Abstract
The azafluoranthene alkaloid eupolauridine has previously been shown to have in vitro antifungal activity and selective inhibition of fungal topoisomerase I. The present study was undertaken to examine further its selectivity and mode of action. Eupolauridine completely inhibits the DNA relaxation activity of purified fungal topoisomerase I at 50 μg/ml, but it does not stabilize the cleavage complex of either human or fungal topoisomerase I. Cleavage complex stabilization is the mode of action of topoisomerase I targeting drugs of the camptothecin family. Also, unlike camptothecin, eupolauridine does not cause significant cytotoxicity in mammalian cells. To determine if the inhibition of topoisomerase I is the principal mode of antifungal action of eupolauridine, Saccharomyces cerevisiae strains with alterations in topoisomerase genes were used in clonogenic assays. The antifungal activity of eupolauridine was not diminished in the absence of topoisomerase I; rather, the cells lacking the enzyme were more sensitive to the drug. Cell-killing activity of eupolauridine was also more pronounced in cells that overexpressed topoisomerase II. In vitro assays with the purified yeast enzyme confirmed that eupolauridine stabilized topoisomerase II covalent complexes. These results indicate that a major target for fungal cell killing by eupolauridine is DNA topoisomerase II rather than topoisomerase I, but does not exclude the possibility that the drug also acts against other targets.
Candida albicans and Cryptococcus neoformans are common causes of life-threatening fungal infections in immunocompromised patients. Increased incidence of aspergillosis also contributes to prevalent mycosis in neutropenic patients. Both drug resistance and toxicity are associated with existing antifungal therapies, and there is a need for new broad-spectrum antifungal drugs to more efficiently manage systemic fungal infections. Several potential new antifungal targets are being investigated in a search for novel drugs with reduced toxicity and less likelihood of resistance.
DNA topoisomerases are the targets of a number of antibacterial and anticancer chemotherapy agents, such as fluoroquinolones, pentamidines, acridines, camptothecins, and epipodophyllotoxins (5, 9, 16). Topoisomerases are ubiquitous enzymes that have a pivotal role in the processes of DNA replication, transcription, and recombination. The topological state of DNA is regulated by topoisomerases through the action of breaking and resealing DNA strands (23, 34). These enzymes have been classified into two major classes, based on their mode of cleaving DNA. Topoisomerase I acts by making a transient nick on one strand of duplex DNA molecule and changing the linking numbers in steps of 1. Topoisomerase II acts by transiently nicking both strands of DNA, passing another double-stranded DNA segment through the gap, and changing the linking number in steps of 2. Topoisomerase II can decatenate or catenate duplex DNA and is involved in the separation and resolution of daughter molecules at the end of replication, while topoisomerase I plays a role in the separation of complementary strands during the process of DNA replication (23, 29, 35).
Many of the topoisomerase-targeting drugs act by converting the enzyme to a DNA-damaging agent by stabilizing the covalent enzyme-DNA intermediate known as the cleavage complex. In this complex, the religation step of the topoisomerase reaction is inhibited. The presence of the cleavage complex interferes with DNA metabolism and ultimately leads to irreversible DNA damage (13, 24, 31). Camptothecins and epipodophyllotoxins are examples of anticancer drugs that target topoisomerases I and II, respectively (23, 24). In prokaryotes, the type II topoisomerases (DNA gyrase and topoisomerase IV) are the targets of cytotoxic action of quinolone antibacterial drugs (17). Early evidence that camptothecin was cytotoxic to Saccharomyces cerevisiae cells, but cells lacking topoisomerase I activity were resistant to camptothecin (15), indicated that topoisomerase I could serve as an antifungal drug target in eukaryotes. Importantly, these studies also showed that a compound could result in substantial cell killing, even though its principal target was not essential for viability. Subsequent studies in eukaryotic pathogens, including fungi and protozoa, have suggested that both topoisomerase I and topoisomerase II are viable targets for antimicrobial therapy (6, 7, 21, 30, 32). The key to successful exploitation of topoisomerase as a target is discovering or designing drugs with selectivity for the microbial enzyme over its mammalian counterpart. Selective inhibition of fungal and protozoal topoisomerase I by a number of topoisomerase-targeting agents has been reported (10, 12, 14). There are few reports of differential inhibitors of mammalian and fungal topoisomerase II (22).
Although not essential for the viability of S. cerevisiae (33), topoisomerase I has recently been shown to be essential in C. albicans and C. neoformans (6, 21). In C. albicans, reduced virulence was seen upon heterozygous TOP1 deletion; when one copy of the TOP1 gene was disrupted and the other copy was placed under a maltose-inducible and glucose-repressible promoter, virulence was further attenuated (21). Expression of TOP1 is important for the normal cellular morphology, germ-tube formation, and virulence of this organism (21). In C. neoformans, modest overexpression of TOP1 conferred sensitivity to camptothecin (6). These observations strongly support the selection of topoisomerase I as a suitable target for antifungal drug discovery against these opportunistic pathogens. The observations further suggest that both stabilization of cleavage complex (in the same manner as camptothecin) and inhibition of catalytic activity (because topoisomerase I is required for viability and virulence) could be useful mechanisms for targeting this enzyme with new antifungal therapies.
As part of a program to identify antifungal agents from natural sources, eupolauridine (molecular weight, 204.23) (Fig. 1) was previously isolated as an antifungal constituent of the root bark of Cleistopholis patens (a West African medicinal tree) and was shown to have significant antifungal activity against C. albicans (18). Larger quantities were then prepared by the procedure of Bowden et al. (3). Eupolauridine was reported by Fostel et al. to stabilize the cleavage complex with selectivity for Candida topoisomerase I, while camptothecin showed selectivity for human topoisomerase I (11). Fostel et al. did not determine the effect of eupolauridine on catalytic activity of the enzyme (11).
