Abstract
Bioactive glass has found extensive application as an orthopedic and dental graft material and most recently also as a tissue engineering scaffold. Here we report an initial investigation of the in vitro antibacterial properties of AgBG, a novel bioactive glass composition doped with Ag2O. The bacteriostatic and bactericidal properties of this new material and of two other bioactive glass compositions, 45S5 Bioglass and BG, have been studied by using Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus as test microorganisms. Concentrations of AgBG in the range of 0.05 to 0.20 mg of AgBG per ml of culture medium were found to inhibit the growth of these bacteria. Not only was AgBG bacteriostatic, but it also elicited a rapid bactericidal action. A complete bactericidal effect was elicited within the first hours of incubation at AgBG concentrations of 10 mg ml−1. 45S5 Bioglass and BG had no effect on bacterial growth or viability. The antibacterial action of AgBG is attributed exclusively to the leaching of Ag+ ions from the glass matrix. Analytical measurements rule out any contribution to AgBG-mediated bacterial killing by changes in pH or ionic strength or the dissolution of other ionic species from the biomaterials. Our observations of the dissolution profiles of Ag+ from AgBG in the presence and absence of bacteria are consistent with silver accumulation by the bacteria.
Bioactive glasses are special glass systems which are generally composed of SiO2, CaO, P2O5, and Na2O. They can be produced by the traditional melting process or by the more versatile sol-gel process (14, 15). The bioactive behavior of these glasses is defined as the ability to bond to soft and hard tissues by means of a series of reactions, which produces a strong, compliant interface between the glass and the tissue (14). Due to their high level of tissue integration and regeneration (15, 34), bioactive glasses have been used clinically in a variety of situations. Bioactive glass devices are now available to treat conductive deafness and alveolar ridge resorption and bone loss due to periodontal disease and to fill cystic and surgically created defects, particularly in craniomaxillofacial sites (19, 25, 33).
The material under investigation in the present work is a novel bioactive glass system composed of SiO2, CaO, P2O5, and Ag2O. The introduction of Ag2O into the bioactive glass composition is aimed at minimizing the risk of microbial contamination through the potential antimicrobial activity of the leaching Ag+ ions (7, 13). The introduction of silver has recently become one of the preferred methods to confer microbial resiliency on biomedical materials and devices (1, 6, 17, 18, 21), since the incidence of biomaterial-centered infections is one of the main causes of revision surgery (12). The production of the material via the sol-gel process allows the tailoring of the textural characteristics of the matrix in order to obtain a controlled Ag+ delivery system.
Here we report an initial investigation of the antibacterial properties of three different bioactive glasses: 45S5 Bioglass, a melt-derived, dense bioactive glass which has been commercially available since 1985; BG, a sol-gel-derived porous bioactive glass; and AgBG, an Ag2O-doped sol-gel-derived porous bioactive glass (2, 3). BG and AgBG possess the same textural characteristics and have similar levels of bioactivity (3). Three bacterial species were selected for this preliminary investigation. Escherichia coli and Pseudomonas aeruginosa were chosen as examples of gram-negative bacteria which have been found in biomaterial-related infection sites (16), with the high intrinsic antibiotic resistance of P. aeruginosa also being a consideration (24). Staphylococcus aureus is a gram-positive bacterium which represents a major concern for prosthetic devices and in other surgery-related infections (16, 23).
MATERIALS AND METHODS
Material preparation.
AgBG and BG were prepared via an acid-catalyzed sol-gel route, as previously described by Bellantone et al. (2). The resulting materials are mesoporous gel-glasses (i.e., they possess pore diameters in the range of 20 to 500 Å) with a density of 1.2 g cm−3. 45S5 Bioglass, a melt-derived bioactive glass with a density of 2.7 g cm−3, was supplied by US Biomaterials Corp. The chemical compositions of the glass systems under investigation were (in percentage of weight) as follows: BG (SiO2, 76; CaO, 22; P2O5, 2), AgBG (SiO2, 76; CaO, 19; P2O5, 2; Ag2O, 3), and 45S5 (SiO2, 45; CaO, 24.5; P2O5, 6; NaO2, 24.5). AgNO3, purchased from Alpha (Johnson Matthey), was used as an alternative source of Ag+.
All specimens were ground with mortar and pestle and sieved in the particle size range of 90 to 710 μm. AgBG was stored and handled in the dark to preserve the +1 oxidation state of the silver ion. This was not necessary for the other two materials, since they do not contain silver and therefore are not light sensitive.
