Abstract
The BlaB metallo-β-lactamase of Chryseobacterium meningosepticum CCUG4310 was overproduced in Escherichia coli by means of a T7 promoter-based expression system. The overproducing system, scaled up in a 15-liter fermentor, yielded approximately 10 mg of BlaB protein per liter, mostly released in the culture supernatant. The enzyme was purified by two ion-exchange chromatographic steps with an overall yield of 66%. Analysis of the kinetic parameters revealed efficient activities (kcat/Km ratios of >106 M−1 s−1) toward most penam and carbapenem compounds, with the exception of the 6-α-methoxypenam derivative temocillin and of biapenem, which were poorer substrates. Hydrolysis of cephalosporins was overall less efficient, with a remarkable variability that was largely due to variable affinities of the BlaB enzyme for different compounds. BlaB was also able to hydrolyze serine-β-lactamase inhibitors, including β-iodopenicillanate, sulbactam and, although less efficiently, tazobactam.
Metallo-β-lactamases are emerging resistance determinants among gram-negative pathogens (15-17, 20). They can contribute to the intrinsic β-lactam resistance of some species that carry resident metallo-β-lactamase genes (e.g., Bacillus cereus, a cluster of Bacteroides fragilis, Stenotrophomonas maltophilia, some Aeromonas spp., Chryseobacterium meningosepticum, Chryseobacterium indologenes, and Legionella gormanii) and to acquired β-lactam resistance in Enterobacteriaceae, pseudomonads, and other nonfastidious gram-negative nonfermenters following acquisition of metallo-β-lactamase determinants carried by mobile genetic elements (3).
Metallo-β-lactamases are included in molecular class B and in group 3 of the functional classification (5). Remarkable structural and functional heterogeneities exist among these enzymes. Structural analysis identified three major molecular lineages, subclasses B1, B2, and B3, with a degree of sequence divergence that can be higher than 90% between members of different subclasses (14, 19). Functional analysis revealed variable substrate specificities and heterogeneous kinetic behaviors and identified three different subgroups, determined to be 3a (showing a broad substrate specificity), 3b (acting as specific carbapenemases), and 3c (overall preferring cephalosporin substrates) (22). However, a constant feature of metallo-β-lactamases that contributes to their clinical relevance is the ability to efficiently hydrolyze carbapenem compounds, which are not hydrolyzed by the majority of active-site serine β-lactamases (4).
Although some of these enzymes have been subjected to detailed biochemical (2, 11-13, 19) and structural analysis (6, 7, 8, 9), the catalytic mechanism(s), interaction with metal, and structure-function relationships are only partially understood. Additional biochemical and structural analyses are essential to the understanding of these aspects and are also strategic to the development of new metallo-β-lactamase inhibitors.
In this work we developed a system for overproduction and purification of the BlaB metallo-β-lactamase of C. meningosepticum, and we carried out a biochemical characterization of the enzyme.
MATERIALS AND METHODS
Bacterial strains and genetic vectors.
Escherichia coli DH5α (25) was used as the host for recombinant plasmids. E. coli BL21(DE3) (Novagen Inc., La Jolla, Calif.) was used as host for overproduction of the BlaB enzyme using the T7 promoter-based expression vectors. Plasmid pSK16CR (24) was used as the source for the C. meningosepticum blaB gene. Plasmid pBC-SK (Stratagene Inc., La Jolla, Calif.) was used as a cloning vector for the PCR-amplified blaB gene. Plasmids pET-9a and pET-24a were used for overexpression of the blaB gene under the control of the T7 promoter.
Media and culture conditions.
Luria-Bertani medium was routinely used for the propagation of E. coli strains. SB medium (yeast extract, 20 g/liter; Bacto tryptone, 35 g/liter; NaCl, 5 g/liter; buffered with 50 mM phosphate buffer [PB; pH 7.0]) was used in the expression experiments. TB medium (yeast extract, 24 g/liter; tryptone, 12 g/liter; glycerol, 4 ml/liter; buffered with 50 mM potassium-phosphate buffer [pH 7.0]) was used for large-scale production of the BlaB enzyme.
