Abstract
Micafungin (FK463) is an echinocandin that demonstrates potent in vitro antifungal activities against Candida and Aspergillus species. However, little is known about its comparative antifungal activities in persistently neutropenic hosts. We therefore investigated the plasma micafungin pharmacokinetics and antifungal activities of micafungin against experimental disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. The groups with disseminated candidiasis studied consisted of untreated controls (UCs); rabbits treated with desoxycholate amphotericin B (DAMB) at 1 mg/kg of body weight/day; or rabbits treated with micafungin at 0.25, 0.5, 1, and 2 mg/kg/day intravenously. Compared with the UCs, rabbits treated with micafungin or DAMB showed significant dosage-dependent clearance of Candida albicans from the liver, spleen, kidney, brain, eye, lung, and vena cava. These in vivo findings correlated with the results of in vitro time-kill assays that demonstrated that micafungin has concentration-dependent fungicidal activity. The groups with invasive pulmonary aspergillosis studied consisted of UCs; rabbits treated with DAMB; rabbits treated with liposomal amphotericin B (LAMB) at 5 mg/kg/day; and rabbits treated with micafungin at 0.5, 1, and 2 mg/kg/day. In comparison to the significant micafungin dosage-dependent reduction of the residual burden (in log CFU per gram) of C. albicans in tissue, micafungin-treated rabbits with invasive pulmonary aspergillosis had no reduction in the concentration of Aspergillus fumigatus in tissue. DAMB and LAMB significantly reduced the burdens of C. albicans and A. fumigatus in tissues (P < 0.01). Persistent galactomannan antigenemia in micafungin-treated rabbits correlated with the presence of an elevated burden of A. fumigatus in pulmonary tissue. By comparison, DAMB- and LAMB-treated animals had significantly reduced circulating galactomannan antigen levels. Despite a lack of clearance of A. fumigatus from the lungs, there was a significant improvement in the rate of survival (P < 0.001) and a reduction in the level of pulmonary infarction (P < 0.05) in micafungin-treated rabbits. In summary, micafungin demonstrated concentration-dependent and dosage-dependent clearance of C. albicans from persistently neutropenic rabbits with disseminated candidiasis but not of A. fumigatus from persistently neutropenic rabbits with invasive pulmonary aspergillosis.
Disseminated candidiasis and invasive pulmonary aspergillosis are important causes of nosocomial fungal infections in immunocompromised patients (1, 14, 27-29, 38, 40). Amphotericin B and its lipid formulations are widely used for the treatment of those fungal infections, but their clinical use are limited by their nephrotoxicities, infusion-related toxicities, and high acquisition costs (12, 15, 31, 32). The antifungal triazoles inhibit sterol biosynthesis and are effective against many pathogenic fungi; however, hepatotoxicity, gastrointestinal intolerance, drug interactions, antifungal spectrum, and emergence of resistance may limit their utilities (9, 26, 30). Flucytosine has activities against yeasts, such as Candida spp. and Cryptococcus neoformans, but its use may be complicated by myelosuppression and gastrointestinal toxicity (8). Given this limited armamentarium, there is an urgent need for new classes of antifungal agents with potent antifungal efficacies and improved safety.
The echinocandins are a novel class of semisynthetic lipopeptide antifungal compounds which inhibit synthesis of 1,3-β-d-glucan, a key component of the cell walls of most pathogenic fungi. Among the current group of agents used for the treatment of Candida and Aspergillus infections, the 1,3-β-d-glucan synthase inhibitors hold great promise as valuable assets. Inhibition of 1,3-β-D-glucan synthesis results in disruption of fungal cell wall biosynthesis, cell wall damage, and ultimately, cell death (10, 18).
Micafungin (FK463) is a novel water-soluble parenterally administered echinocandin lipopeptide derived by semisynthetic modification of ER901379 (2, 36). In vitro studies have demonstrated that micafungin has potent activities against Candida spp. and Aspergillus spp. (21, 22, 35, 37). Recently published studies have demonstrated the potent activities of micafungin in transiently immunocompromised or nonneutropenic animal models of invasive Candida and Aspergillus infections (16, 20, 21).
Little is known, however, about the antifungal activities or pharmacology of micafungin in persistently neutropenic hosts, in which this compound may have important applications (5). Despite the use of dosages that are the same as those used for the treatment of humans and the achievement of concentrations in plasma that also are similar to those observed in clinical trials, we found striking differential effects between the activities of echinocandins against disseminated candidiasis and the activities of echinocandins against invasive pulmonary aspergillosis in persistently neutropenic hosts. We therefore investigated the comparative antifungal efficacies, plasma micafungin pharmacokinetics, and safety of micafungin in models of disseminated candidiasis and invasive primary pulmonary aspergillosis in persistently neutropenic rabbits.
MATERIALS AND METHODS
Animals.
Female New Zealand White rabbits (n = 113; Hazleton Research Products, Inc. Denver, Pa.) weighing 2.5 to 3.5 kg at the time of inoculation were used in all experiments. Rabbits were individually housed and maintained according to guidelines of the National Institutes of Health (NIH) for animal care and in fulfillment of the criteria of the American Association for Accreditation of Laboratory Animal Care (25). Vascular access was established in each rabbit by the surgical placement of a silastic tunneled central venous catheter (41). The silastic catheter permitted nontraumatic venous access for repeated blood sampling for study of biochemical and hematological parameters, plasma micafungin pharmacokinetics, and administration of parenteral agents. Rabbits were euthanized by intravenous (i.v.) administration of sodium pentobarbital (65 mg [1 ml]/kg of body weight; The Butler Company, Columbus, Ohio) at the end of each experiment, 24 h after administration of the last dose of the drug.
Antifungal compounds.
Micafungin (Fujisawa Healthcare, Inc., Deerfield, Ill.) was provided as a sterile powder and was diluted further in sterile normal saline (NS; Quality Biological, Inc., Gaithersburg, Md.) to achieve the desired concentration. Desoxycholate amphotericin B (DAMB; Fungizone; Bristol-Myers Squibb Company, Princeton, N.J.) was resuspended in sterile water, maintained at 4°C, and, immediately prior to use, diluted at a 1:5 ratio with sterile 5% dextrose (Abbott Laboratories, North Chicago, Ill.) to achieve a final concentration of 1 mg/ml, according to the instructions of the manufacturer. Liposomal amphotericin B (LAMB; AmBisome; Fujisawa Healthcare, Inc.) was resuspended in sterile water and further diluted at 1:5 ratios with sterile 5% dextrose to achieve a concentration of 1 mg/ml.
Organisms and inoculation.
NIH isolate Candida albicans 8621 (ATCC MYA-1237) from a granulocytopenic patient with autopsy-proven disseminated candidiasis was used for all experiments involving rabbits with disseminated candidiasis. The MICs for C. albicans were determined by standard methods of NCCLS (23). The MIC was defined as the lowest concentration that caused an optically clear well. The MICs of micafungin and DAMB were 0.125 and 0.125 μg/ml, respectively, in antibiotic medium 3 (AM3; Media Unit, NIH, Bethesda, Md.). Cultures of the isolate were stored at −40°C in a skim milk suspension and at −70°C on potato dextrose agar slants. Cells from this suspension were streaked onto Sabouraud glucose agar (SGA) plates, incubated at 37°C for 24 h, and maintained during the course of these experiments at 4°C. For preparation of the inoculum, three well-isolated colonies were sampled from freshly grown culture plates and suspended in 50 ml of Emmon's modified Sabouraud glucose broth (pH 7.0) in a 250-ml Erlenmeyer flask. The suspension was incubated in a gyratory incubator at 80 oscillations per min at 37°C for 18 h. The Candida suspension was then centrifuged at 3,000 × g for 10 min and washed three times with sterile NS. The concentration was adjusted by use of a of hemacytometer and was confirmed by quantitative cultures of a 10-fold serial dilution. An inoculum of 103 blastoconidia suspended in a 5-ml volume of NS was slowly administered to each rabbit via the intravenous silastic catheter on day 6 of the experiment. The inoculum size was confirmed by plating serial dilutions onto SGA plates. The pattern of infection of disseminated candidiasis permitted survival of nearly all rabbits throughout the experiment.