FIG. 1.
Structure of eupolauridine.
The present study was undertaken to determine the spectrum of antifungal action of eupolauridine and to further characterize its interaction with topoisomerases. In the course of this investigation, the question of whether topoisomerase I is the sole target of its antifungal action was addressed.
MATERIALS AND METHODS
Strains, media, and test compounds.
The yeast strains used in this study are listed in Table 1. S. cerevisiae strains JN362a, JN394, JN394t1, and JN394top2-5 were grown in YPDA medium (1% yeast extract, 2% peptone, 2% dextrose, 10 mg of adenine sulfate per liter), while JN394(YcpPDED1T2) and JN394(Ycp50) were grown in Ura− medium (synthetic medium without uracil) (25, 27).
TABLE 1.
Genetically modified yeast strains used in this study
| Straina | Descriptionb |
|---|---|
| S. cerevisiae | |
| JN362a | DNA repair proficient |
| JN394 | DNA repair deficient |
| JN394t1 | JN394 with TOP1 disruption |
| JN394top2-5 | JN394 with temperature-sensitive allele of TOP2 |
| JN394(YcpPDED1T2) | JN394 with overexpression of TOP2 |
| JN394(Ycp50) | As above without overexpression of TOP2 |
| C. albicans | |
| CAF2-1 | Wild-type TOP1 |
| CWJ429 | Heterozygous TOP1 disruption |
| CWJ477 | Conditional heterozygous or homozygous TOP1 disruption |
C. albicans strains CAF2-1 and CWJ429 were grown in YPD medium, while CWJ477 was grown either in YPD or YPM (1% yeast extract, 2% peptone, 2% dextrose or maltose) (21). Eupolauridine and camptothecin (Sigma Chemical Co.) were dissolved in dimethyl sulfoxide (DMSO) at 4 mg/ml and stored at −20°C until used.
In vitro assays of biological activity. (i) Antifungal activity.
Eupolauridine was evaluated for its antifungal activity against Candida albicans (B311), Cryptococcus neoformans (ATCC 52657), Aspergillus fumigatus (ATCC 26934), Aspergillus flavus (ATCC 9170), and Trichophyton mentagrophytes (ATCC 9972).
Growth inhibition was determined in 96-well microplates by incubating the organism with diluted samples of eupolauridine and assessing growth compared to that of controls. Growth was assessed turbidometrically by measuring A630 in a plate reader (4). Inhibitory activity was expressed as the MIC, defined as the lowest concentration that inhibited 80% of growth compared to controls. The 50% inhibitory concentration (IC50) was estimated graphically from plots of concentration versus percent growth compared to that of controls.
(ii) Cytotoxicity to mammalian cells.
The cytotoxic activity of eupolauridine was determined against human cancer cell lines SK-MEL (malignant, melanoma), KB (epidermal carcinoma, oral), BT-549 (ductal carcinoma, breast), and SK-OV-3 (ovary carcinoma). Vero cells, derived from monkey kidney fibroblasts, were used to represent noncancerous cells. The assay was performed in 96-well tissue culture-treated microplates according to a modification of the neutral red staining procedure of Borenfreund and Puerner (2).
(iii) Interactions with topoisomerases.
Measurement of the catalytic activity of topoisomerase I was based on conversion of supercoiled DNA to relaxed DNA. Cleavage complex stabilization activity was assayed by measuring the nicked DNA produced in the presence of drugs.
Catalytic activity.
Fungal topoisomerase I was purified from C. albicans as described previously (11). The specific activity of the enzyme preparation was 32,000 U/mg. Human topoisomerase I and the Topo I drug kit were purchased from Topogen, Inc. (Columbus, Ohio). A supercoiled plasmid DNA (pHOT1) with a high-affinity topoisomerase I recognition element was used as the substrate for topoisomerase I (supplied with the kit). Enzyme activity was assayed in a total volume of 20 μl (250 ng of DNA, test compound, 2 to 4 U of purified enzyme, 10 mM Tris-HCl [pH 7.9], 1 mM EDTA, 0.15 M NaCl, 0.1% bovine serum albumin, 0.1 mM spermidine, 5% glycerol) by incubating at 37°C for 30 min. The assay was performed according to the procedure provided with the kit. Reactions were terminated by rapid addition of 1% sodium dodecyl sulfate (SDS) followed by treatment with proteinase K (50 μg/ml) at 37°C for 30 min. DNA was extracted with chloroform-isoamyl alcohol (24:1 [vol/vol]) and analyzed by electrophoresis on 1% agarose gel in TAE buffer (40 mM Tris acetate, 2 mM EDTA [pH 8.5]). The gel was stained with ethidium bromide, destained in water, and photographed on a UV transilluminator followed by densitometric analysis by NIH Image 1.52. Enzyme activity was measured in terms of the percentage of substrate DNA converted to product. The concentration of test compound that prevented 50% of the substrate from being converted into the product (IC50) was calculated.
Cleavage complex formation.
For the determination of cleavage complex formation activity with topoisomerase I, the assay was performed with a minimum of 4 U of purified enzyme as described above, except that ethidium bromide was included both in the agarose gel and the buffer to resolve nicked DNA from supercoiled or relaxed species. Drug-induced stabilization of cleavage complex was determined in terms of the percent nicked DNA produced.
Covalent complex formation by topoisomerase II was assessed with purified yeast topoisomerase II prepared as previously described (8). Covalent complex formation with yeast topoisomerase II was assayed with the K+-SDS assay (19).