Antibiotics in culture media were used at the following concentrations: chloramphenicol, 85 μg/ml; kanamycin, 50 μg/ml. Bacteria were always grown aerobically at 37°C. Ingredients for culture media were from Difco Laboratories (Detroit, Mich.). Antibiotics and other chemicals were from Sigma Chemical Co. (St. Louis, Mo.).
Recombinant DNA methodology.
Recombinant DNA methodology was essentially as described by Sambrook et al. (25). Construction of the expression vectors pET9-Chryseo and pET24-Chryseo was performed as follows. The blaB open reading frame was amplified with oligonucleotides C.BlaB-FOR (5′-GCT CTA GAA GGA GAA TAA GAA ATG TTG AAA AAA ATA AAA ATA AGC TTG), which added an XbaI linker (underlined) and a ribosomal-binding site located 8 bp upstream of the blaB start codon, and C.BlaB-REV (5′-GGC GGA TCC GGA AAA AGG CTT CAT TAA TTT G), which added a BamHI linker (underlined) downstream from the blaB stop codon. PCR was performed with 5 ng of template DNA (plasmid pSK16CR), 100 pmol of each primer, and 3.5 U of DNA polymerase (Expand High Fidelity PCR System; Roche Molecular Biochemicals, Mannheim, Germany) in a final volume of 100 μl in the buffer system provided by the manufacturer. Cycling conditions were as follows: 94°C, 1 min; 47°C (with an increment of 0.6°C/cycle, until 53°C), 1 min; 72°C, 1 min; repeated for 35 cycles, and followed by a final extension step of 10 min at 72°C. The resulting amplimer was digested with XbaI and BamHI and cloned in pBC-SK, digested with the same enzymes, to obtain recombinant plasmid pJDD-1. After confirmatory sequencing, the 0.8-kb XbaI-BamHI insert of pJDD-1 was subcloned either in plasmid pET-9a or in plasmid pET24-a, digested with the same enzymes, to obtain the recombinant plasmids pET9-Chryseo and pET24-Chryseo, respectively.
Expression experiments.
Production of the BlaB enzyme by E. coli BL21(DE3) transformed with either pET9-Chryseo or pET24-Chryseo was monitored as a function of time in both supernatants and cell lysates of cultures growing in SB medium containing kanamycin. Each culture (200 ml) was grown to achieve an A600 of approximately 1 and split between two flasks (100 ml per flask), and isopropyl-β-d-thiogalactopyranoside (IPTG) was added, at a 1 mM final concentration, to one flask. Samples, drawn at different times, were centrifuged at 18,000 × g for 5 min. The culture supernatant was stored at 4°C. Cells were resuspended in an equal volume of 50 mM PB (pH 7.0) and disrupted by sonication (4 cycles, each cycle for 20 s at 50 W), and the lysate was centrifuged at 18,000 × g for 15 min at 4°C; the cleared supernatant represented the cellular fraction. BlaB activity in the fractions was determined with 1 mM imipenem as substrate (Δɛ = −9,000 M−1cm−1, at 300 nm) in 50 mM PB (pH 7.0) at 25°C. The amount of BlaB present in the various fractions was calculated from the measured activity based on the following kinetic parameters: Km, 360 μM; kcat, 730 s−1.
Large-scale production and purification of the BlaB enzyme.
