Abstract
N-Chlorotaurine, an endogenous long-lived oxidant, demonstrated fungicidal activity against Candida spp. and a postantifungal effect. Secreted aspartyl proteinases, important fungal virulence factors, proved to be a first target of impact. These results provide support for the topical application of N-chlorotaurine as an antimicrobial agent in yeast infections.
N-Chlorotaurine (NCT) (Cl-HN-CH2-CH2-SO3−), the N-chloro derivative of the amino acid taurine, is the main representative of long-lived oxidants produced by stimulated human leukocytes (19). Besides immune modulatory effects (11), NCT has bactericidal, virucidal, and vermicidal activities (12, 14, 15, 20), including a lag of regrowth of bacteria (postantibiotic effect) after short, sublethal incubation times accompanied by a loss of virulence (13). It has been conceived to be useful as an antimicrobial agent in local treatment of, e.g., eye and urinary tract infections (16).
The aim of this study was a quantitative evaluation of fungicidal and postantifungal effects of NCT against Candida albicans at a concentration of 1%, which is well tolerated by human tissue in vivo (16). In addition, the influence of NCT on the production of secreted aspartyl proteinases, a superfamily of enzymes important for cell adhesion and invasion and thus fungal virulence factors, was investigated (3, 10, 18).
Pure NCT as a crystalline sodium salt (Mr = 181.57 g/mol; kindly prepared by Waldemar Gottardi, Institute of Hygiene and Social Medicine, Innsbruck, Austria) was dissolved in 0.01 M phosphate-buffered saline at pH 7.0 to 7.4.
C. albicans strains CBS 5982 (Centraal Bureau voor Schimmelculturen, Baarn, The Netherlands), ATCC 5314, ATCC 15053, and five clinical Candida sp. isolates, C. krusei ATCC 6128 and a clinical isolate, C. glabrata ATCC 6425, C. tropicalis ATCC 13803, and C. dubliniensis (kindly provided by D. Coleman, University of Dublin, Ireland) were grown on Sabouraud dextrose agar (Oxoid, Basingstoke, United Kingdom) for 48 h. Yeast cells were suspended in saline, washed twice, adjusted to ca. 1 × 108 CFU/ml, and diluted 50-fold (0.1 to 4.9 ml) to 2 × 106 CFU/ml in buffered 1% NCT solutions.
For testing of the fungicidal activity, aliquots of 250 μl were removed after incubation times of 1 to 5 h at 20 or 37°C and mixed with 125 μl of 6% sodium thiosulfate solution to inactivate NCT. Quantitative cultures of adequate dilutions on tryptic soy agar (Merck) were performed by using an automatic spiral plater (model WASP; Whitley, Shipley, United Kingdom), and CFU were counted after incubation at 37°C for 48 h (detection limit, 20 CFU/ml). Controls without NCT and controls with fungi added to NCT previously inactivated by sodium thiosulfate were performed in parallel.
For testing of the postantifungal effect (PAFE), yeasts were incubated at 20°C in 1% NCT solution for sublethal incubation times of 1 to 45 min. Subsequent to inactivation of NCT and a washing step in saline, yeasts were diluted 100-fold in prewarmed tryptic soy broth (Merck) and regrown at 37°C. Quantitative cultures were performed hourly. The lag of regrowth was calculated as the difference between the test and control cultures in the time taken to increase 1 log10 above the count at zero time (2).
For testing of the effect of NCT on production of secreted aspartyl proteinases (Saps), yeasts (2 × 106 CFU) were incubated at 20°C in 1 ml of Sap induction medium (pH 4.0) consisting of 1% bovine serum albumin (fraction V; Sigma), 2% glucose, 0.1% KH2PO4, 0.05% MgSO4, and 1% 100× minimum essential medium vitamins (Sigma). NCT saline solution (50 μl) was added once daily to final concentrations of 0.001, 0.003, 0.01, or 0.03%, or every second day to 0.1% (because of drug accumulation) for 7 days, or only once to 1%. Controls were done with addition of saline instead of NCT. After growth for 7 days under continuous rotation, the CFU count and the Sap concentration were assessed, the latter by a modified version (8) of the original protocol by Ollert et al. (17). Briefly, the suspension was centrifuged at 3,500 × g for 10 min. The amount of proteinase antigen in the supernatant was determined by absorbing aliquots to microwell plates (Greiner, Kremsmünster, Austria). Antigen detection was accomplished by the monoclonal mouse immunoglobulin G antibody FX 7-10 (2 μg/ml), which preferentially reacts with an epitope of Sap2. For better comparison of single experiments, the control values obtained in induction medium were set as 100%, and the values in the presence of NCT were calculated as the percentage in proportion to the controls for each experiment.