NIH isolate Aspergillus fumigatus 4215 (ATCC MYA-1163), obtained from a patient with a fatal case of pulmonary aspergillosis, was used in all experiments. The MICs for A. fumigatus were determined by standard methods of NCCLS for filamentous fungi (24). The MIC of DAMB was defined as the lowest concentration that caused an optically clear well. The minimum effective concentration (MEC) of micafungin was the lowest concentration that caused the formation of microcolonies of A. fumigatus. The MECs of micafungin and DAMB were 0.25 and 1 μg/ml, respectively, in RPMI 1640 with phenol red (BioWhittaker, Walkersville, Md.). Pulmonary aspergillosis was established as described previously (7, 19). Prior to each experiment, the A. fumigatus inoculum was prepared fresh from a frozen isolate that had been stored at −70°C and that was subcultured onto Sabouraud dextrose slants (BBL Microbiology Systems, Cockeysville, Md.). Those slants were incubated for 24 h at 37°C and then kept at room temperature for 5 days before use. The conidia were harvested under a laminar airflow hood with a solution of 10 ml of 0.025% Tween 20 (Fisher Scientific, Fair Lawn, N.J.) in NS, transferred to a 50-ml conical tube, washed, and counted with a hemacytometer. The concentration was adjusted in order to give each rabbit a predetermined inoculum of 1 × 108 to 1.5 × 108 conidia of A. fumigatus in a volume of 250 to 350 μl. The concentrations of the inocula were confirmed by culture of serial dilutions on SGA. Inoculation was performed on day 2 of the experiments while the rabbits were under general anesthesia. Each rabbit was anesthetized with 0.8 to 1.0 ml of a 2:1 mixture (vol/vol) of i.v. ketamine (100 mg/ml; Ketaset; Phoenix Scientific, Inc., St. Joseph, Mo.) and xylazine (20 mg/ml; Rompun; Agriculture Division, Animal Health, Bayer Corp., Shawnee Mission, Kans.). Once satisfactory anesthesia was obtained, a Flagg O straight-blade laryngoscope (Welch Allyn Inc., Skaneateles Falls, N.Y.) was inserted into the oral cavity until the vocal cords were clearly visible. The A. fumigatus inoculum was then administered intratracheally with a tuberculin syringe attached to a 5 1/4-in. 16-gauge Teflon catheter (Becton Dickinson Infusion Therapy Systems Inc., Sandy, Utah).
Immunosuppression and maintenance of neutropenia.
Cytarabine (AraC; Cytosar-U; The Upjohn Company, Kalamazoo, Mich.) was administered i.v. for induction and maintenance of granulocytopenia. Profound granulocytopenia (a granulocyte concentration of <100 granulocytes/μl) was achieved in the disseminated candidiasis model by an initial i.v. course of 440 mg of AraC per m2 daily for 5 days before inoculation of the rabbits. A maintenance dose of 440 mg of AraC per m2 was administered at 2-day intervals during the experiment.
To simulate the conditions of persistent neutropenia in invasive pulmonary aspergillosis, therapy with AraC was initiated i.v. 1 day before the endotracheal inoculation of the animals. Profound and persistent neutropenia (a granulocyte concentration of <100 granulocytes/μl) was achieved by an initial course of 525 mg of AraC per m2 for 5 consecutive days. A maintenance dose of 484 mg of AraC per m2 was administered for 4 additional days (days 8, 9, 13, and 14) of the experiment. Concomitant thrombocytopenia was present in a range of 30,000 to 50,000 platelets/μl. Methylprednisolone (5 mg/kg; Abbott Laboratories) was administered on days 1 and 2 of the experiment to inhibit macrophage activity against conidia in order to facilitate establishment of invasive pulmonary aspergillosis.
All rabbits with disseminated candidiasis and invasive pulmonary aspergillosis received ceftazidime (Glaxo Pharmaceuticals, Division of Glaxo Inc., Research Triangle Park, N.C.) at 75 mg/kg i.v. twice daily, gentamicin (Elkins-Sinn, Inc., Cherry Hill, N.J.) at 5 mg/kg i.v. every other day, and vancomycin (Abbott Laboratories) at 15 mg/kg i.v. daily from day 4 of chemotherapy for the prevention of opportunistic bacterial infections during neutropenia. In order to prevent antibiotic-associated diarrhea due to Clostridium spiriforme, all rabbits received 50 mg of vancomycin per liter of drinking water.
Total leukocyte counts and the percentages of granulocytes were monitored twice weekly with a Coulter counter (Coulter Corporation, Miami, Fla.) and by use of peripheral blood smears and differential counts, respectively.
Treatment groups.
The treatment groups in the model of disseminated candidiasis consisted of untreated control (UC) animals and animals treated with either micafungin or DAMB. Micafungin was administered as an i.v. bolus over 4 min at dosages of 0.25 mg/kg/day (FK0.25), 0.5 mg/kg/day (FK0.5), 1 mg/kg/day (FK1), or 2 mg/kg/day (FK2). DAMB was administered i.v. at 1 mg/kg/day (0.1 ml every 10 s). The treatment groups in the model of invasive pulmonary aspergillosis consisted of UC animals or animals treated i.v. with FK0.25, FK1, FK2, or DAMB or with LAMB at 5 mg/kg/day (0.1 ml every 10 s).
Antifungal therapy in all treatment groups was initiated 24 h after inoculation. Treatment was continued throughout the course of the experiments for a maximum of 10 days in surviving rabbits with disseminated candidiasis and for a maximum of 12 days in rabbits with invasive pulmonary aspergillosis.
In vitro pharmacodynamics.
The time-concentration profile of the antifungal activities of micafungin and DAMB against C. albicans in vitro were assessed by the time-kill methodology. In brief, C. albicans was grown overnight on SGA plates. Three colonies were then inoculated into 50 ml of Sabouraud glucose broth in a 250-ml Erlenmeyer flask and incubated in a gyratory water bath at 37°C for 18 h in order to generate log-phase growth. This suspension was centrifuged and washed twice with NS. Colony counts were adjusted to 1 × 106 to 5 × 106 CFU/ml with a spectrophotometer (transmittance, 74 to 77%). Byadding 1.5 ml of this suspension to 13.5 ml of AM3, a 1:10 dilution that yielded a final inoculum of 1 × 105 to 5 × 105 CFU/ml was obtained.