The BlaB enzyme was purified from E. coli BL21(DE3)(pET9-Chryseo) grown aerobically in a 15-liter fermentor for 6 h at 37°C in TB medium containing kanamycin. Cells were removed by continuous-flow centrifugation. All subsequent steps were carried out at 4°C. The cleared supernatant was diluted fivefold in 50 mM PB (pH 6.5) and added to 2 liters of Sepharose SP Fast Flow (Amersham Pharmacia Biotech, Uppsala, Sweden) equilibrated with the same buffer. After incubation for 2 h under gentle agitation, the gel was collected by sedimentation and filtration and washed with two volumes of PB, and proteins were desorbed by resuspension of the gel in 2 liters of PB containing 0.5 M NaCl. The eluate was concentrated about twice by ultrafiltration (1.5 × 105 Pa; membrane molecular weight cutoff, 10,000) and dialyzed overnight against PB. After centrifugation at 9,750 × g for 30 min to remove any precipitate, the solution was fractionated by ion-exchange chromatography using a fast-performance liquid chromatography system (Pharmacia-LKB, Uppsala, Sweden). The solution was loaded onto a Sepharose SP Fast Flow column (1-liter bed volume) equilibrated with 10% buffer B (PB, 50 mM ZnCl2, 1 M NaCl) in buffer A (PB, 50 mM ZnCl2). The elution conditions were as follows: 10% buffer B in one column volume (CV); 10 to 60% buffer B in two CV; flow rate 5 ml/min. The eluent was monitored at 280 nm and peaks were collected. The elution peak containing β-lactamase activity was concentrated at 0.8 mg/ml as described above and then dialyzed against 30 mM cacodylate buffer (pH 6.5), 50 mM ZnCl2, 0.1 M NaCl. The solution was finally centrifuged at 9,750 × g for 30 min, and the clear enzymatic solution was stored at −80°C until use.
Protein analysis techniques.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out as described by Laemmli (18), with final acrylamide concentrations of 15 and 5% (wt/vol) for the separating and the stacking gels, respectively. After electrophoresis the protein bands were stained with Coomassie brilliant blue R-250. The amino-terminal sequence of the purified protein was determined using a gas-phase sequencer (Procise-492; Applied Biosystems, Foster City, Calif.), after resuspension of the protein (50 pmol) in a 0.1% (vol/vol) trifluoroacetic acid solution and loading of the sample onto a polyvinylidene difluoride (PVDF) membrane (Millipore Corp., Bedford, Mass.).
Determination of kinetic parameters.
Substrate hydrolysis was monitored by following the absorbance variation at 30°C in 30 mM sodium cacodylate buffer (pH 6.5) containing 50 μM ZnCl2, using a lambda 2 spectrophotometer (Perkin-Elmer, Rahway, N.J.) that was equipped with thermostatically controlled cells. The sources of β-lactam compounds, wavelengths, and changes in extinction coefficients have been described previously (19, 20). Bovine serum albumin (final concentration, 20 μg/ml) was added to the diluted solutions of β-lactamase.
The steady-state kinetic parameters (Km and kcat) were determined under initial rate conditions using the Hanes-Woolf plot (26). Km values lower than 20 μM were measured as inhibition constants (Kis) in a competitive model, using nitrocefin (40 μM) or imipenem (400 μM) as the reporter substrate. The Ki value was determined by the plot of Vo/Vi versus I, yielding a line whose slope is Km/[(Km + S) × Ki], where Vo and Vi are the initial rates in the absence or presence of inhibitor, respectively, I is the inhibitor concentration, S is the reporter substrate concentration, and Km is the Michaelis constant of the enzyme for the reporter substrate (10). In these cases, the kcat values were determined by measuring the hydrolysis of the substrate at a saturating concentration that was >Km.
RESULTS AND DISCUSSION
Development of an expression system for overproduction of the BlaB metallo-β-lactamase in E. coli.
Overexpression of the C. meningosepticum blaB gene in E. coli was carried out using two T7 promoter-based expression vectors, pET-9a and pET-24a, characterized by different stringencies of the transcriptional control. Both constructs used to transform E. coli BL21(DE3) yielded high-level production of the BlaB enzyme. With BL21(DE3)(pET9-Chryseo), BlaB production was scarcely influenced by IPTG induction and, regardless of induction, the protein was progressively released in the medium (Table 1; Fig. 1). With BL21(DE3)(pET24-Chryseo), production of the BlaB enzyme was remarkably influenced by IPTG induction. In induced cultures, the yield of enzyme was comparable to that obtained with pET9-Chryseo and production followed a similar pattern, including the eventual release in the medium. In cultures not induced the yield was significantly lower and the enzyme remained cell associated (Table 1). After 24 h and under the tested conditions, the BlaB activity was detected only in minor amounts in the cell fraction.
TABLE 1.