One-way analysis of variance and Dunnett's multiple comparison Test (Graphpad Software Inc., Calif.) were applied for statistical analysis. The data of each time point were compared for the kinetic experiments. P values of <0.05 were considered significant.
Fungicidal activity.
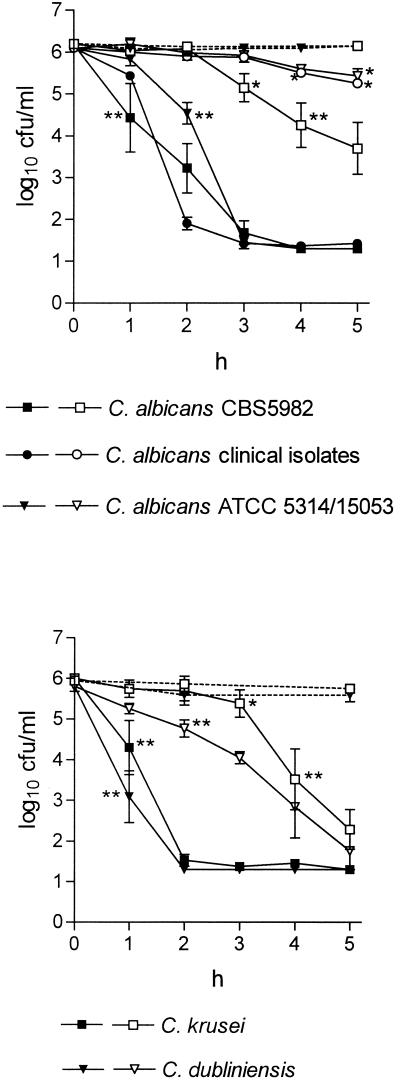
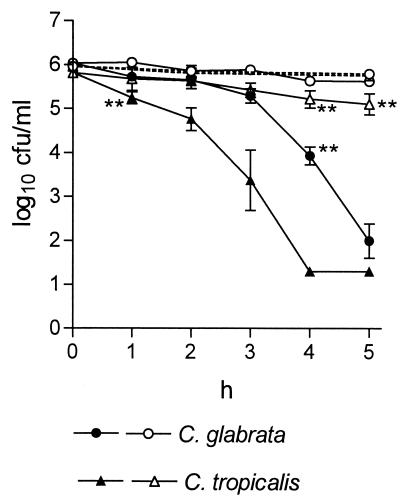
NCT demonstrated fungicidal activity enhanced by increasing temperature against all tested strains, with C. glabrata being the most resistant one, followed by C. tropicalis and then C. albicans and C. dubliniensis (Fig. 1). This activity is comparable to that found when bacteria are used (12), with the difference that incubation times needed for killing the yeasts were 5 to 10 times longer.
FIG. 1.
Fungicidal activity of 1% NCT against Candida spp. at pH 7.2 and 20°C (open symbols) or 37°C (filled symbols). Mean values ± standard errors of the mean of two (eight for CBS 5982) separate experiments. Dotted lines show controls without NCT. ∗, P < 0.05; ∗∗, P < 0.01 (borderline values of statistical significance compared to the controls are marked).
PAFE.
This study is the first report which demonstrates a yeast lag of regrowth caused by an active chlorine compound. The PAFE increased with the incubation time, similarly to what was seen in previous studies with other antifungal agents (5, 6, 9, 13), and could be detected in all strains tested. For C. albicans CBS 5982 the lag time was 0.7 to 1.4 h for 1min of incubation, 1.1 to 1.4 h for 10 min, 1.1 to 2.3 h for 30 min, and 2.6 to 2.7 h for 45 min of incubation in NCT solution (ranges of three duplicate experiments; P < 0.01 for all times). For C. glabrata (C. krusei) the values were 0.4 to 0.6 h (0.0 to 0.2 h) for 10 min, 0.4 to 0.6 h (0.4 to 0.6 h) for 30 min, and 0.5 to 0.9 h (0.8 to 1 h) for 45 min of incubation (ranges of two duplicate experiments; P < 0.01 for all times except for 10 min in C. krusei). The susceptibility of the strains in PAFE tests did not correlate with the one found in killing assays (Fig. 1), in which C. glabrata was significantly more resistant than C. krusei. The PAFE of 1% NCT on C. albicans CBS 5982 occurred as rapidly as on bacteria (1 min [13]). Although the fungal target molecules of NCT which might explain these differences between the single strains are unknown, the delayed regrowth clearly demonstrates an early impact on the fungal cell long before irreversible inactivation starts.