The drugs were diluted in AM3 to concentrations of 100 times the final concentration. The appropriate final drug concentrations were obtained by diluting 150 μl of the drug concentrate into the final volume of 15 ml. A growth control was included, as was a flask containing only 15 ml of AM3 as a sterility control. The following drug concentrations were used for C. albicans: 0.01, 0.1, 0.25, 1, 2, and 4 μg/ml for DAMB and 0.01, 0.1, 0.25, 1, 2, and 4 μg/ml for micafungin. Test solutions were placed in 150-ml flasks, and the flasks were placed in a shaking water bath at 37°C. Samples of the suspensions of growth were obtained at 0, 2, 4, 6, and 24 h; 100-μl aliquots of the original broth and serial 10-fold dilutions were plated onto individual SGA plates; and the plates were incubated at 37°C. The colonies were counted after 24 h, and the calculated number of CFU per milliliter was plotted for each time point. The lower limit of quantitation for the time-kill assay was 10 CFU/ml. The use of in vitro pharmacodynamic assays for C. albicans refines the selection of dosage groups and reduces the number of animals required for in vivo studies.
Assessment of antifungal efficacy against experimental disseminated candidiasis.
Antifungal efficacy in the model of disseminated candidiasis was determined by quantitative measurement of the clearance of C. albicans from tissue. Representative sections of liver, spleen, kidney, lung, anterior vena cava, and brain were weighed; and each tissue sample was then homogenized (Stomacher 80; Tekmar Corp., Cincinnati, Ohio) in sterile reinforced polyethylene bags (Tekmar) with sterile NS for 30 s (39). The globes of the eyes were carefully dissected by the use of aseptic technique. The globe was transferred to a sterile petri dish (Falcon; Becton Dickinson Labware, Becton Dickinson and Co., Franklin Lakes, N.J.), and remaining parts of ocular muscles and orbital fat tissue were carefully cleaned from the globe. The posterior pole of the globe was gently rubbed with sterile gauze to remove any remaining capillaries. The sclera was incised with sharp scissors at the posterior pole, and 0.3 to 0.4 ml of vitreous was slowly aspirated into a sterile tuberculin syringe. The specimens of vitreous from both globes were pooled. Each tissue homogenate or vitreous specimen was serially diluted 10−1 to 10−4 in sterile NS. Aliquots (100 μl) of undiluted tissue homogenate or vitreous and of each dilution were separately plated onto Emmon's modified SGA containing chloramphenicol and gentamicin. Culture plates were incubated at 37°C for 24 h, after which the numbers of CFU were counted and the numbers of CFU per gram of tissue were calculated for each organ. The method was sensitive for the detection of ≥10 CFU/g. The culture-negative plates were considered to have 0 CFU/g. The data were plotted as the mean ± standard error of the mean (SEM) log10 CFU per gram.
Assessment of antifungal efficacy against experimental invasive pulmonary aspergillosis.
The following panel of outcome variables was used to assess antifungal efficacy: residual fungal burden in lung tissue (numbers of CFU per gram), pulmonary infarct score, lung weight, and survival.
Fungal cultures.
Lung tissue from each rabbit was sampled and cultured by excision of tissue from each lobe by standard methods. Each tissue fragment was weighed individually, placed in a sterile polyethylene bag, and homogenized with sterile saline for 30 s (38). Lung homogenate dilutions (10−1 and 10−2) were prepared in sterile saline. Aliquots (100 μl) from homogenates and homogenate dilutions were plated onto SGA plates, and the plates were incubated at 37°C for the first 24 h and then at room temperature for another 24 h. The number of CFU of A. fumigatus was counted and recorded for each lobe, and the number of CFU per gram was calculated. A finding of one colony of A. fumigatus was considered a positive result.
Pulmonary lesion scores.
The entire heart-lung block was carefully resected at autopsy. The heart was then dissected away from the lungs, leaving the tracheobronchial tree and lungs intact. The lungs were weighed and inspected by at least two observers who were blinded to the treatment group and who recorded the hemorrhagic infarct lesions (if any) in each individual lobe. Hemorrhagic infarcts were dark red consolidated lesions that corresponded histologically to coagulative necrosis and intra-alveolar hemorrhage. The number of positive lobes was added together, and the mean value for all positive lobes was calculated for each treatment group.
Survival time.
The survival time (in days postinoculation) was recorded for each rabbit in each treatment group. The surviving rabbits were euthanized by sodium pentobarbital anesthesia on day 13 postinoculation.
Galactomannan assay.
Blood from each rabbit infected with A. fumigatus was collected every other day for determination of serum galactomannan concentrations. Serum galactomannan concentrations were determined by the Platelia Aspergillus EIA (Genetic Systems/Sanofi Diagnostics Pasteur, Redmond, Wash.) one-stage immunoenzymatic sandwich microplate assay method (Platelia Aspergillus 62797; Immunoenzymatic Detection of Galactomannan Antigen of Aspergillus in Serum; Sanofi Diagnostics Pasteur). The assay used rat monoclonal antibody EB-A2, which is directed against Aspergillus galactomannan (33, 34). The monoclonal antibody is used to sensitize the wells of the microplate and to bind to the antigen. Peroxidase-linked rat monoclonal antibody is used as the detector antibody.
A 300-μl volume of a rabbit serum sample was mixed with 100 μl of 4% EDTA treatment solution, and the mixture was boiled for 3 min in order to dissociate the immune complexes and to precipitate serum proteins. After centrifugation at 10,000 × g for 10 min, 50 μl of the supernatant was added to 50 μl of a reaction mixture containing peroxidase-conjugated antigalactomannan monoclonal antibody EB-A2. The 100-μl mixture was added to the wells of a microtitration plate coated with the same monoclonal antibody (EB-A2), and the plate was then incubated for 90 min at 37°C. A monoclonal antibody-galactomannan-monoclonal antibody-peroxidase complex was formed in the presence of Aspergillus antigen. The microtiter plates were washed to remove any unbound material. Next, 200 μl of a substrate solution which reacted with the complexes bound to the well to form a blue color reaction was added to each well. The plates were incubated for another 30 min in darkness at room temperature. The enzymatic reaction was stopped by the addition of 100 μl of stopping solution (1.5 N sulfuric acid), which changed the blue color to yellow. The optical absorbances of the specimens and the controls were determined with a microplate spectrophotometer equipped with 450- and 620-nm filters (Multiscan MMC/340; Titertek, Huntsville, Ala.).
Enzyme immunoassay data were expressed as a serum galactomannan index (GMI) and were plotted over time. The GMI for each test serum sample is equal to the optical density of the sample divided by the optical density of a serum sample with a threshold value provided with the test kit. Sera with GMIs less than 1 were considered negative. Sera with GMIs greater than 1.5 were considered positive. Sera with GMIs between 1 and 1.5 were considered indeterminate. Serial serum galactomannan levels were plotted over time of administration of antifungal compound.
Histopathology.
Representative sections of kidney in the model of disseminated candidiasis and representative sections of lung in the model of pulmonary aspergillosis were prepared for histologic studies. Tissue specimens were excised and fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and then stained with either periodic acid-Schiff or Grocott-Gomori methenamine silver. The C. albicans and A. fumigatus organisms in the tissue specimens were microscopically examined for structural changes, and the tissue specimens were microscopically examined for visual confirmation with microbiologic clearance.
Toxicity studies.
Blood was collected from each rabbit every other day, starting from the first day after inoculation and continuing throughout treatment. Plasma samples were stored in Sarstedt tubes (Sarstedt Inc., Newton, N.C.) at −70°C until all samples were processed simultaneously. Potassium, aspartyl aminotransaminase, alanine aminotransaminase, serum creatinine, serum urea nitrogen, alkaline phosphatase, and total bilirubin levels were determined in the penultimate sample drawn from each rabbit.
Pharmacokinetic studies.
