Asikainen, M. Saarela, S. Alaluusua, and H. Jousimies-Somer. 1993. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand. J. Dent. Res. 101:299-303. [DOI] [PubMed] [Google Scholar]
- 18.Pavicic, M. J., A. J. Van Winkelhoff, and J. de Graaff. 1992. In vitro susceptibilities of Actinobacillus actinomycetemcomitans to a number of antimicrobial combinations. Antimicrob. Agents Chemother. 36:2634-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peman, J., E. Canton, M. T. Hernandez, and M. Gobernado. 1994. Intraphagocytic killing of Gram-positive bacteria by ciprofloxacin. J. Antimicrob. Chemother. 34:965-974. [DOI] [PubMed] [Google Scholar]
- 20.Perea, E. J., I. Garcia, and A. Pascual. 1992. Comparative penetration of lomefloxacin and other quinolones into human phagocytes. Am. J. Med. 92(Suppl. 4A):48-51. [DOI] [PubMed] [Google Scholar]
- 21.Saglie, F. R., F. A. Carranza, M. G. Newman, L. Cheng, and K. I. Lewin. 1982. Identification of tissue invading bacteria in human periodontal disease. J. Periodontol. 17:452-455. [DOI] [PubMed] [Google Scholar]
- 22.Sanders, C. C. 1990. Microbiology of fluoroquinolones, p. 1-27. In W. E. Sanders and C. C. Sanders (ed.), Fluoroquinolones in the treatment of infectious diseases. Physicians and Scientists Publishing, Glenview, Ill.
- 23.Slots, J., D. Feik, and T. Rams. 1990. In vitro antimicrobial sensitivity of enteric rods and pseudomonads from advanced adult periodontitis. Oral Microbiol. Immunol. 5:298-301. [DOI] [PubMed] [Google Scholar]
- 24.Smith, J. A. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 56:672-686. [DOI] [PubMed] [Google Scholar]
- 25.Tonetti, M. S., and A. Mombelli. 1999. Early-onset periodontitis. Ann. Periodontol. 4:39-52. [DOI] [PubMed] [Google Scholar]
- 26.Van Dyke, T. E., H. U. Horoszewicz, and R. J. Genco. 1982. The polymorphonuclear leukocyte (PMNL) locomotor defect in juvenile periodontitis. J. Periodontol. 53:682-687. [DOI] [PubMed] [Google Scholar]
- 27.Van Dyke, T. E., M. Schweinebraten, L. J. Cianociola, S. Offenbacher, and R. J. Genco. 1985. Neutrophil chemotaxis in families with localized juvenile periodontitis. J. Periodontal Res. 20:503-514. [DOI] [PubMed] [Google Scholar]
- 28.van Furth, R., T. L. VanZwet, and P. C. Leijh. 1978. In vitro determination of phagocytosis and intracellular killing by polymorphonuclear and mononuclear phagocytes, p. 1-19. In D. M. Weir (ed.), Handbook of experimental immunology, 3rd ed. Blackwell Scientific Publications, Oxford, England.
- 29.van Rensberg, C. E. J., G. Joone, and R. Anderson. 1990. Interactions of the oxygen-dependent antimicrobial system of the human neutrophil with difloxacin, ciprofloxacin, pefloxacin and fleroxacin in the intraphagocytic eradication of Staphylococcus aureus. J. Med. Microbiol. 32:15-17. [DOI] [PubMed] [Google Scholar]
- 30.Walker, C. B., J. D. Pappas, K. Z. Tyler, S. Cohen, and J. M. Gordon. 1985. Antimicrobial susceptibilities of periodontal bacteria. In vitro susceptibilities to eight antimicrobial agents. J. Periodontol. 56(Suppl.):67-74. [DOI] [PubMed] [Google Scholar]
- 31.Walters, J. D., R. J. Nakkula, and F. Zhang. 1999. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob. Agents Chemother. 43:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson, M., and B. Henderson. 1995. Virulence factors of Actinobacillus actinomycetemcomitans relevant to the pathogenesis of inflammatory periodontal diseases. FEMS Microbiol. Rev. 17:365-379. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi, N., M. Kawasaki, Y. Yamashita, K. Nakashima, and T. Koga. 1995. Role of the capsular polysaccharide-like serotype specific antigen in resistance of Actinobacillus actinomycetemcomitans to phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 63:4589-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zambon, J. J., V. I. Haraszthy, G. Hariharan, E. T. Lally, and D. R. Demuth. 1996. The microbiology of early-onset periodontitis: association of highly toxic Actinobacillus actinomycetemcomitans strains with localized juvenile periodontitis. J. Periodontol. 67:282-290. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerli, W., P. D. Lew, S. Suter, M. Wyss, and F. A. Waldvogel. 1983. In vitro efficacy of several antibiotics against intracellular S. aureus in chronic granulomatous disease. Helv. Paediatr. Acta 38:51-61. [PubMed] [Google Scholar]