Bacterial strains.
The following bacteria were used in this study: E. coli MG1655; P. aeruginosa PAO6049 (met-9011 amiE200 strA [streptomycin resistant]), a derivative of PAO1; and S. aureus NCIMB 11852. All strains were grown aerobically. P. aeruginosa cultures were grown at 30°C (4), and E. coli and S. aureus were grown at 37°C, in 50 ml of medium in 250-ml conical flasks with shaking at 200 rpm. Luria-Bertani medium (26) was used for E. coli and P. aeruginosa, and nutrient broth (Difco Laboratories) was used for S. aureus. Streptomycin was added at 1 mg ml−1 for P. aeruginosa cultures.
Determination of the antibacterial activity of the AgBG.
Two practical problems prevent the direct determination of a conventional MIC of AgBG by using culture turbidity as a qualitative measure of cell growth. These are the potential optical interference due to the light-scattering properties of the powdered glass and the visible light absorption spectrum of silver in solution, which overlaps the wavelengths used in optical density measurements. Consequently, the bacteriostatic effect of AgBG was determined by performing a viable count after exposure of the bacteria to AgBG for a fixed time period of 20 h.
(i) Concentration dependence.
A single bacterial colony was used to inoculate a 50-ml starter culture, which was grown overnight. Growth medium was inoculated with an aliquot of the overnight culture. The initial cell concentration was 5 × 107 CFU ml−1 for E. coli and S. aureus and 106 CFU ml−1 for P. aeruginosa. Assay mixtures containing AgBG, BG, and 45S5 Bioglass in concentrations ranging from 0.05 to 1.00 mg ml−1 were prepared in four replicates. Negative control assay mixtures, containing only the cell inoculum in growth medium, were cultured in triplicate. Positive control assay mixtures, containing AgNO3 in concentrations up to 0.1 mM, were cultured in triplicate. This range was chosen to cover the concentration of Ag+ eluted from AgBG during the time course of the dissolution experiment. Following inoculation, the cultures were incubated for 20 h at 30 or 37°C. After a 20-h incubation period a 1-ml sample was taken and serial dilutions in the range of 10−1 to 10−7 were prepared. One hundred microliters of each dilution was plated onto the same growth medium employed in the corresponding liquid culture. The number of colonies was counted after overnight incubation (8). We estimated the approximate minimal bactericidal concentration (MBC) as the concentration of AgBG leading to a 99.9% reduction in viability.
(ii) Time dependence.
Each bacterial strain was grown into early stationary phase, and cultures were harvested by centrifugation at 10,000 × g for 10 min. The cell pellets were stored at −80°C until needed. To evaluate the time dependence of the bactericidal activity of the powdered materials on stationary-phase bacteria, the cell pellets were thawed and resuspended in 50 mM phosphate buffer (pH 7) containing 10 mg of AgBG, BG, or 45S5 Bioglass ml−1. Control experiments in phosphate buffer containing no material were also performed. All assays were replicated four times. The phosphate buffer solutions containing the cell cultures were incubated in an orbital shaker (rotating speed, 200 rpm) at an appropriate temperature for 24 h. One-milliliter samples were taken at the time intervals shown in Fig. 1 and were left briefly to allow the particulate biomaterial to settle out. The supernatant solution was then centrifuged at 13,000 × g for 5 min, the bacterial pellet was washed in phosphate buffer solution and centrifuged again, and the final pellet was resuspended in 0.9% NaCl. Serial dilutions in the range of 10−1 to 10−7 were prepared. This procedure ensured the removal of soluble silver ions (or any other soluble species that might affect bacterial viability) released by the biomaterials into the culture medium. One hundred microliters of each dilution was plated out onto the same growth medium employed in the corresponding starter cultures. After overnight incubation the number of colonies was counted.
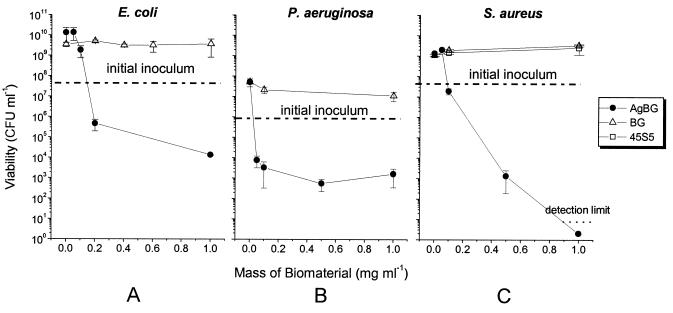
FIG. 1.