The plasma micafungin pharmacokinetics were investigated in 6 to 12 infected animals in each dosage cohort by using optimal plasma sampling. Time points for optimal plasma sampling were determined on the basis of full profiles of the concentration in plasma obtained with healthy rabbits (13) with the aid of the ADAPT II computer program (D. Z. D'Argenio and A. Schumitzky, Adapt II user's guide, Biomedical Simulations Resource, University of Southern California, Los Angeles). Plasma sampling was performed on day 6 of antifungal therapy. Blood samples were drawn at 0.1, 0.3, 2, 6, and 24 h postdosing in heparinized syringes. The plasma was immediately separated by centrifugation and stored at −80°C until assay.
Micafungin was extracted from acidified heparinized plasma by liquid-liquid extraction with acetonitrile-based organic solvents and was diluted with phosphate buffer. The concentrations of micafungin were determined by reversed-phase high-performance liquid chromatography (13). External standards and quality control samples were prepared by adding known amounts of micafungin to plasma samples from healthy rabbits (Gibco Laboratories, Grand Islands, N.Y.). Prior to extraction, 0.5 μg of LY306168, the internal standard, was added to 300 μl of the sample, the external standard, or the quality control sample to serve as an internal control for the accuracy and precision of the procedure. The mobile phase consisted of 20 mM KH2PO4-acetonitrile (59:41; vol/vol) delivered at 1 ml/min. The samples were maintained in the autosampler at room temperature in amber glass vials. The injection volume was 75 μl. Micafungin eluted at 10.3 to 13.8 min with a 5 μ TSK-GEL silica-based analytical column (150 by 4.6 mm; ODS80TM; TosoHaas, Montgomeryville, Pa.) maintained at 50°C in conjunction with a precolumn filter containing a 5-μm insert and fluorometric detection (excitation wavelength, 273 nm; emission wavelength, 464 nm). Quantitation was based on the peak height of micafungin and the nonweighted concentration response of the external calibration standard. Eight- to 10-point standard curves (range, 0.05 to 25 μg/ml) were linear, with r2 values being greater then 0.987. The lower limit of quantitation in plasma was 0.100 μg/ml. Accuracies were within 1.7 to 12.8%, and intra- and interday variabilities (precision) ranged from 1.2 to 6.8%.
Pharmacokinetic parameters for micafungin were determined by compartmental analysis and a Bayesian approach with microconstants derived from previous pharmacokinetic studies with healthy rabbits (13). Pharmacokinetic modeling was performed with the ADAPT II (7) computer program by using iterative weighted nonlinear least-squares regression analysis. Model selection was guided by Akaike's information criterion (43). The concentration-time profiles of micafungin were best described by a two-compartment open model with an i.v. bolus input and linear first-order elimination from the central compartment. The model fit the optimal sampling-derived data well. The regression lines through the plot of the observed concentrations versus the estimated concentrations did not differ from the line of identity, and no bias was observed. r2 values for the individual fits ranged from 0.947 to 1.00 (mean, 0.996). The maximum concentrations of drug in serum (Cmaxs) were determined as model-estimated concentrations 6 min after the start of administration of the i.v. bolus, and the concentrations of drug in serum 24 h after dosing (Cmins) were determined as model-estimated concentrations 24 h postdosing, respectively. Areas under the concentration-time curve (AUC) from time zero to 24 h (AUC0-24s) were calculated from estimated profiles of the concentration in plasma by using the trapezoidal rule, and the AUCs from time zero to infinity (AUC0-∞s) were calculated by extrapolation to infinity by a standard technique (11). Dose linearity was determined by comparison of the dose-normalized AUC0-∞ across dosage levels.
Statistical analysis.
Values are expressed as means ± SEMs. All treatment groups were compared against the untreated control group by analysis of variance (ANOVA) with Bonferroni's correction for multiple comparisons. The central hypothesis of this analysis is based upon the response of the treated rabbits in comparison to that of the UCs. A two-tailed P value of <0.05, which had already been adjusted for multiple comparisons by Bonferroni's method, was considered statistically significant. Survival was plotted by Kaplan-Meier analysis. Differences in survival times between the treatment groups and the UCs were analyzed by the log rank test.
Differences between the means for the pharmacokinetic parameters across dosage levels were evaluated by ANOVA and the Mann-Whitney U test, as appropriate. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Time-kill assay.
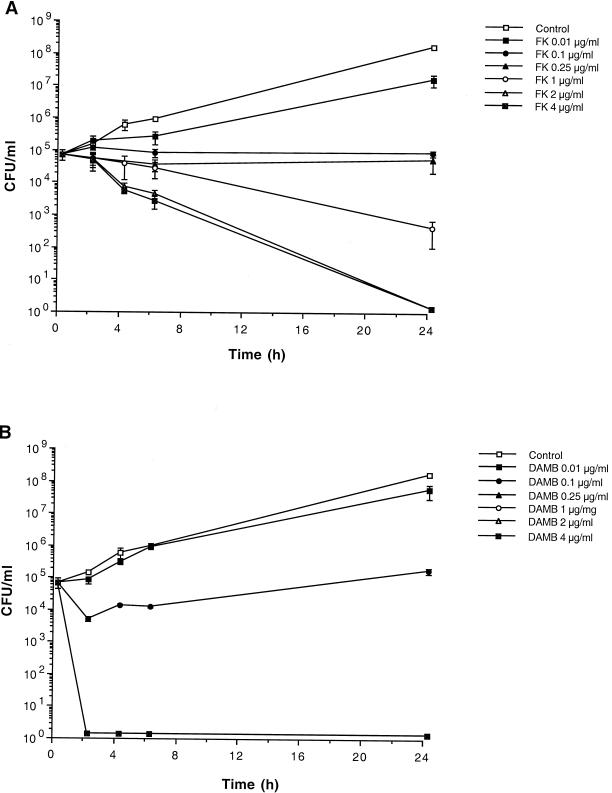
Micafungin and DAMB had concentration-dependent fungicidal activities against C. albicans, as demonstrated by time-kill curves (Fig. 1). FK0.1 to FK0.25 caused inhibition of growth, while FK1 caused a 90% reduction in the C. albicans burden at 24 h and FK2 and FK4 caused a >99.9% reduction in the C. albicans burden at 24 h. Inhibition of growth by DAMB occurred at 0.01 μg/ml, while it had a very rapid fungicidal effect at ≥0.25 μg/ml. The rate of killing of C. albicans appeared to be faster for DAMB than for micafungin at concentrations ≥0.25 μg/ml.
FIG. 1.
Time-kill assay of micafungin (A) and amphotericin B (B) against C. albicans in AM3. The effects of treatment with concentrations of micafungin and amphotericin B at 0.01, 0.1, 0.25, 1, 2, and 4 μg/ml were studied in relation to no treatment (growth control). Data are plotted as the means ± SEMs from three separate experiments for each growth curve. As the SEM was small for several time points, the error bars may not always be apparent in the time-kill curves.
Treatment of disseminated candidiasis.