BlaB production by E. coli BL21 (DE3)(pET9-Chryseo) and E. coli BL21 (DE3)(pET24-Chryseo)a
| Time after induction (h) | Enzyme amount (mg/liter)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| BL21(DE3)(pET9-Chryseo)
|
BL21(DE3)(pET24-Chryseo)
|
|||||||
| Noninduced
|
Induced
|
Noninduced
|
Induced
|
|||||
| Cells | Supernatant | Cells | Supernatant | Cells | Supernatant | Cells | Supernatant | |
| 1 | 26 | 2 | 33 | 29 | 0.3 | 0.1 | 38 | 0.3 |
| 3 | 80 | 18 | 23 | 24 | 39 | 0.1 | 43 | 46 |
| 5 | 82 | 28 | 20 | 28 | 36 | 1 | 14 | 85 |
| 24 | 2 | 85 | 2 | 70 | 2 | 0.6 | 0.1 | 82 |
The enzyme amount was calculated on the basis of the kinetic parameters with imipenem (see Materials and Methods for details). Data are the means of three measurements. The standard deviations were always lower than 10%.
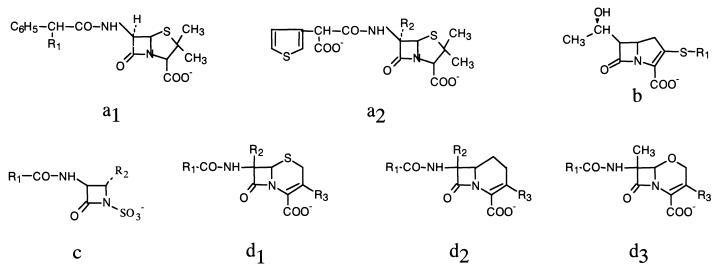
FIG. 1.
General structure of various groups of β-lactams: penams (a1, a2); carbapenems (b); monobactams (c); cephems (d1); carbacephems (d2); and oxacephems (d3).
Large-scale production and purification of the BlaB enzyme.
The BlaB enzyme produced by E. coli BL21(DE3)(pET9-Chryseo) was purified from the supernatant of a culture grown in a 15-liter fermentor by means of two ion-exchange chromatography steps, with an overall yield of 10 mg/liter of culture.
The protein was estimated to be >95% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). The amino-terminal sequence was determined to be NH2-QENPD and was identical to that of the protein purified from C. meningosepticum (20). Electrospray mass spectrometry yielded a value of 25,805 Da, which is consistent with the calculated molecular mass of the mature protein (25,803 Da) (24).
Kinetic parameters of the BlaB enzyme.
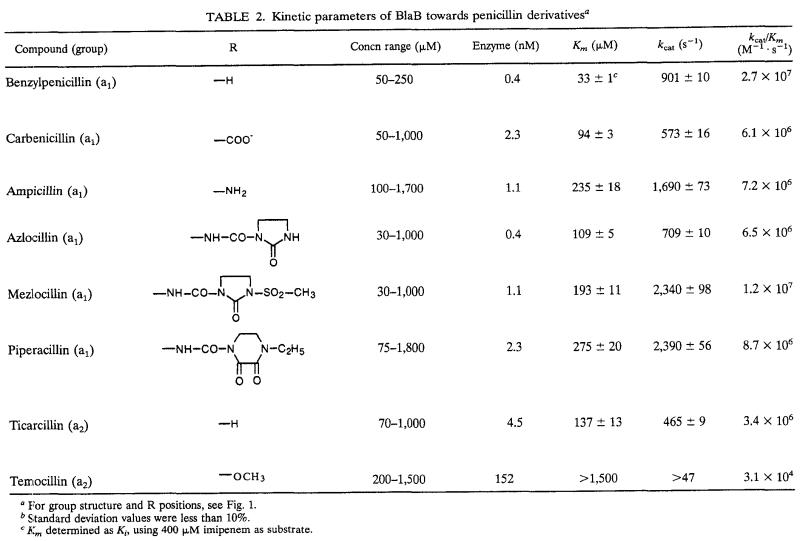
Under the experimental conditions adopted, the enzyme hydrolyzed all of the tested compounds, with the only exception being monobactams (aztreonam and carumonam, with Km s of >1,500 μM and kcats of <0.01 s−1). The steady-state kinetic parameters of the BlaB enzyme with the hydrolyzed compounds are reported below in Tables 2 to 5.