The effects of variable concentrations of AgBG, BG, and 45S5 on growth and viability of E. coli, P. aeruginosa, and S. aureus. Bacteria were inoculated into the appropriate growth medium containing a known amount of the relevant biomaterial. Cultures were incubated for 20 h, after which time samples were taken for viable count determinations. The initial inoculation density is shown on each panel. Error bars represent ±1 standard deviation.
Inductively coupled plasma (ICP) analysis.
ICP is an analytical technique based on atomic emission spectroscopy which allows the quantitative elemental determination of inorganic species present in solution (31). This technique was used in order to monitor the dissolution of ionic species leaching from the materials into the culture medium during the assays. A 1-ml sample was collected from each assay after 20 h of incubation and centrifuged at 13,000 × g for 5 min. The supernatant solution, free of cells and particulate biomaterial, was then subjected to ICP analysis. In addition, control assay mixtures containing culture medium and AgBG in the absence of bacteria were subjected to the same experimental conditions as those of the bacterial assays and were subsequently analyzed by ICP. The instrument used was an ARL 3580 B ICP analyzer. The detection limits for the elements of interest were as follows: Si, 0.050 ppm; Ca, 0.100 ppm; P, 0.200 ppm; Na, 0.100 ppm; and Ag, 0.020 ppm.
RESULTS
Determination of the antibacterial activity of AgBG.
We investigated the bacteriostatic effects of low concentrations of AgBG on bacteria by determining the viable cell count after exposure of the bacterial population to AgBG for a fixed time period of 20 h. The dependence of viability on the concentration of AgBG is shown in Fig. 1. We can estimate an approximate MIC from the concentration of AgBG at which the total viable cell numbers after 20 h of incubation remain at or close to the initial inoculation value. This estimates the concentration at which AgBG prevents significant growth, which is what is measured by standard MIC determinations using optical density. These data suggest that AgBG markedly inhibits the growth of both the gram-negative and gram-positive bacteria, when tested at concentrations in the range of 0.05 to 0.20 mg ml−1 (Fig. 1). This, as can be seen from the Ag+ dissolution profile of AgBG in the absence of bacteria (Fig. 2A), corresponds to dissolved silver concentrations of ∼25 μM.
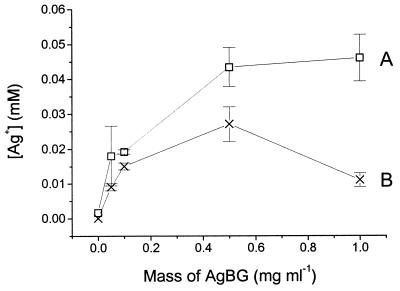
FIG. 2.
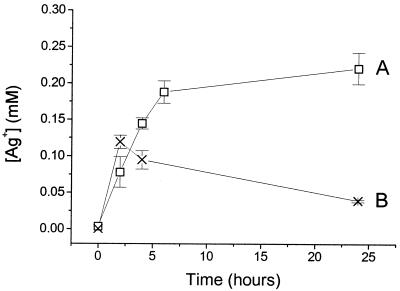
The dissolution of Ag+ from AgBG in the presence and absence of S. aureus. Different amounts of AgBG were added to nutrient broth, and the medium was then inoculated with S. aureus to a density of 5 × 107 bacteria ml−1. Cultures with (×) and without (□) S. aureus were then incubated at 30°C for 20 h under conditions identical to those described in the legend to Fig. 1. The Ag+ content of the culture medium was then determined by ICP analysis as described in Materials and Methods. Error bars represent ±1 standard deviation.