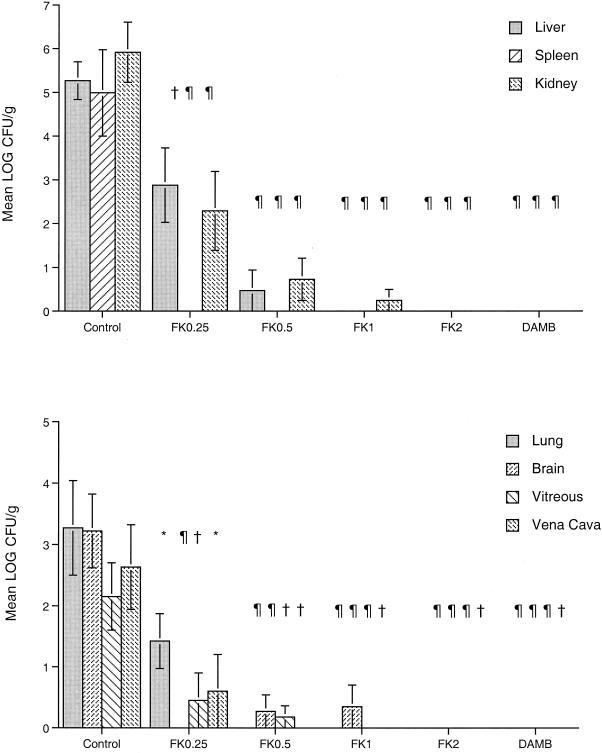
Micafungin demonstrated significant dose-dependent antifungal efficacy in the treatment of disseminated candidiasis (Fig. 2). The clearance of C. albicans from the liver (P ≤ 0.001), spleen (P ≤ 0.001), kidney (P ≤ 0.001), lung (P ≤ 0.001), brain (P < 0.001), vena cava (P < 0.01), and vitreous (P < 0.01) of rabbits treated with FK0.5, FK1, FK2, and DAMB was significantly better than that from the tissues of the UCs. In comparison with UC rabbits, FK0.25 also significantly reduced the numbers of C. albicans CFU per gram in all tissues (P < 0.05). FK2 and DAMB at 1 mg/kg reduced the C. albicans burdens to below the limit of detection in all tissues.
FIG. 2.
Responses of disseminated candidiasis in persistently neutropenic rabbits to antifungal therapy, measured by determination of the mean concentrations (in log CFU per gram) of the organism in the liver, spleen, kidney, lung, brain, vena cava, and vitreous of UCs (n = 11) and rabbits treated with FK0.25 (n = 8), FK0.5 (n = 8), FK1 (n = 8), FK (n = 6), and DAMB at 1 mg/kg/day (n = 6). Values are means ± SEMs. ∗, P < 0.05; †, P < 0.01; ¶, P < 0.001. P values are for the results for treated rabbits in comparison to those for the UCs, as determined by ANOVA with Bonferroni's correction for multiple comparisons.
Treatment of pulmonary aspergillosis.
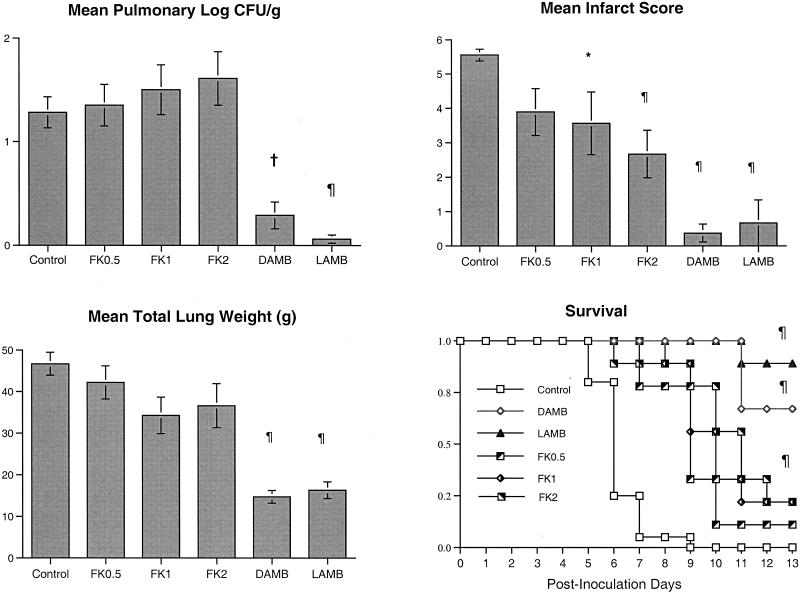
Rabbits treated with micafungin at all dosages had no quantitative reductions in A. fumigatus growth in their lung tissues in comparison to the growth in the lung tissues of the UCs. In contrast, there was a significant reduction in A. fumigatus growth in the lung tissues of rabbits treated with either LAMB or DAMB in comparison to the growth in the lung tissues of the UCs (P < 0.01 and P < 0.001, respectively) (Fig. 3). Despite the lack of microbiologic clearance of A. fumigatus from lung tissue, other parameters demonstrated the antifungal effect of micafungin.
FIG. 3.
Responses of primary pulmonary aspergillosis in persistently neutropenic rabbits to antifungal therapy are measured by determination of the mean concentrations of residual organisms (in log CFU per gram) in pulmonary tissue from the pulmonary infarct score and total lung weight for UCs (n = 20) and rabbits treated with FK0.5 (n = 9), FK1 (n = 9), FK2 (n = 9), DAMB (n = 8), and LAMB (n = 9). A Kaplan-Meier plot of the survival times of persistently neutropenic rabbits receiving micafungin, DAMB, or LAMB versus those for UCs is shown at the bottom right. Differences in the survival times of the treated groups versus those of the UCs were analyzed by the log rank test. Values are given as means ± SEMs. ∗, P < 0.05; †, P < 0.01; ¶, P < 0.001. P values are for the results for treated rabbits in comparison to those for the UCs, as determined by ANOVA with Bonferroni's correction for multiple comparisons.
There was a significant reduction in the level of organism-mediated pulmonary tissue injury, as measured by the pulmonary infarct score, in rabbits treated with FK1, FK2, DAMB, and LAMB in comparison to the level of injury in the UCs (P < 0.05, P < 0.01, P < 0.01, and P < 0.01, respectively) (Fig. 3). There was a significant reduction in the mean lung weights for rabbits treated with DAMB and LAMB in comparison to the mean lung weights for the UCs (P < 0.01 and P < 0.01, respectively). On the other hand, no significant differences in lung weights were observed between animals treated with FK0.5, FK1, and FK2 and the UCs. Corresponding to this reduction in organism-mediated pulmonary injury, there was a significant improvement in the survival rates of rabbits treated with FK0.5, FK1, FK2, DAMB, and LAMB in comparison to those of the UCs (P < 0.001).
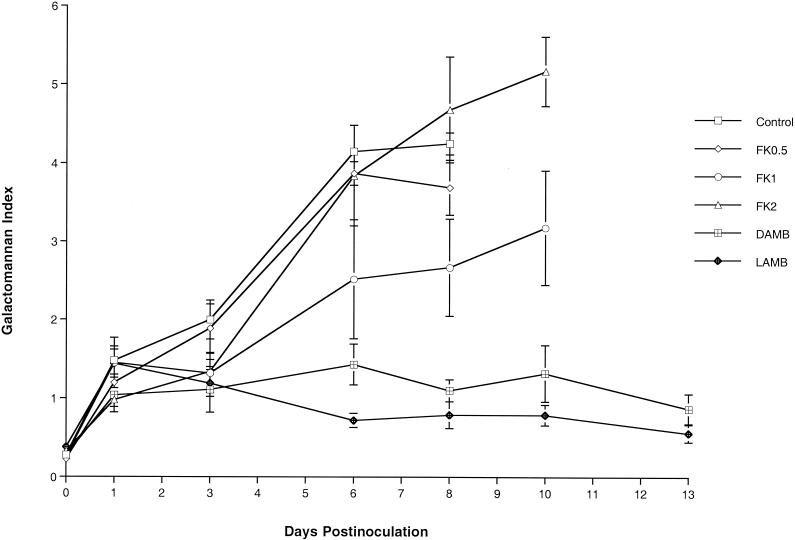
We further investigated the expression of galactomannan in the sera of rabbits with pulmonary aspergillosis as a surrogate marker of the therapeutic response. Expression of galactomannan was examined in the following groups: UCs and rabbits treated with FK0.5, FK1, FK2, DAMB, and LAMB (Fig. 4). The GMI was positive (≥1.5) for the UCs and FK0.5-treated rabbits 3 days after inoculation and for the FK1- and FK2-treated rabbits 6 days after inoculation. No galactomannan antigenemia values are available for the UCs and the FK0.5-treated animals after day 8 because all animals died. Serial serum samples from the UCs and from FK0.5-, FK1-, and FK2-treated rabbits showed that progressive galactomannan antigenemia correlated with increased residual fungal burdens (in log CFU per gram). By comparison, the GMIs for the sera of rabbits treated with DAMB and LAMB were negative during the entire treatment period, while the GMIs correlated with low residual fungal burdens (in log CFU per gram) in those groups (P < 0.001 by ANOVA).