(iv) Cell viability assay in yeast.
Yeast strains were grown in appropriate media and at appropriate temperatures until the cells reached an A630 of 0.5 to 1.0. Cells were harvested and washed with saline twice prior to enumeration with a hemacytometer. After adjustment to a concentration of 3 × 106 cells per ml in the appropriate media, cells were incubated with the test compound or DMSO at 30°C in a total volume of 1 ml for a period of 24 h. At different time intervals, aliquots were removed, serially diluted, and plated on the appropriate agar plates. Plates were incubated at 30°C, and colonies were counted after 2 to 3 days. Percent survival is expressed relative to the number of viable colonies at the time of drug addition (0 h) and plotted against time on a logarithmic scale (25, 27).
All assays (described above) were repeated at least in duplicate, and representative data are shown in the figures and tables. Results measuring catalytic activity and covalent complex formation are shown as the mean ± standard error of three independent experiments. The DMSO concentration was 2.5% or below in all of the assays, and a DMSO control was always included in each assay.
RESULTS
The antifungal activity of eupolauridine and its cytotoxicity to mammalian cells.
The in vitro antifungal activity of eupolauridine against a wide range of pathogens is summarized in Table 2. Eupolauridine is active against C. albicans, C. neoformans, A. fumigatus, A. flavus, and T. mentagrophytes. The activity of eupolauridine against C. albicans has been reported previously (18). This is the first report of activity of eupolauridine against C. neoformans, A. fumigatus, A. flavus, and T. mentagrophytes. Eupolauridine showed no cytotoxicity to any of the mammalian cell lines tested at concentrations up to 60 μg/ml (data not shown).
TABLE 2.
Antifungal activity of eupolauridinea
| Microorganism | IC50 (μg/ml) | MIC (μg/ml) |
|---|---|---|
| Candida albicans | 5.25 | 12.5 |
| Cryptococcus neoformans | 4.8 | 50 |
| Aspergillus flavus | NDb | 2 |
| Aspergillus fumigatus | ND | 2 |
| Trichophyton mentagrophytes | ND | 2 |
Amphotericin B was used as control drug, with MICs of 0.156 for C. albicans, 0.078 for C. neoformans, and 1.25 for A. fumigatus.
ND, not determined.
Action of eupolauridine on topoisomerases.
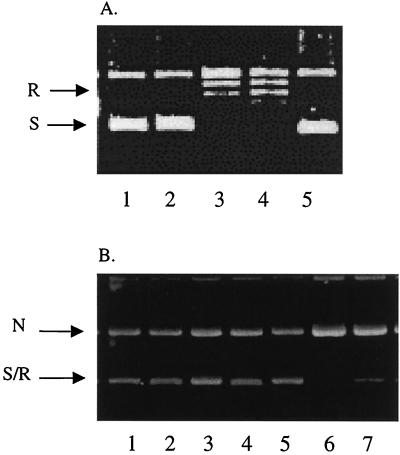
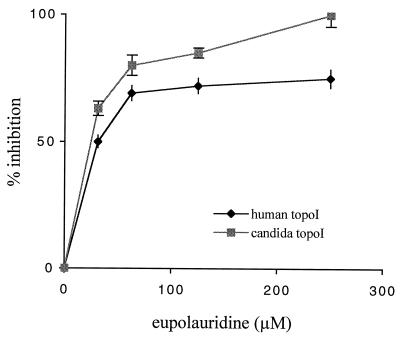
At a concentration of 250 μM, eupolauridine completely inhibited the DNA relaxation activity of fungal topoisomerase I, and no relaxed DNA was seen in lanes 1 and 2 in Fig. 3A. Conversion of supercoiled DNA (substrate) to relaxed DNA (product) in the absence of eupolauridine is shown in lanes 3 and 4 (Fig. 3A), representing enzyme controls. Eupolauridine was slightly less potent in inhibiting human topoisomerase I (IC50, 33 μM) than fungal topoisomerase I (IC50, 20 μM), as shown in Fig. 2. Although eupolauridine caused a plateau effect at 75% inhibition of human topoisomerase I, with the fungal enzyme, 100% inhibition was observed (no detectable product), so the plateau effect does not seem to be due to the limited solubility of the drug in the assay medium.
FIG. 3.
Interaction with topoisomerase I. Supercoiled plasmid DNA (250 ng) was incubated with purified topoisomerase I (2 U for the catalytic assay in panel A, 4 U for the cleavage assay in panel B) in the presence or absence of eupolauridine (250 μM) or camptothecin (100 μM). Products were analyzed by agarose gel electrophoresis. N, nicked DNA; R, relaxed DNA; S, supercoiled DNA. (A) Catalytic activity. Lanes: 1 and 2, activity of human and Candida topoisomerase I, respectively, in the presence of eupolauridine; 3 and 4, human and Candida topoisomerase I controls with no drug; 5, DNA substrate control. (B) Cleavage activity. Lanes: 1 to 5, same as in panel A; 6 and 7, activity of human and Candida topoisomerase I, respectively, in the presence of camptothecin.
FIG. 2.
Inhibition of DNA relaxation activity of topoisomerase I by eupolauridine. Indicated concentrations of eupolauridine were incubated with 250 ng of supercoiled DNA and 2 U of human or Candida topoisomerase I (topoI) in the catalytic assay. Percent inhibition was calculated after determining enzyme activity in the absence or presence of eupolauridine, as described in Materials and Methods.