With an AgBG concentration of 0.2 mg ml−1 the E. coli viable cell population did not increase above the inoculation density; indeed, there was evidence of bactericidal activity at this concentration. The viable population was 4 to 5 orders of magnitude lower than that of the culture without AgBG and the culture containing BG and over 1 order of magnitude lower than that of the initial inoculum concentration (Fig. 1A). P. aeruginosa did not increase viability above the inoculation density at an AgBG concentration of 0.05 mg ml−1, and there was a significant loss of viability relative to the inoculum, indicating a true MIC of less than this value (Fig. 1B). Finally, AgBG was bacteriostatic for S. aureus at 0.1 mg ml−1, and at higher concentrations significant bactericidal activity was observed (Fig. 1C). Thus, the AgBG concentrations used exhibited a bacteriostatic effect at similar concentrations for each of the bacteria under investigation. From the data in Fig. 1 we estimate MBCs (>99.9% killing) for AgBG of 1, 0.5, and 0.5 mg ml−1 for E. coli, P. aeruginosa, and S. aureus, respectively. The mean cell viabilities for the cultures containing BG are not significantly different from those for the control cultures at 0 mg ml−1 for all three bacterial species tested (Fig. 1). Thus, BG does not affect the growth of the three bacterial species tested here. In addition, the melt-derived bioactive glass 45S5 Bioglass had no effect on the viability of S. aureus.
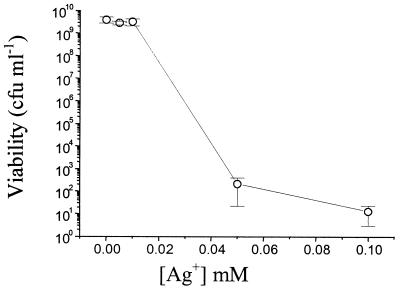
The direct effect of Ag+, introduced by dissolving AgNO3 in the culture medium, on the viability of S. aureus is shown in Fig. 3. These data should be considered with the dissolution profile of Ag+ from AgBG shown in Fig. 2A, which shows the amount of Ag+ dissolved from different concentrations of AgBG after a fixed incubation period. The data show that Ag+ is toxic to S. aureus at concentrations similar to those released over the same incubation period during the experiment described in the legend to Fig. 1. That is, 0.5 mg of AgBG ml−1 had a significant bactericidal effect on S. aureus (Fig. 1C), and this dissolved ∼45 μM Ag+ over 20 h (Fig. 2), a concentration of Ag+ that leads to significant killing of S. aureus (Fig. 3).
FIG. 3.
The effect of AgNO3 on the growth and viability of S. aureus. S. aureus cultures were set up, at an initial inoculation density of 5 × 107 CFU ml−1, in nutrient broth containing different concentrations of AgNO3. Cultures were incubated for 20 h, after which they were sampled for viable counting. Error bars represent ±1 standard deviation.
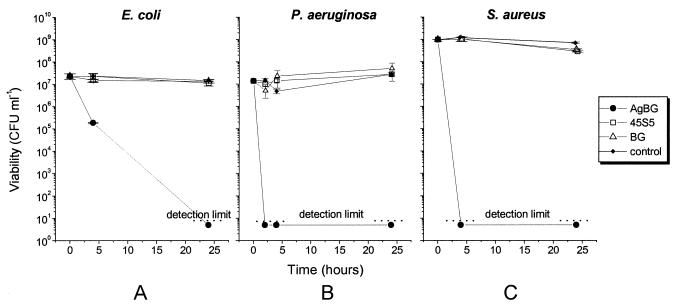
Figure 4 shows the time-dependent killing of stationary-phase populations of bacteria by a bactericidal concentration of AgBG (10 mg ml−1). After 4 h E. coli cultures containing 10 mg of AgBG ml−1 decreased in cell viability by 2 orders of magnitude, and after 24 h the viable cell number was below the detection limit (Fig. 4A). For P. aeruginosa, the first cell viability measurement was taken 2 h after AgBG addition, at which time the bacterial population was already below the detection limit (Fig. 4B). For S. aureus the viability was below the detection limit after the first 4 h of incubation (Fig. 4C). The experiments were terminated after 24 h, at which time the bacterial cultures containing AgBG were all below the experimental detection limit of 10 CFU ml−1. In all assays BG and 45S5 Bioglass had no effect on the viability of any of the bacterial strains under investigation even at a 10-mg ml−1 concentration (Fig. 4).
FIG. 4.
The time-dependent killing of stationary-phase bacteria by AgBG. Bacterial cultures were grown into early stationary phase, harvested by centrifugation, and resuspended in phosphate buffer containing the biomaterial under study. After incubation for 24 h samples were taken for viability measurement as described in Materials and Methods. Error bars represent ±1 standard deviation.
Dissolution of Ag+ from AgBG.