TABLE 2.
Kinetic parameters of BlaB towards penicillin derivativesa
For group structure and R positions, see Fig. 1.
Standard deviation values were less than 10%.
Km determined as Ki, using 400 μM imipenem as substrate.
(i) Penicillins.
All penam substrates were efficiently hydrolyzed (kcat/Km ratios, >106 or even >107 M−1 s−1), with the exception of temocillin (Table 2). Individual kinetic parameters for penam substrates were characterized by low to intermediate affinities (the Km values were in the range of 33 to 275 μM) and high kcat values, resulting in catalytic efficiencies that are comparable or superior to those of other metallo-β-lactamases of subgroup 3a (11, 13, 19, 27). The presence of a small side chain (such as in penicillin G, ampicillin, and carbenicillin) or of a bulky side chain (such as in azlocillin, mezlocillin, or piperacillin) did not significantly affect the binding or the turnover process. On the other hand, the presence of an α-methoxy group in position C-6 caused a dramatic decrease of the catalytic efficiency that was due, at least in part, to an impairment of the recognition process, as shown by comparison of the kinetic parameters measured with temocillin and ticarcillin (Table 2). A similar behavior does not appear to be a constant feature among metallo-β-lactamases of subgroup 3a, which can be affected quite differently by the presence of an α-methoxy group in position C-6 of the penam nucleus.
The high catalytic efficiency of BlaB for penicillins makes this enzyme one of the most efficient penicillin-hydrolyzing metallo-β-lactamases, together with the L1 enzyme from Pseudomonas maltophilia ULA-511 (11).
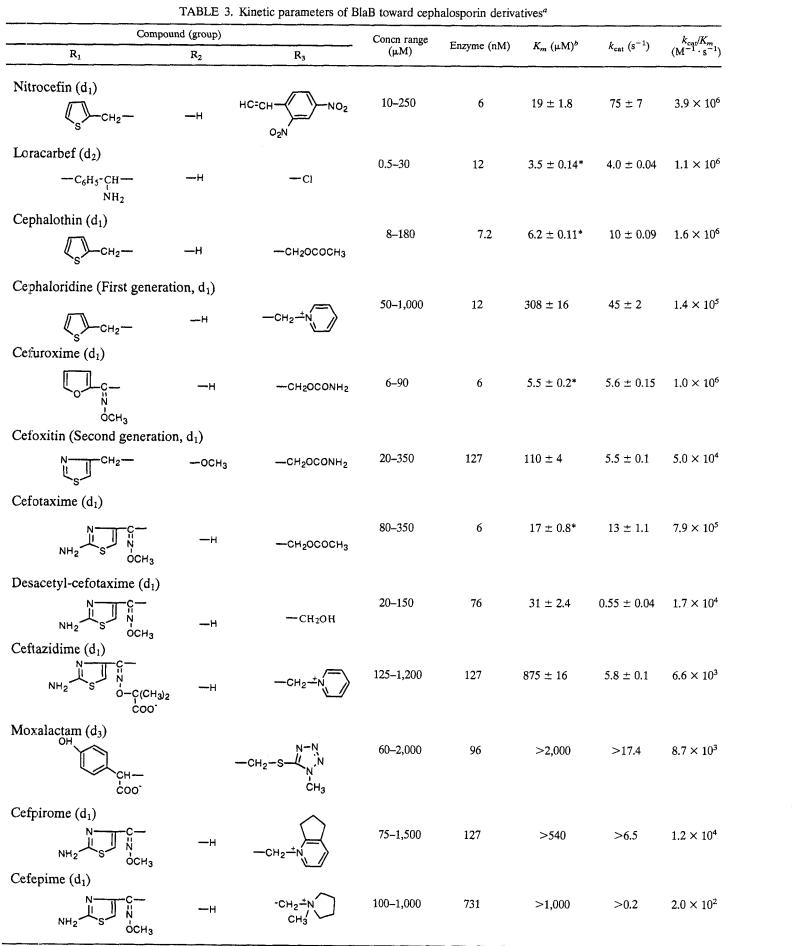
(ii) Cephalosporins.