In the cleavage assay, drug-stabilized enzyme-DNA complexes are trapped by SDS and digested with proteinase K to remove denatured enzyme. The resulting nicked DNA is then measured (top band in Fig. 3B). Eupolauridine did not stabilize the cleavage complex with either fungal or human topoisomerase I and failed to increase the formation of nicked DNA (lanes 1 and 2, Fig. 3B), suggesting that it does not act as a topoisomerase I poison. At the same time, camptothecin stabilized the cleavage complex and resulted in an increase of nicked DNA with both fungal and human enzyme as shown in lanes 6 and 7, Fig. 3B. These results do not agree with previous observations in which eupolauridine was found to stabilize the cleavage complex with fungal topoisomerase I and act as a topoisomerase I poison (11); however, this effect of eupolauridine was modest and required a concentration of 50 μg/ml or higher. The effect of eupolauridine on the catalytic activity of topoisomerase I was not determined in that report (11). Our results clearly show that eupolauridine inhibits the catalytic activity of both human and fungal topoisomerase I (Fig. 3A, lanes 1 and 2), but does not stabilize covalent topoisomerase-DNA complexes (Fig. 3B, lanes 1 and 2).
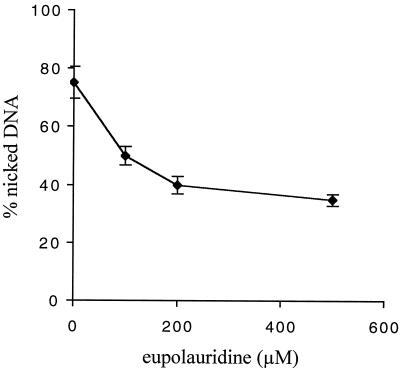
The stabilization of the cleavage complex of human topoisomerase I in the presence of 100 μM camptothecin, a known topoisomerase I poison, was monitored in the presence of increasing concentrations of eupolauridine (100 to 500 μM). Eupolauridine inhibited camptothecin-induced stabilization of covalent topoisomerase I-DNA complexes in a dose-dependent manner (Fig. 4). These results are further evidence that eupolauridine is not acting as a topoisomerase I poison and suggest that the inhibition of catalytic activity is the basis for the antagonistic activity against camptothecin.
FIG. 4.
Inhibition of camptothecin-induced stabilization of cleavage complex in the presence of eupolauridine. Cleavage assay was performed with 4 U of human topoisomerase I, 250 ng of DNA, and 100 μM camptothecin in the presence of increasing concentrations of eupolauridine (0 to 500 μM). The percent nicked DNA produced was measured as described in Materials and Methods.
Drug sensitivity in S. cerevisiae.
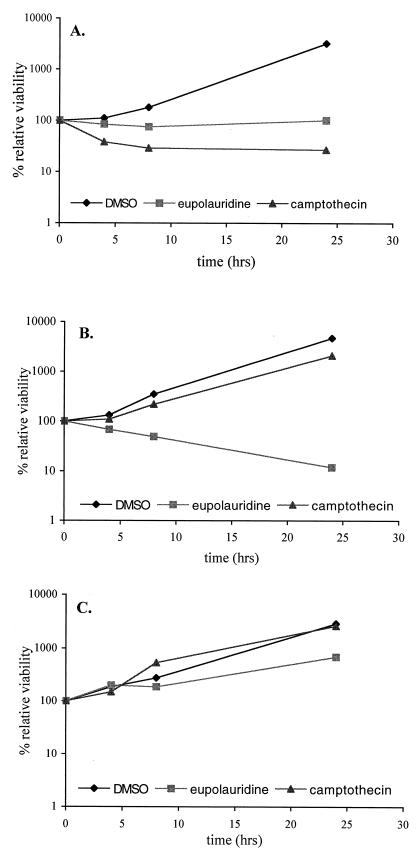
To further determine the differences in the ability of these compounds to affect topoisomerase I, we compared the effect of eupolauridine and camptothecin on clonogenic survival of drug-permeable, DNA repair-deficient strains of S. cerevisiae, JN394 and JN394t1, which carry ISE2 and rad52 mutations. These mutations increase the sensitivity of these cells to drugs (26). In addition, JN394t1 cells contain a disrupted TOP1 gene compared to the wild-type gene in JN394 cells. Consistent with previous reports (27, 28), the absence of the target enzyme resulted in diminished cytotoxicity of camptothecin in JN394t1 cells compared to JN394 cells (Fig. 5A and B). Eupolauridine remained active under conditions of topoisomerase I deletion and inhibited growth to a greater degree in JN394t1 cells than in cells of the parent strain, JN394 (Fig. 5A and B). In the repair-proficient strain (JN362a), eupolauridine had a very mild effect on cell viability (Fig. 5C) compared to its effect in JN394 (Fig. 5A). The presence of a DNA repair pathway (RAD52) in JN362a seems to be responsible for overcoming the cell-killing action of eupolauridine. This suggests that eupolauridine treatment results in DNA damage. Camptothecin did not have any cell-killing effect in this drug-permeable, repair-proficient strain (Fig. 5C).
FIG. 5.
Sensitivity of JN394 (A), JN394t1 (B), and JN362a (C) cells to camptothecin and eupolauridine. Cells were treated with drug (25 μg/ml) or DMSO (2.5%), and percent viability was determined at the indicated times relative to the time of drug addition (0 h), as described in Materials and Methods.