In the absence of bacteria it is expected that dissolution profiles would show Ag+ concentrations proportional to either the quantity of material in solution or the time of residence in solution, as shown in previous studies of similar glass materials (17).
In the present study the amount of Ag+ dissolved from AgBG during a typical concentration dependence experiment (Fig. 1) was measured in the presence and absence of bacteria (after a fixed experimental time of 20 h) (Fig. 2A). A normal dissolution profile is observed in the absence of cells, with the Ag+ content of the medium increasing with the AgBG concentration until a plateau is reached (Fig. 2A). However, in the presence of cells the Ag+ concentration is lower than in the absence of cells at the same AgBG concentration (Fig. 2B). At an AgBG concentration of 1 mg ml−1 the cell pellet was dark gray, whereas the cell pellet obtained from all the other cultures (containing BG, 45S5 Bioglass, and control) was white-yellow.
Figure 5 depicts the solution concentration profile of the silver ion during the experimental conditions used in the time dependency study (shown in Fig. 4). After 5 h the Ag+ concentration in solution is markedly lower in the presence of bacteria than in their absence. The color of the bacterial pellet changes from white-yellow to dark gray during the time course. The difference between the profiles in the presence and absence of cells is consistent with Ag+ being bound or taken up into the cells.
FIG. 5.
Kinetics of Ag+ dissolution from AgBG in the presence and absence of stationary-phase S. aureus. AgBG (10 mg ml−1) was added to 50 mM potassium phosphate, which was then inoculated with stationary-phase S. aureus, as described in the legend to Fig. 4. Cultures with (×) and without (□) S. aureus were incubated at 37°C for 24 h under conditions identical to those described in the legend to Fig. 4. The Ag+ content of the culture medium was then determined by ICP analysis as described in Materials and Methods. Error bars represent ±1 standard deviation.
pH and mineral content of the cell cultures.
Ionic strength and pH of the medium can affect the growth and survival of microorganisms. We monitored and compared the pH rise and ionic concentrations of species released by the biomaterials under investigation to determine whether differential changes in these factors between the different bioactive glasses could contribute to the antibacterial properties of AgBG.
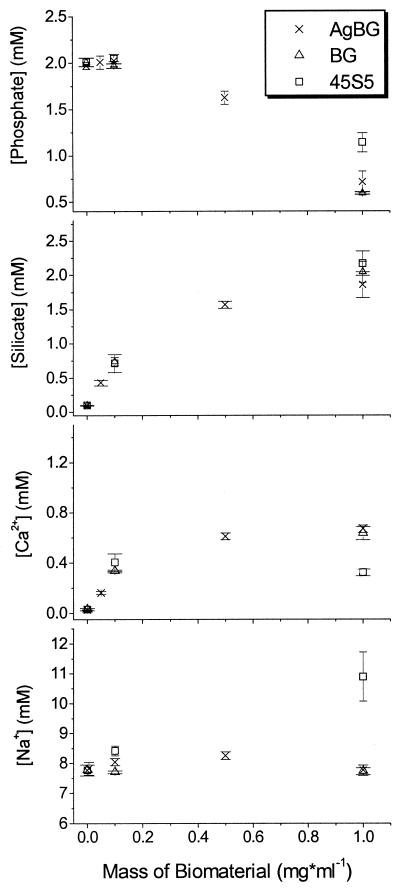
Due to their alkaline and/or alkaline-earth oxide content (see Materials and Methods), bioactive glasses have a basic reaction to water, causing the pH to rise. Cultures containing 45S5 Bioglass increased by nearly half a pH unit, and those containing BG and AgBG both increased by more than 1 pH unit, but their pH values were not significantly different (Table 1). Therefore, a differential change in pH cannot explain the bactericidal effects of AgBG. Figure 6 shows that the concentration profiles of Si, Ca, Na, and P ionic species released from the biomaterials after 20 h of incubation in culture medium were similar for each of the three biomaterials. There is only a slight discrepancy in the 45S5 Bioglass profiles: its phosphate content decreases less, and its Ca2+ content increases less, than do those of BG and AgBG. This can be attributed to the fact that 45S5 Bioglass is a melt-derived nonporous glass which, having a smaller surface area than that of porous glasses, is therefore less reactive. In addition, of all the biomaterials under investigation, 45S5 Bioglass is the only one containing Na2O (Table 1); thus, its Na+ profile shows an increase of approximately 1.5 mM (about 17.5% over the initial 8.5 mM Na+ concentration). Therefore, we conclude that the bactericidal effect observed with AgBG cannot be explained by changes in pH or ionic strength or dissolution of other ionic species from the biomaterial.