Hydrolysis of cephalosporins was overall less efficient than that of penicillins. kcat/Km ratios around 106 M−1 s−1 were found only with nitrocefin, cephalothin, cefuroxime, cefotaxime, and loracarbef, while with the other compounds they were 10- to 10,000-fold lower (Table 3). This remarkable variability was largely due to a broad range of affinities that characterized the interaction of BlaB with different cephalosporins (Km ranged from 3.5 to >2,000 μM). The presence of a charged bulky side chain in the R3 substituent seems to be relevant to decreased affinity, as suggested by comparing the Km values for cephalotin (6 μM) with that for cephaloridine (308 μM), or that for cefotaxime (17 μM) with those for cefpirome (>540 μM) and cefepime (>1,000 μM). Affinity was also decreased by the presence of an α-methoxy group at C-7, as suggested by comparison of the Km values for cefuroxime (5.5 μM) or cefotaxime (17 μM) with that for cefoxitin (110 μM), while this modification did not significantly affect the catalytic constant kcat (Table 3). A similar behavior toward the presence of an α-methoxy group at C-7, which parallels that seen with penicillins (see above), was also observed with Bc-II (11), but it does not appear to be a constant feature among metallo-β-lactamases of subgroup 3a. Turnover rates for cephalosporins were constantly lower than those observed with penicillins and, for most compounds, kcat values varied in the range of 4 to 75 s−1. The removal of the acetyl moiety from the R3 substituent of cefotaxime (yielding desacetyl-cefotaxime) caused a 20-fold reduction of the turnover rate with only a minor effect on the recognition step (Table 3).
TABLE 3.
Kinetic parameters of BlaB toward cephalosporin derivativesa
For group structure and R positions, see Fig. 1.
∗, Km determined as Ki, using 40 μM nitrocefin as reporter substrate.
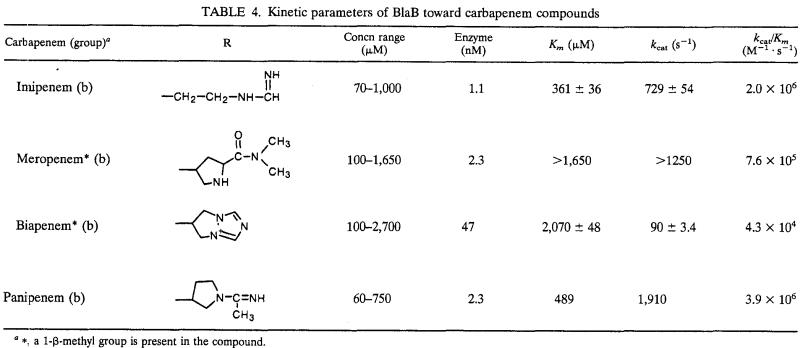
(iii) Carbapenems.
Most carbapenems behaved as good substrates (kcat/Km around 106 M−1 s−1), with the exception of biapenem, which was hydrolyzed 20- to 100-fold less efficiently (Table 4). Affinities for carbapenems were quite low, especially for meropenem and biapenem, and the high kcat/Km ratios observed with imipenem, meropenem, and panipenem always resulted from very high turnover rates. The 10- to 20-fold lower kcat values observed with biapenem significantly contributed to the reduced efficiency observed with this compound, which could be related to the unique presence of a quaternary ammonium on the C-2 substituent (Table 4).
TABLE 4.
Kinetic parameters of BlaB toward carbapenem compounds
∗, a 1-β-methyl group is present in the compound.
The efficiency of BlaB on imipenem is similar or higher than that of the most efficient imipenem-hydrolyzing enzymes of group 3, such as CphA, CcrA, IMP-1, VIM-1, and L1 (11, 13, 19, 27). Similar to CphA and CcrA, BlaB owes this feature to a combination of relatively low affinity but high turnover rates, while with L1, IMP-1, and VIM-1 the equilibrium is increasingly shifted toward increased affinities and decreased turnover rates (11, 13, 19). Also with meropenem, BlaB showed an efficiency similar to those of other metallo-β-lactamases, while with biapenem the BlaB efficiency was notably lower than that exhibited by VIM-1 and IMP-1 (13, 19).