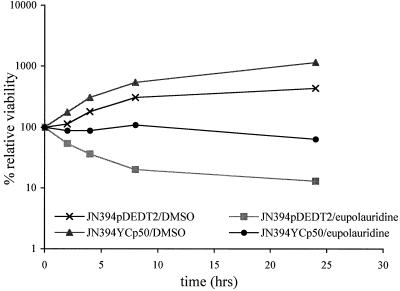
These observations (increased sensitivity of topoisomerase I-deficient cells and the involvement of a repair pathway) led to the hypothesis that another DNA enzyme might be involved in the antifungal action of eupolauridine. To test this, strains of S. cerevisiae with alterations involving the TOP2 gene were included in the clonogenic assay. The S. cerevisiae strain JN394(pDEDT2) harbors a single-copy plasmid, YcpPDED1T2, in which the yeast TOP2 gene is overexpressed from a constitutive DED1 promoter. A control strain harbors the vector Ycp50 and expresses normal levels of the TOP2 gene (25). In the TOP2-overexpressing strain JN394(pDEDT2), eupolauridine was more effective in reducing cell viability than in the corresponding control strain, as shown in Fig. 6. After incubation with eupolauridine (25 μg/ml) for 24 h in the TOP2-overexpressing strain, the viable count dropped to a value of 13% of the initial (time zero) counts, compared to 64% in the control strain; without the drug, the two strains had relative viabilities of 500 and 1,100%, respectively (Fig. 6). During the first 8 h after addition of eupolauridine (rapid growth phase), the viable counts were 20% in the overexpressing strain and 109% in the control strain. These results suggest that eupolauridine interacts with fungal topoisomerase II and are consistent with stabilization of a covalent complex by topoisomerase II.
FIG. 6.
Sensitivity of JN394(pDEDT2) and JN394(Ycp50) cells to eupolauridine. Cells were treated with drug or DMSO, and percent viability was determined relative to the time of drug addition (0 h) as described in Materials and Methods. Overexpression of TOP2 resulted in higher activity of eupolauridine.
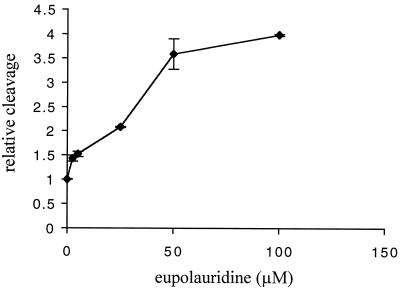
To verify that eupolauridine can stabilize topoisomerase II covalent complexes, we directly tested eupolauridine with purified S. cerevisiae topoisomerase II by using the K+-SDS assay. In this assay, enzyme is incubated with the drug and 32P-labeled DNA. The enzyme reaction is terminated by the addition of SDS, and addition of K+ results in precipitation of protein and covalent protein-DNA complexes. DNA that does not have protein covalently bound remains in solution. Therefore, the level of 32P precipitated is proportional to the level of covalent protein-DNA complexes. As shown in Fig. 7, eupolauridine increases the levels of topoisomerase II covalent complexes. The level of covalent complexes is increased by a factor of 2 at 25 μM eupolauridine. In comparison, the anticancer drug etoposide, a specific topoisomerase II poison, increases covalent complexes by a factor of 2 at a concentration of 2 μM (data not shown). Thus, eupolauridine is an active, potent topoisomerase II poison.
FIG. 7.
Covalent complex formation activity of eupolauridine. Purified yeast topoisomerase II (8 U) was incubated with the indicated concentration of eupolauridine and 32P-labeled DNA. Covalent enzyme-DNA complexes were determined by K+-SDS assay as described in Materials and Methods.
To determine whether the cytotoxicity of eupolauridine was mainly due to its effect on topoisomerase II, a strain of S. cerevisiae was used that carries a TOP2 allele that is resistant to multiple classes of topoisomerase II poisons. The top2-5 allele confers temperature-sensitive growth, and strains carrying this allele are highly resistant to mAMSA, etoposide, and other topoisomerase II poisons at its permissive temperature, 25°C. JN394 and JN394top2-5 are isogenic strains that differ only at the top2 allele. Both strains were exposed to eupolauridine at 25°C for 24 h, and cell viability was assessed. The results shown in Table 3 indicate that at 25 μg/ml, eupolauridine does not cause cell killing in the top2-5 strain, but inhibits growth (only 27% increase in cells at 24 h). In contrast, cell killing is seen with the strain with wild-type topoisomerase II (JN394), since only 25% of the cells survived compared to 100% at time 0 h of drug addition. Higher eupolauridine concentrations cause equivalent levels of cell killing for both strains (14 to 36% cell survival at 24 h after drug addition). This result agrees with the results described above that topoisomerase II is a cytotoxic target of eupolauridine. However, there may be other important targets that contribute to growth inhibition and cell killing.
TABLE 3.
Sensitivity of JN394 and JN394top2-5 to eupolauridine following a 24-h exposure
| Eupolauridine dose (μg/ml) | % Relative survival
|
|
|---|---|---|
| JN394 | JN394top2-5 | |
| 25 | 25 | 127 |
| 50 | 14 | 36 |
| 100 | 19 | 24 |
Drug sensitivity in Candida albicans.
To determine if observations in S. cerevisiae held true in C. albicans, genetically mutated strains of C. albicans were also included in the study. CWJ429 and CWJ477 have heterozygous and conditional homozygous TOP1 disruptions, respectively (21). CWJ429 is a heterozygous TOP1 knockout strain, with one copy of the gene replaced by a hisG-URA3-hisG cassette and one wild-type copy of TOP1. CWJ477 is derived from CWJ429, in which the wild-type copy of TOP1 is placed under the regulation of a maltose-inducible and glucose-repressible promoter (21). When grown in the presence of glucose (YPD), expression of the TOP1 gene is suppressed and the strain approximates the homozygous TOP1 knockout. In the presence of maltose (YPM), a single copy of TOP1 is expressed, thus representing a condition analogous to a heterozygous knockout. Strain CAF2-1 is the parent strain and was used as the control for wild-type TOP1.