TABLE 1.
pH values of phosphate buffer (50 mM) containing 10 mg of material ml−1, after bactericidal assays
| Biomaterial | pH
|
|
|---|---|---|
| Mean | SD | |
| Control | 7.11 | 0.01 |
| BG | 8.08 | 0.10 |
| AgBG | 8.52 | 0.85 |
| 45S5 | 7.41 | 0.01 |
FIG. 6.
Comparison of the phosphate, silicate, calcium, and sodium content of nutrient broth containing AgBG, BG, and 45S5, after 20 h of incubation in the presence of S. aureus. S. aureus cultures in nutrient broth, containing different amounts of biomaterial, were set up as described in the legend to Fig. 1. After 20 h of incubation the phosphate, silicate, calcium, and sodium contents of the growth medium were determined, after removal of the bacteria, by ICP analysis as described in Materials and Methods. Error bars represent ±1 standard deviation.
DISCUSSION
AgBG is a novel porous glass designed to combine bioactive and antimicrobial properties. The latter are studied herein by using single strains of E. coli, P. aeruginosa, and S. aureus as test microorganisms. Furthermore the antimicrobial properties of BG (analogous to AgBG in morphology and composition with the exception of Ag2O) and 45S5 Bioglass (dense bioactive glass) are investigated and compared.
AgBG features bactericidal properties towards each of the bacteria under investigation. We have demonstrated that, with powdered AgBG, a killing effect occurs in liquid culture medium and in phosphate buffer solution which is not observed in the presence of bioactive glasses that are not doped with Ag2O. We estimate MBCs for AgBG of 1, 0.5, and 0.5 mg ml−1 for E. coli, P. aeruginosa, and S. aureus, respectively. However, AgBG is present in the culture as a separate solid phase that results in continuous dissolution of ionic species. Therefore, the situation here is different from conventional MIC and MBC determinations for antimicrobial agents, in which a single bolus of the compound is given at the outset of the experiment, although the data in Fig. 5 indicate that Ag+ is dissolved from the AgBG fairly rapidly. This work is an initial study of a novel biomaterial that suggests that AgBG may have useful antibacterial properties.
BG and 45S5 Bioglass show neither bacteriostatic nor bactericidal effects on any of the studied microorganisms. Several researchers have tried to establish whether the success of bioactive glasses and glass-ceramics as bone graft materials could be due partly to antibacterial effects (9, 29, 30). In agreement with the results presented here, Geyer et al. (9) and Stoor et al. (29) report that bioactive glasses and glass-ceramics are not antibacterial; in specific cases they transiently promote growth and are subject to bacterial adhesion. Antibacterial properties of a dense bioactive glass were subsequently reported (30), although the material concentration investigated was over 3 orders of magnitude greater than the maximum concentration studied here. It is questionable whether the observed antimicrobial effect was related to intrinsic properties of the bioactive glass or arose as a result of the dramatic changes in the physicochemical characteristics of the culture medium (osmotic pressure, pH, ionic strength, and composition) which occur as a consequence of the dissolution of such a large quantity of the material.
In the present study neither 45S5 Bioglass nor BG affects significantly the viability of the bacterial strains under investigation. The changes induced in pH (Table 1) and ionic content (Fig. 6) are similar to those provoked by AgBG (particularly in comparison with BG). Therefore, the marked antibacterial effect observed for AgBG is attributed exclusively to the leaching of ionic silver from the glass matrix. Comparing the antibacterial action of AgBG and AgNO3, it can be inferred that the silver delivered by AgBG shows antibacterial power similar to that of Ag+ on its own. This result suggests that the presence of the porous bioactive glass in liquid bacterial cultures neither enhances nor impairs the antibacterial action of Ag+. Furthermore the fact that the silver species is released together with other ionic species (such as Ca2+, phosphate, and silicate) does not alter its antibacterial properties.
Although the efficacy of silver as an anti-infection and anticontamination agent is well established, the mechanism by which the silver ion exerts its toxicity towards bacteria is not yet fully understood. It is believed that the driving force of the interaction is the complexation reaction involving sulfhydryl (20), amino, and hydroxyl groups, which are present in various cellular components (11, 22). It has been postulated elsewhere that Ag+ competes with Cu+ for cellular entry by an essential copper transport mechanism (28) and also for specific enzymatic sites at which Ag+ exerts its toxic action (27).