FIG. 4.
Expression of galactomannan antigenemia in persistently neutropenic rabbits with pulmonary aspergillosis that were left untreated (controls) or treated with FK0.1, FK1, FK2, DAMB, or LAMB.
Histopathology.
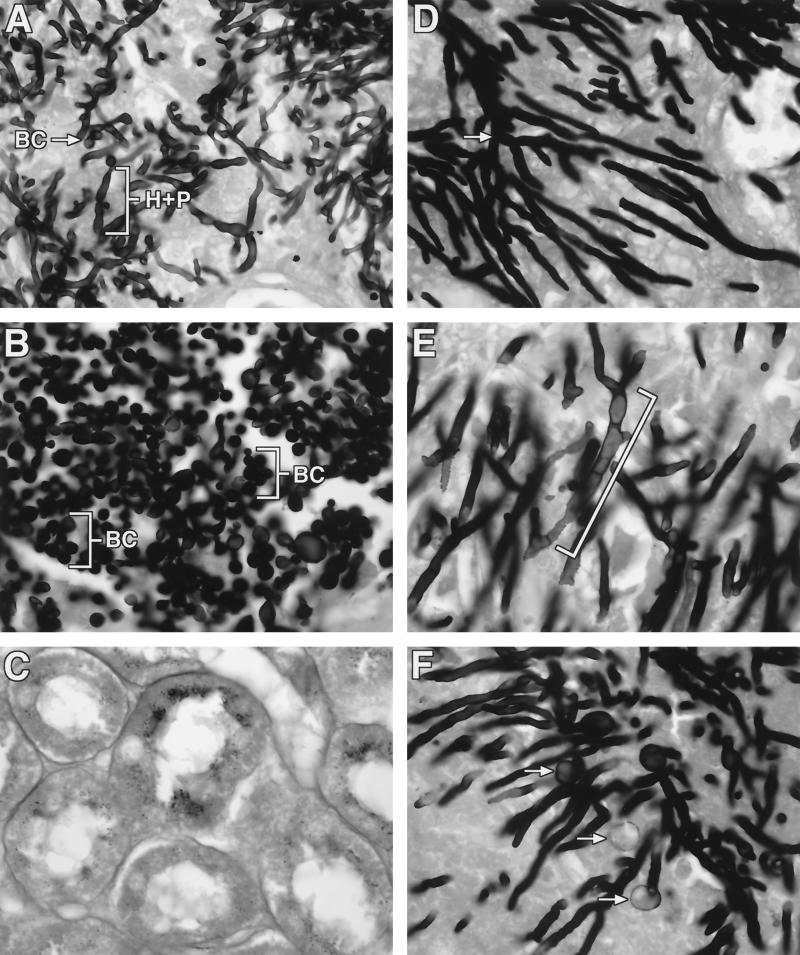
Micafungin had a concentration-dependent effect on the clearance of histologically detectable C. albicans organisms from tissue and also a dose-dependent effect on the microscopic morphology of the Candida cell wall structure in the rabbits with disseminated candidiasis. A transition from a predominance of hyphae and pseudohyphae in UCs to a predominance of yeast-like structures in rabbits treated with FK0.25 occurred. There was no histologic evidence of organisms in rabbits treated with micafungin at ≥0.5 mg/kg/day (Fig. 5A to C). By comparison, micafungin caused dose-dependent damage of hyphal structures in the lung tissues of animals with invasive pulmonary aspergillosis but did not clear histologically detectable A. fumigatus from the lung tissues. Organisms from UC rabbits (Fig. 5D) had typical elongated branching septate hyphae. Organisms from rabbits treated with FK0.5 (Fig. 5E) demonstrated fragmented and shortened hyphal elements with progressive hyphal swelling. Organisms from rabbits treated with FK2 (Fig. 5F) had the greatest level of apparent cell wall damage, as evidenced by the presence of spheroplast-like structures.
FIG. 5.
Effects of micafungin in vivo on hyphal structures of C. albicans and A. fumigatus in rabbits with experimental candidiasis and experimental pulmonary aspergillosis. All specimens were stained with Grocott-Gomori methalamine silver. (A) Hyphae (H), pseudohyphae (P), and blastoconidia (BC) of C. albicans in kidney tissue from a neutropenic UC rabbit with disseminated candidiasis. (B) Multiple blastoconidia (BC) (budding yeast forms) and a loss of hyphae and pseudohyphae in kidney tissue from a neutropenic rabbit receiving FK0.25. (C) Complete histologic eradication of C. albicans from kidney tissue from a neutropenic rabbit receiving FK0.5. (D) Branching septate hyphae (arrow) in lung tissue from a neutropenic UC rabbit with invasive pulmonary aspergillosis. (E) A. fumigatus hyphae from a neutropenic rabbit receiving FK0.5; the hyphae are wider and shorter (bracket) than those in the lung tissues of UCs. (F) Chlamydospore-like structures (arrows) and truncated hyphae in lung tissue from a neutropenic rabbit receiving FK2. Magnification, ×540.
Plasma micafungin pharmacokinetics.
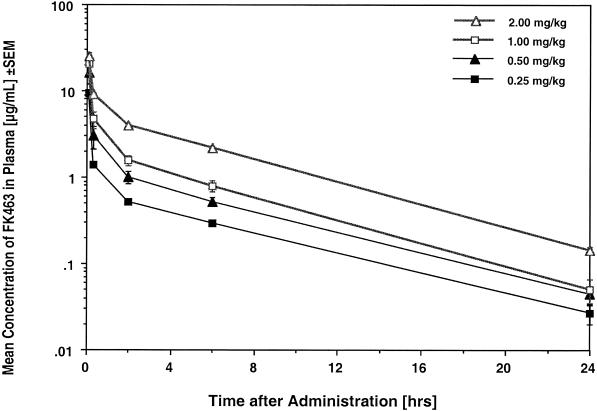
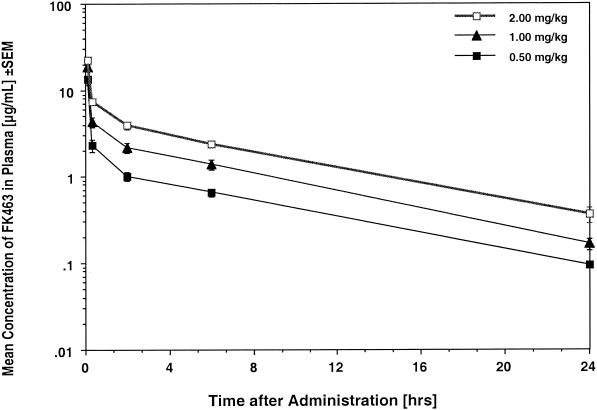
The estimated plasma concentration-versus-time profiles of micafungin in rabbits with disseminated candidiasis and invasive pulmonary aspergillosis are shown in Fig. 6 and 7, respectively, and the corresponding compartmental pharmacokinetic parameters are shown in Table 1 and Table 2, respectively. Treatment with FK0.25 to FK2.0 resulted in escalating mean peak levels in plasma that ranged from 9.31 ± 1.11 to 25.21 ± 2.39 μg/ml, which exceeded the MICs and MECs for the experimental isolates. Mean levels in plasma remained above these concentrations for 8 to 24 h. Micafungin was eliminated from plasma with mean terminal half-lives ranging from 4.34 to 6.78 h. Micafungin demonstrated linear plasma micafungin pharmacokinetics, with constant clearances and overall unchanged dose-normalized AUC0-∞ values across the dosage range investigated.