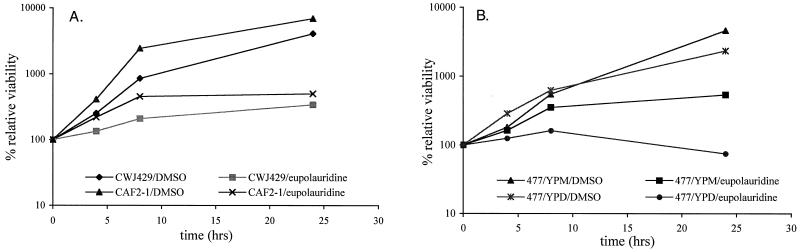
Eupolauridine was observed to inhibit growth in CAF2-1 cells as well as CWJ429 cells (Fig. 8A). It was also effective in inhibiting growth of CWJ477 cells grown in YPM (Fig. 8B). When CWJ477 cells were grown in YPD, growth inhibition by eupolauridine was enhanced in these cells with reduced expression of TOP1 (Fig. 8B). These observations are similar to the effect of eupolauridine on comparable S. cerevisiae strains (JN394 and JN394t1, Fig. 5A and B) and are consistent with growth inhibition mediated by inhibition of topoisomerase II.
FIG. 8.
(A) Sensitivity of CAF2-1 and CWJ429 cells to eupolauridine. Cells were treated with drug or DMSO, and percent viability was determined relative to the time of drug addition (0 h). Eupolauridine was effective in reducing the cell viability of the parental strain as well as the strain with heterozygous TOP1 disruption. (B) Sensitivity of CWJ477 cells to eupolauridine. Cells were grown in YPD or YPM to represent the conditions of homozygous or heterozygous TOP1 disruption, respectively. They were treated with drug or DMSO in the presence of YPD or YPM, and percent viability was determined relative to the time of drug addition (0 h). Eupolauridine was more effective in reducing cell viability of CWJ477 in the presence of dextrose than in the presence of maltose.
DISCUSSION
The results presented here demonstrate that the effect of eupolauridine on topoisomerase I does not resemble that of camptothecin. Eupolauridine is not a topoisomerase I poison, but does appear to inhibit the catalytic activity of the enzyme. Eupolauridine showed no growth inhibition activity in a number of mammalian cell lines tested, although this lack of cytotoxicity might be in part attributable to the binding of eupolauridine to serum albumin; such binding is characteristic of polyaromatic hydrocarbons. In our study, eupolauridine inhibited the DNA relaxation activity (catalytic activity) of topoisomerase I with modest selectivity towards the fungal enzyme in comparison to human enzyme (Fig. 2). There was no increase in the formation of nicked DNA in the cleavage assay with either human or fungal topoisomerase I in the presence of eupolauridine at concentrations up to 250 μM (Fig. 3B), indicating that eupolauridine did not stabilize the enzyme-DNA cleavable complex. Furthermore, assessment of cleavable complex formation with purified S. cerevisiae topoisomerase I also failed to detect any increase in cleavage in the presence of various concentrations of eupolauridine (data not shown). Taken together, these results indicate that eupolauridine does not have a topoisomerase I-poisoning activity.
Agents that act as inhibitors of topoisomerase I activity might be expected to antagonize the cleavage complex stabilization activity of a topoisomerase I poison such as camptothecin. When human topoisomerase I was incubated with supercoiled DNA substrate and camptothecin in the presence of increasing concentrations of eupolauridine, a decrease in the amount of nicked DNA was observed (Fig. 4). This activity of eupolauridine is similar to the activity of pyrazoloacridine, a catalytic inhibitor of mammalian topoisomerase I that antagonizes topotecan-induced DNA cleavage (1). Similarly, aclarubicin is a catalytic inhibitor of topoisomerase II and was reported to antagonize cytotoxicity of topoisomerase II poisons such as etoposide and amsacrine (20). It was suggested earlier (11) that eupolauridine may bind or intercalate into DNA. However, no experimental documentation was presented. Nevertheless, this characteristic might account for the enzyme-inhibitory activity of this compound and, if so, would limit its therapeutic usefulness.
In the present investigation, results from clonogenic assays in drug-permeable yeast cells confirm the in vitro studies with purified enzyme and support the conclusion that eupolauridine is not acting as a topoisomerase I poison: cells lacking topoisomerase I (JN394t1) were more sensitive to eupolauridine than the cells with wild-type amounts of topoisomerase I (JN394). If a drug is a topoisomerase poison, such as camptothecin, the absence of the target enzyme results in resistance to the drug. On the other hand, an increased amount of target enzyme results in increased efficacy. For example, overexpression of TOP1 in C. neoformans conferred sensitivity to camptothecin (6). For an inhibitor of catalytic activity, decreased target enzyme would be expected to result in higher efficacy. In our studies with C albicans, the wild type (CAF2-1) as well as a strain with heterozygous TOP1 deletion (CWJ429) were sensitive to eupolauridine. When the expression of TOP1 was further suppressed by growing CWJ477 in glucose, eupolauridine was more effective in decreasing the cell viability. Although inhibition of catalytic activity of topoisomerase I may play some role in sensitivity to eupolauridine in C. albicans, the results obtained with S. cerevisiae cells strongly suggest that topoisomerase I is not an important target for this drug.