The results reported here are consistent with silver accumulation by the sensitive bacteria tested (5, 10). In the absence of cells the expected dissolution trends are observed for the silver ion released from the AgBG glass matrix (Fig. 2A and 5A): the Ag+ concentration rises as the amount of material introduced in the solution and the residence time increase. In contrast, anomalous trends were observed when the same dissolution processes were monitored in the presence of bacteria (Fig. 2B and 5B). It was found that, as the material concentration and the incubation time increased, Ag+ seemed to be depleted from the solution instead of increasing. The difference between the two profiles observed is most likely due to the silver binding to or accumulating in the cells.
Although we cannot yet predict how efficient this material will be in its prospective applications, where various biological molecules may be present, one of the main advantages of incorporating silver ions in a gel-glass system is that the porous glass matrix can allow a controlled sustained delivery of the antibacterial agent (3). This may be useful for the preservation of its antibacterial efficiency in body fluids containing species such as Cl− or proteins capable of complexing ionic silver, preventing its bactericidal action (32), and also for providing a long-term action required for systems which are constantly at risk of microbial contamination, such as tissue culture systems. These aspects will be addressed with in vitro and in vivo studies in future research.
Acknowledgments
This work was supported by the EPSRC. Research in H.D.W.'s laboratory was funded by the BBSRC, the British Lung Foundation, and the Wellcome Trust.
US Biomaterials Corporation kindly provided 45S5 Bioglass. We gratefully acknowledge Barry Coles for his help with the ICP analysis. M.B. also thanks all the staff in H.D.W.'s laboratory for their kindness and helpfulness.
REFERENCES
- 1.Adams, A. P., E. M. Santschi, and M. A. Mellencamp. 1999. Antibacterial properties of a silver chloride-coated nylon wound dressing. Vet. Surg. 28:219-225. [DOI] [PubMed] [Google Scholar]
- 2.Bellantone, M., N. J. Coleman, and L. L. Hench. 2000. Bacteriostatic action of a novel four-component bioactive glass. J. Biomed. Mater. Res. 51:484-490. [DOI] [PubMed] [Google Scholar]
- 3.Bellantone, M., and L. L. Hench. 2000. Bioactive behaviour of sol-gel derived antibacterial bioactive glass. Key Eng. Mater. 192-195:617-660. [Google Scholar]
- 4.Cunningham, L., M. Pitt, and H. D. Williams. 1997. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol. Microbiol. 24:579-591. [DOI] [PubMed] [Google Scholar]
- 5.Efrima, S., and B. V. Bronk. 1998. Silver colloids impregnating or coating bacteria. J. Phys. Chem. B 102:5947-5950. [Google Scholar]
- 6.Gatter, N., W. Kohnen, and B. Jansen. 1998. In vitro efficacy of hydrophilic central venous catheter loaded with silver to prevent microbial colonization. Zentbl. Bakteriol. 287:157-169. [DOI] [PubMed] [Google Scholar]
- 7.George, N., J. Faoagali, and M. Muller. 1997. Silvazine (silver sulfadiazine and chlorexidine) activity against 200 clinical isolates. Burns 23:493-495. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.). 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 9.Geyer, G., C. Schott, and A. Swarzkopf. 1999. Effect of alloplastic bone substitutes on bacterial growth. HNO 47:25-32. [DOI] [PubMed] [Google Scholar]
- 10.Ghandour, W., J. A. Hubbard, J. Deistrung, M. N. Hughes, and R. K. Poole. 1988. The uptake of silver ions by Escherichia coli K12: toxic effects and interaction with copper ions. Appl. Microbiol. Biotechnol. 28:314-319. [Google Scholar]
- 11.Grier, N. 1977. Silver and its compounds, p. 68-72. In S. S. Block (ed.), Disinfection, sterilization and preservation. Lea & Febiger, Philadelphia, Pa.