FIG. 6.
Plasma micafungin (FK463) concentration profiles for persistently neutropenic rabbits with experimental subacute disseminated candidiasis. All values are means ± SEMs for six to eight rabbits each.
FIG. 7.
Plasma micafungin (FK463) concentration profiles for persistently neutropenic rabbits with experimental invasive pulmonary aspergillosis. All values are means ± SEMs for 10 to 12 rabbits each.
TABLE 1.
Compartmental pharmacokinetic parameters in persistently neutropenic rabbits with experimental subacute disseminated candidiasisa
| Micafungin dosage (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0-24 (μg · h/ml) | AUC0-∞/dose | VSS (liter/kg) | CL (liter/h/kg) | t112 (h) |
|---|---|---|---|---|---|---|---|
| 0.25 | 9.31 ± 1.11 | 0.02 ± 0.00 | 5.32 ± 0.25 | 9.16 ± 0.67 | 0.283 ± 0.04 | 0.043 ± 0.00 | 5.37 ± 0.77 |
| 0.5 | 16.27 ± 4.17 | 0.04 ± 0.01 | 9.75 ± 1.29 | 8.26 ± 1.12 | 0.298 ± 0.03 | 0.051 ± 0.00 | 4.91 ± 0.41 |
| 1.0 | 20.44 ± 4.92 | 0.05 ± 0.01 | 14.47 ± 2.03 | 5.90 ± 0.72 | 0.277 ± 0.03 | 0.052 ± 0.00 | 4.34 ± 0.44 |
| 2.0 | 25.21 ± 2.39 | 0.14 ± 0.01 | 36.33 ± 1.28 | 6.92 ± 0.30 | 0.326 ± 0.01 | 0.053 ± 0.00 | 4.56 ± 0.11 |
| P (ANOVA) | 0.0275 | <0.0001 | <0.0001 | 0.0413 | 0.8247 | 0.2915 | 0.5508 |
Values are means ± SEMs for six to eight rabbits each. VSS, apparent volume of distribution at steady state; CL, total clearance from plasma; t112, elimination half-life.
TABLE 2.
Compartmental pharmacokinetic parameters in persistently neutropenic rabbits with experimental invasive pulmonary aspergillosisa
| Micafungin dosage (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0-24 (μg · h/ml) | AUC0-∞/dose | VSS (liter/kg) | CL (liter/h/kg) | t112 (h) |
|---|---|---|---|---|---|---|---|
| 0.5 | 13.36 ± 1.61 | 0.09 ± 0.00 | 11.47 ± 1.25 | 9.44 ± 1.07 | 0.407 ± 0.07 | 0.045 ± 0.00 | 6.77 ± 0.51 |
| 1.0 | 18.48 ± 1.40 | 0.16 ± 0.02 | 22.86 ± 2.86 | 9.15 ± 1.10 | 0.391 ± 0.05 | 0.049 ± 0.00 | 5.64 ± 0.43 |
| 2.0 | 22.49 ± 1.22 | 0.36 ± 0.07 | 45.57 ± 3.60 | 9.22 ± 0.74 | 0.419 ± 0.05 | 0.045 ± 0.00 | 6.78 ± 0.74 |
| P (ANOVA) | 0.0008 | 0.0020 | <0.0001 | 0.9789 | 0.9440 | 0.7695 | 0.2667 |
Values are means ± SEMs for 10 to 12 rabbits each. VSS, apparent volume of distribution at steady state; CL, total clearance from plasma; t1/2, elimination half-life.
The level of exposure to drug was greater in a dose-dependent manner for rabbits with invasive pulmonary aspergillosis than for rabbits with subacute disseminated candidiasis, as reflected by significantly higher mean AUC0-24 values (P = 0.0075) and trough concentrations (P = 0.0420), slower clearances from plasma (P = 0.0075), and longer elimination half-lives (P = 0.0420) for the 2-mg/kg dosage group. Similar trends were observed for the 0.5- and 1.0-mg/kg dosage groups.
Safety.
The mean serum creatinine and serum urea nitrogen levels were significantly greater in DAMB-treated rabbits than in the UCs (4.97 ± 1.54 versus 0.77 ± 0.01 and 139.89 ± 36.51 versus 21.78 ± 2.25 mg/dl, respectively) (P < 0.001). By comparison, the serum creatinine levels in micafungin- and LAMB-treated rabbits were unchanged in comparison to those in the UCs. There were no differences in aspartyl aminotransaminase, alanine aminotransaminase, serum potassium, and bilirubin levels in any of the treatment groups compared to those in the UCs.
DISCUSSION
This study demonstrated that micafungin has differential antifungal activities in the treatment of disseminated candidiasis and invasive pulmonary aspergillosis in profoundly neutropenic hosts. The in vivo effects of this echinocandin against the tissue burden of C. albicans were dosage dependent and correlated with the concentration-dependent fungicidal activities demonstrated in time-kill assays. By comparison, the drug had no activity in the eradication of the residual burden of A. fumigatus (in CFU per gram) from pulmonary tissue. The elevation of the level of galactomannan antigenemia in the animal model of invasive pulmonary aspergillosis treated with micafungin correlates with these findings. Further demonstration of the differential activities of micafungin against disseminated candidiasis compared with those against invasive aspergillosis is the histologic eradication of fungal elements of C. albicans from tissue in a dosage-dependent manner but the persistence of hyphal elements of A. fumigatus in tissue. This is the first study to our knowledge to demonstrate the differential effects of micafungin against C. albicans and A. fumigatus in persistently neutropenic hosts.
The dosage-dependent antifungal effects of micafungin were observed in all tissues, including the liver, spleen, kidney, lung, vena cava, and vitreous. Micafungin completely eradicated C. albicans from brain tissue when it was used at 2 mg/kg/day, while it cleared non-central nervous system tissues with the exception of the kidney of C. albicans when it was used at ≤1 mg/kg/day. These findings suggest that even though the blood-brain barrier was disrupted due to Candida meningoencephalitis, it may limit the access of the relatively large echinocandin molecule to brain parenchymal tissue. As micafungin is only minimally excreted in urine, higher dosages may be necessary to eradicate C. albicans from renal tissue (15). Eradication of renal candidiasis by echinocandins may occur more readily in the vascularized interstitium and less so in the renal tubules, where drug concentrations will likely be lower.