The clonogenic studies led to a second line of investigation, since these results suggested that topoisomerase I was not the target responsible for the antifungal activity of eupolauridine. Since several compounds have been found to be active against both enzymes (topoisomerases I and II), we examined the activity of eupolauridine against topoisomerase II. One test of an agent as a topoisomerase II poison is to determine whether increased expression of topoisomerase II leads to hypersensitivity to the agent (25). In the present study, we observed higher sensitivity of a topoisomerase II-overexpressing strain of S. cerevisiae to eupolauridine (Fig. 6). This observation suggests the involvement of topoisomerase II in the cell-killing action of eupolauridine and guided us to do a biochemical investigation to examine whether eupolauridine affects topoisomerase II. Assays with purified yeast topoisomerase II confirmed that eupolauridine stabilizes topoisomerase II cleavage complexes. Although topoisomerase II has been partially purified from C. albicans (32), we have utilized yeast topoisomerase II in this study. The possibility that topoisomerase II is a target for eupolauridine is consistent with the observation that cells that lacked topoisomerase I were hypersensitive to eupolauridine. It has previously been demonstrated that yeast cells that lack topoisomerase I are hypersensitive to the topoisomerase II poisons such as amsacrine (28). This has been attributed to increased reliance on topoisomerase II during replication for processes requiring a topoisomerase. However, yeast cells carrying the top2-5 allele showed only slight resistance to eupolauridine. The top2-5 allele confers resistance to many topoisomerase II poisons, including both intercalating and nonintercalating poisons (19). The sensitivity of the top2-5 strain suggests that the cytotoxic targets of eupolauridine may include enzymes other than DNA topoisomerases.
The present investigation extends our understanding of the mechanism of antifungal activity of eupolauridine and suggests the utility of this class of compounds as tools for understanding topoisomerase-related mechanisms of action. The observations made in the cell-based assays are fully supported by the biochemical studies. The findings indicate that eupolauridine's antifungal activity is mediated via interaction with topoisomerase II, as opposed to topoisomerase I, as previously suggested. Of course, other cellular mechanisms are not excluded. However, these findings suggest that fungal topoisomerases represent viable targets for exploration in the search for new antifungal agents.
Acknowledgments
This work was supported by a grant from the Public Health Service, National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant no. AI-33865). J.L.N. and M.M. were supported by grant CA52814, by core grant CA21765, and by the American Lebanese Syrian Associated Charities (ALSAC). The United States Department of Agriculture, Agriculture Research Service Specific Cooperative Agreement no. 58-6408-7-012 is also acknowledged for partial support of the work.
We thank William Fonzi, Georgetown University, and Yigal Koltin, Millennium Pharmaceuticals, Inc., for providing C. albicans strains used in this study.
REFERENCES
- 1.Adjei, A. A., M. Charron, E. K. Rowinksky, P. A. Svigen, J. Miller, J. M. Reid, J. Sebolt-Leopold, M. M. Ames, and S. H. Kaufmann. 1998. Effect of pyrazoloacridine (NSC 366140) on DNA topoisomerases I and II. Clin. Cancer Res. 4:683-691. [PubMed] [Google Scholar]
- 2.Borenfreund, E., and J. A. Puerner. 1985. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 24:119-124. [DOI] [PubMed] [Google Scholar]
- 3.Bowden, B. F., K. Picker, E. Ritchie, and W. C. Taylor. 1975. Constituents of eupoma species. IV. Structure and synthesis of eupolauridine (EL Base-1) and some observations on the structure of eupolauramine (EL Base-2) and hydroxyeupolauramine (EL Base-3). Aust. J. Chem. 28:2681. [Google Scholar]
- 4.Chand, S., I. Lusunzi, D. A. Veal, L. R. Williams, and P. Karuso. 1994. Rapid screening of the antimicrobial activity of extracts and natural products. J. Antibiot. 47:1295-1304. [DOI] [PubMed] [Google Scholar]
- 5.Chen, A. Y., and L. F. Liu. 1994. DNA topoisomerases: essential enzymes and lethal targets. Annu. Rev. Pharmacol. Toxicol. 34:191-218. [DOI] [PubMed] [Google Scholar]
- 6.Del Poeta, M., D. L. Toffaletti, T. H. Rude, C. C. Dykstra, J. Heitman, and J. R. Perfect. 1999. Topoisomerase I is essential in Cryptococcus neoformans: role in pathobiology and as an antifungal target. Genetics 152:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykstra, C. C., D. R. McClernon, L. P. Elwell, and R. R. Tidwell. 1994. Selective inhibition of topoisomerases from Pneumocystis carinii compared with that of topoisomerases from mammalian cells. Antimicrob. Agents Chemother. 38:1890-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsea, S. H., Y. Hsiung, J. L. Nitiss, and N. Osheroff. 1995. A yeast type II topoisomerase selected for resistance to quinolones. Mutation of histidine 1012 to tyrosine confers resistance to nonintercalative drugs but hypersensitivity to ellipticine. J. Biol. Chem. 270:1913-1920. [DOI] [PubMed] [Google Scholar]