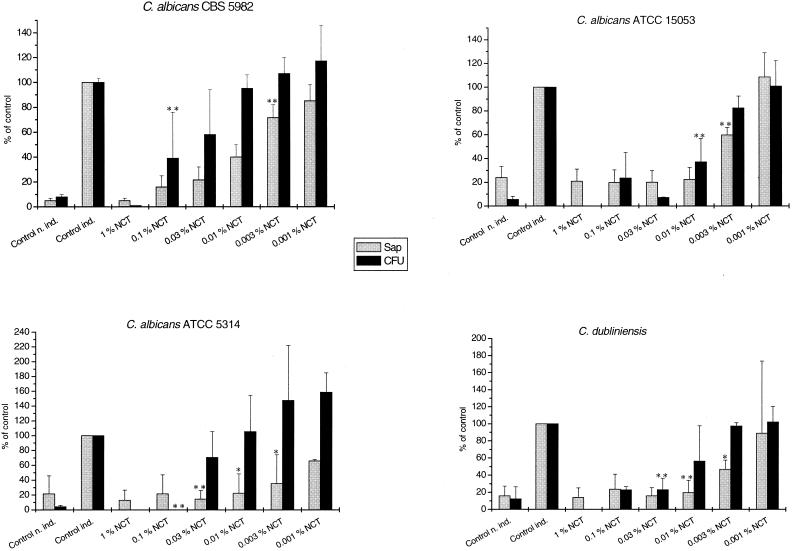
Effect of NCT on the production of Saps. C. albicans (2 × 106 CFU/ml) grown in Sap induction medium for 7 days reached 1 × 108 to 4 × 108 CFU/ml (3 × 107 to 4 × 107 CFU/ml for C. dubliniensis). When NCT was added daily to the induction medium, the Sap production was clearly impaired already at NCT concentrations that did not inhibit fungal growth (Fig. 2). If it was added once to the medium and stored until a complete decay of activity was achieved, i.e., 24 h for 0.01% and 3 weeks for 1.0% at 20°C, before addition of yeasts, neither growth nor Sap production was affected, which rules out a destruction of the medium by NCT.
FIG. 2.
Production of Saps (grey bars) and growth (black bars) of Candida albicans incubated for 7 days at 20°C in the presence of NCT. Mean values ± standard deviations of two (three to five for C. albicans CBS 5982) separate experiments. Control ind., control treated with saline in induction medium; control n. ind., control not induced (in medium without bovine serum albumin and vitamins). ∗, P < 0.05; ∗∗, P < 0.01 (borderline values are marked).
When 0.01, 0.1, or 1% NCT was mixed with the supernatants of control yeasts which had been grown in induction medium for 7 days, there was no decrease in the Sap concentration after 24 h of incubation, indicating the absence of a direct inactivation of Saps by NCT. Therefore, the mechanism of action of NCT on the proteinases remains unclear. Conceivable are a direct or indirect impact on Sap induction or production, inactivation of protein pumps located in the cell membrane, and inhibition of secretion of Saps by this means. The effect was reversible, demonstrated by full Sap production when C. albicans was regrown for 7 days after the end of an incubation period in NCT. This can be explained by repair mechanisms within the lag time (2, 5).
As in earlier findings with staphylococci and streptococci (13, 14), decrease of yeast virulence has to be considered one of the earliest reactions caused by NCT. Candida Sap mutants have been demonstrated to lose virulence in a vaginitis model (4), so that the inhibition of Sap production by drugs is expected to contribute to a therapeutic effect in opportunistic yeast infections (1, 7). Investigation of the mechanisms of action of NCT and evaluation of its therapeutical efficacy in such infections remain challenges for the future.
Acknowledgments
This study was supported by the Austrian Science Fund (grants no. P15240 and P13182-MOB) and by the Jubiläumsfonds of the Austrian National Bank (grants no. 6801/1 and 8366).
We acknowledge Manfred P. Dierich, head of our institute, and Ilse Jenewein for support.