(iv) Active-site serine β-lactamase inhibitors.
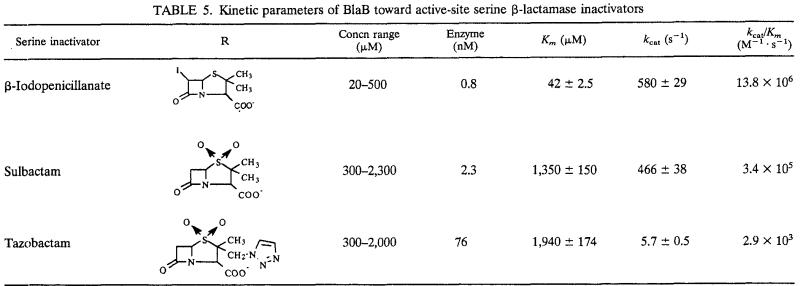
BlaB hydrolyzed all of the tested active-site serine β-lactamase inhibitors, but the behavior was quite heterogenous (Table 5). β-Iodopenicillanate was the best substrate, and a combination of high affinity (Km, 42 μM) with high turnover rate (kcat, 579 s−1) yielded a kcat/Km ratio second only to that of penicillin G. This excellent activity for β-iodopenicillanate suggests that the thiazolidine nucleus could be of great relevance to the binding of the substrate to the active site of BlaB, and it differentiates BlaB from other members of subclass B1, such as Bc-II, IMP-1, and VIM-1 (11, 13, 19). With sulbactam, kcat was similar to that observed for β-iodopenicillanate, but the affinity was remarkably lower, likely due to the presence of the sulfone moiety. However, with a kcat/Km ratio of >105 M−1 s−1, BlaB remains one the most efficient sulbactam-hydrolyzing metallo-β-lactamases (21). With tazobactam, which was the least efficiently hydrolyzed compound, a dramatic decrease in the turnover rate was also observed, likely due to the presence of the methyl-triazole group in C-2 (Table 5). The kcat/Km ratio observed with tazobactam is similar to those reported for VIM-1, CcrA, and IMP-1 (13, 21).
TABLE 5.
Kinetic parameters of BlaB toward active-site serine β-lactamase inactivators
Concluding remarks.
Some data presented in this paper seem to be in disagreement with those previously reported (24). This could be explained on the basis of a partial inactivation of the enzyme during its purification and mainly during the dialysis process. Despite this consideration, the high catalytic efficiency of BlaB for several β-lactams, its distribution in a species of clinical relevance, and its kinetic peculiarities suggest that this enzyme should be further investigated with spectroscopic and crystallographic methods to evaluate the role of specific amino acid residues in the catalytic mechanism and substrate specificity, how zinc ions bind in the active site, and which is the role of these ions in the reaction mechanism.
The substrate profile of the BlaB enzyme, more oriented toward penicillins, appears to be somewhat complementary to that of the GOB metalloenzyme, the other resident metallo-β-lactamase of C. meningosepticum, which shows a 15- to 1,000-fold higher catalytic efficiency than those found for BlaB relative to expanded-spectrum cephalosporins such as moxalactam, ceftazidime, and cefepime (1). This finding could explain the simultaneous presence of these two different metallo-β-lactamases in the same organism, which is a quite unusual finding. The presence of both metalloenzymes, together with a class A serine β-lactamase active on monobactams (23), enables C. meningosepticum to efficiently degrade virtually all β-lactam antibiotics.
Acknowledgments
S.V., J.-D.D., and S.R. are fellows of the European Research Network on Metallo-β-lactamases within the Training and Mobility of Researchers (TMR) Program (contract no. FMRX-CT98-0232). This work was partially supported also by grants from MURST, PRIN 1999 to G. Amicosante and G. M. Rossolini.