- 12.Gristina, A. G. 1987. Biomaterial-centered infections: microbial adhesion versus tissue integration. Science 237:1588-1595. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, A., and S. Silver. 1998. Silver as a biocide: will resistance become a problem? Nat. Biotechnol. 16:888.. [DOI] [PubMed] [Google Scholar]
- 14.Hench, L. L., R. J. Splinter, W. C. Allen, and T. K. Greenlee. 1972. Bonding mechanism at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 2:117-141. [Google Scholar]
- 15.Hench, L. L. 1998. Bioactive materials: the potential for tissue regeneration. J. Biomed. Mater. Res. 41:511-518. [DOI] [PubMed] [Google Scholar]
- 16.Hughes, S. P. F. 1988. The role of antibiotics in preventing infections following total hip replacement. J. Hosp. Infect. 11:41-47. [DOI] [PubMed] [Google Scholar]
- 17.Kawashita, M., S. Tsuneyama, F. Miyaji, T. Kokubo, and K. Yamamoto. 2000. Antibacterial silver-containing silica glass prepared by the sol-gel method. Biomaterials 21:393-398. [DOI] [PubMed] [Google Scholar]
- 18.Kim, T. N., Q. L. Feng, J. O. Kim, J. Wu, H. Wang, and G. C. Chen. 1998. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 9:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Kinnunen, I., K. Aitasalo, M. Pollonen, and M. Varpula. 2000. Reconstruction of orbital floor fractures using bioactive glass. J. Cranio-Maxillo-Fac. Surg. 28:229-234. [DOI] [PubMed] [Google Scholar]
- 20.Liau, S. Y., D. C. Read, W. J. Pugh, J. R. Furr, and A. D. Russel. 1997. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 25:279-283. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura, T., Y. Abe, Y. Sato, K. Okamoto, M. Ueshige, and Y. Akagawa. 1997. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J. Dent. 25:373-377. [DOI] [PubMed] [Google Scholar]
- 22.Modak, S. M., and C. L. Fox. 1973. Binding of silver sulfadiazine to the cellular components of Pseudomonas aeruginosa. Biochem. Pharmacol. 22:2391-2404. [DOI] [PubMed] [Google Scholar]
- 23.Naylor, P. T., Q. N. Myrvik, and A. G. Gristina. 1990. Antibiotic resistance of biomaterial-adherent coagulase-negative and coagulase-positive Staphylococci. Clin. Orthop. 261:126-133. [PubMed] [Google Scholar]
- 24.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltola, M., J. Suonpaa, K. Aitasalo, H. Maattanen, O. Andersson, A. Yli-Urpo, and P. Laippala. 2000. Experimental follow-up model for clinical frontal sinus obliteration with bioactive glass (S53P4). Acta Otolaryngol. Suppl. 543:167-169. [DOI] [PubMed] [Google Scholar]
- 26.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusion. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Slawson, R. M., H. Lee, and J. T. Trevors. 1990. Bacterial interactions with silver. Biol. Metals 3:151-154. [DOI] [PubMed] [Google Scholar]
- 28.Solioz, M., and A. Odermatt. 1995. Copper and silver transport by CopB-ATPase in membrane vesicles of Enterococcus hirae. J. Biol. Chem. 270:9217-9221. [DOI] [PubMed] [Google Scholar]
- 29.Stoor, P., V. Kirstila, E. Soderling, I. Kangasniemi, K. Hebst, and A. Yli-Urpo. 1996. Interactions between bioactive glass and periodontal pathogens. Microb. Ecol. Health Dis. 9:109-114. [Google Scholar]
- 30.Stoor, P., E. Soderling, and J. I. Salonen. 1998. Antibacterial effect of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 56:161-165. [DOI] [PubMed] [Google Scholar]
- 31.Thompson, M., and J. N. Walsh. 1983. Handbook of inductively coupled plasma spectroscopy. Blackie Academic and Professional, Glasgow, United Kingdom.
- 32.Williams, R. L., P. J. Doherty, D. G. Vince, G. J. Grashoff, and D. F. Williams. 1989. The biocompatibility of silver. Crit. Rev. Biocompat. 5:221-243. [Google Scholar]
- 33.Wilson, J., A. E. Clark, E. Douek, J. Krieger, W. King Smith, and J. Saville Zamet. 1994. Clinical applications of Bioglass implants, p. 415-422. In O. H. Andersson and A. Yli-Urpo (ed.), Bioceramics, vol. 7. Butterworth-Heinemann Ltd., Oxford, United Kingdom. [Google Scholar]
- 34.Xynos, I. D., A. J. Edgar, L. D. K. Buttery, L. L. Hench, and J. M. Pollak. 2000. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 276:461-465. [DOI] [PubMed] [Google Scholar]