REFERENCES
- 1.Cassone, A., F. De Bernardis, A. Torosantucci, E. Tacconelli, M. Tumbarello, and R. Cauda. 1999. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J. Infect. Dis. 180:448-453. [DOI] [PubMed] [Google Scholar]
- 2.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 3.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187-218. [DOI] [PubMed] [Google Scholar]
- 4.De Bernardis, F., S. Arancia, L. Morelli, B. Hube, D. Sanglard, W. Schafer, and A. Cassone. 1999. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J. Infect. Dis. 179:201-208. [DOI] [PubMed] [Google Scholar]
- 5.Fuursted, K., A. Hjort, and L. Knudsen. 1997. Evaluation of bactericidal activity and lag of regrowth (postantibiotic effect) of five antiseptics on nine bacterial pathogens. J. Antimicrob. Chemother. 40:221-226. [DOI] [PubMed] [Google Scholar]
- 6.Garcia, M. T., M. T. Llorente, F. Minguez, and J. Prieto. 2000. Influence of pH and concentration on the postantifungal effect and on the effects of sub-MIC concentrations of 4 antifungal agents on previously treated Candida spp. Scand. J. Infect. Dis. 32:669-673. [DOI] [PubMed] [Google Scholar]
- 7.Gruber, A., J. Berlit, C. Speth, C. Lass-Floerl, G. Kofler, M. Nagl, M. Borg von Zepelin, M. P. Dierich, and R. Wurzner. 1999. Dissimilar attenuation of Candida albicans virulence properties by human immunodeficiency virus type 1 protease inhibitors. Immunobiology 201:133-144. [DOI] [PubMed] [Google Scholar]
- 8.Gruber, A., E. Lukasser Vogl, M. Borg von Zepelin, M. P. Dierich, and R. Wurzner. 1998. Human immunodeficiency virus type 1 gp160 and gp41 binding to Candida albicans selectively enhances candidal virulence in vitro. J. Infect. Dis. 177:1057-1063. [DOI] [PubMed] [Google Scholar]
- 9.Gunderson, S. M., H. Hoffman, E. J. Ernst, M. A. Pfaller, and M. E. Klepser. 2000. In vitro pharmacodynamic characteristics of nystatin including time-kill and postantifungal effect. Antimicrob. Agents Chemother. 44:2887-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hube, B., and J. Naglik. 2001. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology 147:1997-2005. [DOI] [PubMed] [Google Scholar]
- 11.Marcinkiewicz, J., B. Nowak, A. Grabowska, M. Bobek, L. Petrovska, and B. Chain. 1999. Regulation of murine dendritic cell functions in vitro by taurine chloramine, a major product of the neutrophil myeloperoxidase-halide system. Immunology 98:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagl, M., and W. Gottardi. 1996. Enhancement of the bactericidal efficacy of N-chlorotaurine by inflammation samples and selected N-H compounds. Hyg. Med. 21:597-605. [Google Scholar]
- 13.Nagl, M., P. Hengster, E. Semenitz, and W. Gottardi. 1999. The postantibiotic effect of N-chlorotaurine on Staphylococcus aureus. Application in the mouse peritonitis model. J. Antimicrob. Chemother. 43:805-809. [DOI] [PubMed] [Google Scholar]
- 14.Nagl, M., M. W. Hess, K. Pfaller, P. Hengster, and W. Gottardi. 2000. Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. Antimicrob. Agents Chemother. 44:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagl, M., C. Larcher, and W. Gottardi. 1998. Activity of N-chlorotaurine against herpes simplex- and adenoviruses. Antivir. Res. 38:25-30. [DOI] [PubMed] [Google Scholar]
- 16.Nagl, M., B. Teuchner, E. Pöttinger, H. Ulmer, and W. Gottardi. 2000. Tolerance of N-chlorotaurine, a new antimicrobial agent, in infectious conjunctivitis—a phase II pilot study. Ophthalmologica 214:111-114. [DOI] [PubMed] [Google Scholar]
- 17.Ollert, M. W., C. Wende, M. Gorlich, C. G. McMullan Vogel, M. Borg von Zepelin, C. W. Vogel, and H. C. Korting. 1995. Increased expression of Candida albicans secretory proteinase, a putative virulence factor, in isolates from human immunodeficiency virus-positive patients. J. Clin. Microbiol. 33:2543-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staib, P., M. Kretschmar, T. Nichterlein, H. Hof, and J. Morschhauser. 2000. Differential activation of a Candida albicans virulence gene family during infection. Proc. Natl. Acad. Sci. USA 97:6102-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Test, S. T., M. B. Lampert, P. J. Ossanna, J. G. Thoene, and S. J. Weiss. 1984. Generation of nitrogen-chlorine oxidants by human phagocytes. J. Clin. Investig. 74:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazdanbakhsh, M., C. M. Eckmann, and D. Roos. 1987. Killing of schistosomula by taurine chloramine and taurine bromamine. Am. J. Trop. Med. Hyg. 37:106-110. [DOI] [PubMed] [Google Scholar]