REFERENCES
- 1.Bellais, S., L. Poirel, S. Leotard, T. Naas, and P. Nordmann. 2000. Genetic diversity of carbapenem-hydrolyzing metallo-β-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob. Agents Chemother. 44:3028-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bounaga, S., A. M. Laws, M. Galleni, and M. I. Page. 1998. The mechanism of catalysis and the inhibition of the Bacillus cereus zinc-dependent β-lactamase. Biochem. J. 331:703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush, K. 1998. Metallo-β-lactamases: a class apart. Clin. Infect. Dis. 27:48-53. [DOI] [PubMed] [Google Scholar]
- 4.Bush, K., and S. Mobashery. 1998. How β-lactamases have driven pharmaceutical drug discovery. From mechanistic knowledge to clinical circumvention. Adv. Exp. Med. Biol. 456:71-98. [PubMed] [Google Scholar]
- 5.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfi, A., E. Duee, M. Galleni, J. M. Frère, and O. Dideberg. 1998. 1.85 Å resolution structure of the zinc(II) β-lactamase from Bacillus cereus. Acta Chrystallogr. D 54(Pt. 3):313-323. [DOI] [PubMed] [Google Scholar]
- 7.Carfi, A., S. Pares, E. Dueè, M. Galleni, C. Duez, J. M. Frère, and O. Dideberg. 1995. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 14:4914-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concha, N. O., B. A. Rasmussen, K. Bush, and O. Herzberg. 1996. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure 4:823-836. [DOI] [PubMed] [Google Scholar]
- 9.Concha, N. O., C. A. Janson, P. Rowling, S. Pearson, C. A. Cheveer, B. P. Clarke, C. Lewis, M. Galleni, J. M. Frère, D. J. Payne, J. H. Bateson, and S. S. Abdel-Meguid. 2000. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39:4288-4298. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, M. 1953. The determination of enzyme-inhibitor constants. Biochem. J. 55:170-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felici, A., G. Amicosante, A. Oratore, R. Strom, P. Ledent, B. Joris, L. Fanuel, and J. M. Frère. 1993. An overview of the kinetic parameters of class B β-lactamases. Biochem. J. 291:151-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felici, A., and G. Amicosante. 1995. Kinetic analysis of extension of substrate specificity in Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob. Agents Chemother. 39:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschini, N., B. Caravelli, J. Docquier, M. Galleni, J. M. Frère, G. Amicosante, and G. M. Rossolini. 2000. Purification and biochemical characterization of the VIM-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:3003-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J. M. Frère. 2001. Standard numbering for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, H., Y. Arakawa, S. Ohsuka, R. Wacharotayankun, N. Kato, and M. Ohta. 1995. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 39:824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyobe, S., H. Yamada, and S. Minami. 1996. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J. Antimicrob. Chemother. 38:1114-1115. [DOI] [PubMed] [Google Scholar]
- 17.Koh, T. H., G. S. Babini, N. Woodford, L. H. Sing, L. M. Hall, and D. M. Livermore. 1999. Carbapenem-hydrolysing IMP-1 β-lactamase in Klebsiella pneumoniae from Singapore. Lancet 353:2162.. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frère, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prosperi Meys, C., G. Llabres, D. de Seny, R. P. Soto, M. H. Valladares, N. Laraki, J. M. Frère, and M. Galleni. 1999. Interaction between class B β-lactamases and suicide substrates of active-site serine β-lactamases. FEBS Lett. 443:109-111. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossolini, G. M., N. Franceschini, L. Lauretti, B. Caravelli, and G. Amicosante. 1999. Cloning of a Chryseobacterium (Flavobacterium) meningosepticum chromosomal gene (blaACME) encoding an extended-spectrum class A β-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER β-lactamases. Antimicrob. Agents Chemother. 43:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossolini, G. M., N. Franceschini, M. L. Riccio, P. S. Mercuri, M. Perilli, M. Galleni, J. M. Frère, and G. Amicosante. 1998. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem. J. 332:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Segel, I. H. 1976. Biochemical calculations, 2nd ed., p. 236-241. John Wiley & Sons, New York, N.Y.
- 27.Yang, Y., B. A. Rasmussen, and K. Bush. 1992. Biochemical characterization of the metallo-β-lactamase CcrA from Bacteroides fragilis TAL 3636. Antimicrob. Agents Chemother. 36:1155-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]