- 9.Figgitt, D. P., S. P. Denyer, P. M. Dewick, D. E. Jackson, and P. Williams. 1989. Topoisomerase II: a potential target for novel antifungal agents. Biochem. Biophys. Res. Commun. 160:257-262. [DOI] [PubMed] [Google Scholar]
- 10.Fostel, J., and D. Montgomery. 1995. Identification of the aminocatechol A-3253 as an in vitro poison of DNA topoisomerase I from Candida albicans. Antimicrob. Agents Chemother. 39:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fostel, J. M., D. Montgomery, and L. L. Shen. 1992. Characterization of DNA topoisomerase I from Candida albicans as a target for drug discovery. Antimicrob. Agents Chemother. 36:2131-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fostel, J. M., D. Montgomery, and P. Lartey. 1996. Comparison of responses of DNA topoisomerases I from Candida albicans and human cells to four new agents which stimulate topoisomerases-dependent DNA nicking. FEMS Microbiol. Lett. 138:105-111. [DOI] [PubMed] [Google Scholar]
- 13.Froelich-Ammon, S. J., and N. Osheroff. 1995. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J. Biol. Chem. 270:21429-21432. [DOI] [PubMed] [Google Scholar]
- 14.Goldman, G. H., C. Yu, H.-Y. Wu, M. M. Sanders, E. J. La Voie, and L. F. Liu. 1997. Differential poisoning of human and Aspergillus nidulans DNA topoisomerase I by bi- and terbenzimidazoles. Biochemistry 36:6488-6494. [DOI] [PubMed] [Google Scholar]
- 15.Goto, T., and J. C. Wang. 1985. Cloning of yeasts TOP1, the gene encoding DNA topoisomerase I, and construction of mutants defective in both DNA topoisomerase I and DNA topoisomerase II. Proc. Natl. Acad. Sci. USA 82:7178-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, M., A. Fujimori, and Y. Pommier. 1995. Eukaryotic DNA topoisomerases I. Biochim. Biophys. Acta 1262:1-14. [DOI] [PubMed] [Google Scholar]
- 17.Hooper, D. C. 1999. Mode of action of fluoroquinolones. Drugs 58:6-10. [DOI] [PubMed] [Google Scholar]
- 18.Hufford, C. D., S. Liu, A. M. Clark, and B. O. Oguntimein. 1987. Anticandidal activity of eupolauridine and onychine, alkaloids from Cleistopholis patens. J. Nat. Prod. 40:961-964. [DOI] [PubMed] [Google Scholar]
- 19.Jannatipour, M., Y. X. Liu, and J. L. Nitiss. 1993. The top2-5 mutant of yeast topoisomerase II encodes an enzyme resistant to etoposide and amsacrine. J. Biol. Chem. 268:18586-18592. [PubMed] [Google Scholar]
- 20.Jensen, P. B., B. S. Sorensen, E. J. F. Demant, M. Sehested, P. S. Jensen, L. Vindelov, and H. H. Hansen. 1990. Antagonistic effect of aclarubicin on the cytotoxicity of etoposide and 4′-(9-acridinylamino)methanesulfon-m-aniside. Cancer Res. 50:3311-3316. [PubMed] [Google Scholar]
- 21.Jiang, W., D. Gerhold, E. B. Kmiec, M. Hauser, J. M. Becker, and Y. Koltin. 1997. The topoisomerase I gene from Candida albicans. Microbiology 143:377-386. [DOI] [PubMed] [Google Scholar]
- 22.Keller, B. A., S. Patel, and L. M. Fisher. 1997. Molecular cloning and expression of the Candida albicans TOP2 gene allows study of fungal DNA topoisomerase II inhibitors in yeast. Biochem. J. 324:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, L. F. 1989. DNA topoisomerase poisons as antitumor drugs. Annu. Rev. Biochem. 58:351-375. [DOI] [PubMed] [Google Scholar]
- 24.Liu, L. F., P. Duann, C. T. Lin, P. D'Arpa, and J. Wu. 1996. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 13:803-844. [DOI] [PubMed] [Google Scholar]
- 25.Nitiss, J. L., Y. X. Liu, P. Harbury, M. Jannatipour, R. Wasserman, and J. C. Wang. 1992. Amsacrine and etoposide hypersensitivity of yeast cells overexpressing DNA topoisomerase II. Cancer Res. 52:4467-4472. [PubMed] [Google Scholar]
- 26.Nitiss, J. L. 1994. Yeast as a genetic model system for studying topoisomerase inhibitors. Adv. Pharmacol. 29B:201-226. [DOI] [PubMed] [Google Scholar]
- 27.Nitiss, J. L., and J. C. Wang. 1996. Mechanisms of cell killing by drugs that trap covalent complexes between DNA topoisomerase and DNA. Mol. Pharmacol. 50:1095-1102. [PubMed] [Google Scholar]
- 28.Nitiss, J. L., P. Pourquier, and Y. Pommier. 1997. Aclacinomycin A stabilizes topoisomerase I covalent complexes. Cancer Res. 57:4564-4569. [PubMed] [Google Scholar]
- 29.Pommier, Y. 1998. Diversity of DNA topoisomerases I and inhibitors. Biochimie 80:255-270. [DOI] [PubMed] [Google Scholar]
- 30.Ray, S., P. K. Sadhukhan, N. B. Mandal, S. B. Mahato, and H. K. Majumdar. 1997. Dual inhibition of DNA topoisomerases of Leishmania donovani by novel indolyl quinolines. Biochem. Biophys. Res. Commun. 230:171-175. [DOI] [PubMed] [Google Scholar]
- 31.Rothenberg, M. L. 1997. Topoisomerase I inhibitors: review and update. Ann. Oncol. 8:837-855. [DOI] [PubMed] [Google Scholar]
- 32.Shen, L. L., J. Baranowski, J. Fostel, D. A. Montgomery, and P. A. Lartey. 1992. DNA topoisomerases from pathogenic fungi: targets for the discovery of antifungal drugs. Antimicrob. Agents Chemother. 36:2778-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thrash, C., A. T. Bankier, B. G. Barrell, and R. Sternglanz. 1985. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc. Natl. Acad. Sci. USA 82:4374-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vosberg, H. P. 1985. DNA topoisomerases: enzymes that control DNA conformation. Curr. Top. Microbiol. Immunol. 114:19-102. [DOI] [PubMed] [Google Scholar]
- 35.Wang, J. C. 1985. DNA topoisomerase. Annu. Rev. Biochem. 54:665-697. [DOI] [PubMed] [Google Scholar]