The effects of micafungin on C. albicans viability are apparent at dosages as low as 0.25 mg/kg/day. For all tissues, the morphological effects of micafungin on C. albicans resulted in a transition from a predominance of pseudohyphae to a predominance of blastoconidia and subsequently to histologic eradication. These morphological effects of the echinocandin appear to be the result of disruption of essential cell wall structures and are especially evident by the lack of formation of hyphal elements and the formation of large chlamydospore-like yeast forms, as seen in Fig. 5B. Earlier work by Cole and colleagues (6) found that those vesicular structures induced by echinocandins have many of the histochemical properties of chlamydospores of C. albicans. Klepser et al. (17) also described the effects of sublethal concentrations of echinocandins on cell wall morphology by using scanning electron microscopy to evaluate cell wall changes.
Micafungin successfully reduced the burden of C. albicans in the vitreous by >103-fold. No C. albicans organisms were detectable in the vitreous of animals treated with dosages ≥1.0 mg/kg. Although the cyclic hexapeptide molecule of echinocandins is a relatively large structure, it penetrated into the vitreous to produce a significant reduction in organism burden. This finding may have important implications for the use of echinocandins in patients with Candida endophthalmitis.
Despite the lack of eradication of A. fumigatus from lung tissue, treatment with micafungin resulted in prolonged survival times comparable to those achieved by treatment with LAMB. The basis for this improved survival may be related to the favorable safety profile of micafungin and its ability to damage hyphae, thereby reducing the levels of angioinvasion and pulmonary infarct scores. The antifungal effect of micafungin at 0.5 mg/kg/day on hyphal structures was apparent, with fragmentation and truncation of hyphal elements. A further increase in the dosage was associated with more hyphal fragmentation as well as chlamydospore-like formation. These alterations in hyphal structures were associated with reductions in the level of pulmonary injury, as measured by the mean pulmonary infarct score, and suggest that damaged hyphal elements appear to have reduced angioinvasive properties, as observed histologically. Notably, however, increasing dosages were also associated with a trend toward an increasing fungal organism load, consistent with the observation of increasing fragmentation and potentially more viable units of the organism.
By comparison, DAMB- and LAMB-treated animals demonstrated greater clearance of the residual fungal burden, as well as decreases in mean pulmonary infarct score and total lung weight. Evidence from a comparison of the two treatment groups (micafungin- versus amphotericin B-treated animals) suggests that the echinocandin may be altering the hyphal structure to prevent progressive pulmonary angioinvasion through a mechanism that continues to permit the fungal elements to survive. This process may be more gradual than the rapid fungicidal effect of amphotericin B against Aspergillus hyphae.
Further evidence consistent with the persistence of viable fungal elements is the continued increase in the GMI for micafungin-treated rabbits with invasive pulmonary aspergillosis. By comparison, the rapid fungicidal effects of DAMB and LAMB are reflected in the early and sustained suppression of serum galactomannan levels below a GMI of 1.5. The apparent paradox between the micafungin dose escalation and an increase in the GMI is possibly due to increased drug-mediated hyphal fragmentation. Micafungin treatment leads to the recovery of increased amounts of galactomannan in serum as the A. fumigatus hyphae are increasingly damaged. The fact that echinocandin-treated animals had progressive rises in galactomannan levels in serum suggests that the continued persistence of viable organisms may be important in understanding the effect of micafungin on the GMI in patients receiving an echinocandin for invasive aspergillosis. Such patients may have a rising GMI despite clinical improvement.
Recent studies of micafungin in the treatment of invasive candidiasis and aspergillosis in transiently neutropenic or hydrocortisone-immunosuppressed mice found significant improvement in survival times and a reduction in the levels of viable C. albicans organisms in kidney tissue (16). Those experiments also showed that micafungin incompletely eradicated C. albicans from tissue. No additional information on the eradication of C. albicans from other tissues was reported. When micafungin was administered by continuous infusion in a transiently neutropenic mouse model of pulmonary aspergillosis, a reduction of the residual fungal burden in the lungs was observed (20). However, despite the administration of 4 mg/kg, there was a concentration-dependent reduction of the counts from 3.99 to 1.71 log CFU/g but no eradication of the organisms. Whether maintenance of a continuous high dose of echinocandin could improve the outcome of aspergillosis requires further investigation. Earlier studies with cilofungin, another echinocandin, demonstrated that it had superior activity against experimental disseminated candidiasis when it was given as a continuous infusion than when it was given as an intermittent infusion (42). Further adding to the reduced residual fungal burden in transiently neutropenic mice is the presence of phagocytic cells (neutrophils and monocytes). By comparison, the rabbits used in our experiments were persistently neutropenic, and the conditions of the rabbits more closely correlate with the conditions that one encounters in oncology patients. The histologic findings for our persistently neutropenic animals also reflect in vitro conditions, in which fragmentation and microcolony formation are readily observed. The activities of neutrophils and monocytes, when they are present, may act in synergy with the activities of the echinocandins, resulting in enhanced clearance of Aspergillus hyphae from tissue (4). However, in the profoundly neutropenic host, in which fungicidal activity is critical for the eradication of Aspergillus, the organism may persist, and indeed, a paradoxical increase in the log CFU per gram due to the fragmentation effect of echinocandins on the hyphal elements may actually be detected. Maintenance of a constant concentration of drug may impair the activity of the drug against Aspergillus spp. because echinocandins appear to require an actively growing cell to exert their antifungal effects.
The in vitro effect of hyphal fragmentation by echinocandins has been observed by Kurtz et al. (18). This has given rise to the concept of MEC, which is the lowest drug concentration at which microcolony formation and fragmentation become apparent in vitro. Our study demonstrates that the same effect occurs in profoundly neutropenic hosts in vivo. This hyphal fragmentation and the persistence of organisms are due to the antifungal effects of echinocandins at the growing tips as well as at the branching points of hyphal elements (C. M. Douglas, J. C. Bowman, G. K. Abruzzo, A. M. Flattery, C. J. Gill, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, M. B. Kurtz, and H. Rosen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1683, 2000). Since 1,3-β-d-glucan synthase is more widely distributed in C. albicans than in A. fumigatus, its inhibition may have a more profound effect on both pseudohyphal and yeast formation. Cabib et al. (3) also reported on the localization of the 1,3-β-d-glucan synthase complex at the cell membrane of Candida and reported that this enzyme system is involved in budding as well as septum formation. This process of budding and septum formation may be ongoing throughout the life cycle of the entire population of yeast cells. Inhibition of this more widely distributed 1,3-β-d-glucan synthase complex in C. albicans by echinocandins results in a fungicidal effect and the eradication of organisms in vitro and in vivo. Thus, A. fumigatus appears to be limited in its susceptibility to echinocandins at the level of cells undergoing apical growth or branching. The result is a modest impact on the total viable fungal burden and a pattern of hyphal fragmentation. Measurement of this more viable fungal burden may be facilitated by the use of quantitative PCR techniques; however, these methods remain investigational and unstandardized at present (Douglas et al., 40th ICAAC).
The plasma micafungin pharmacokinetics demonstrate that the Cmax of micafungin is multiple times the MIC of micafungin for C. albicans and is similarly elevated above the MEC for A. fumigatus. The amount of time that the level of drug exposure is above the MIC and MEC ranges from approximately 6 to 8 h for a dosage of 0.25 mg/kg to 24 h for a dosage of 2 mg/kg/day in a dosage-dependent manner. For invasive aspergillosis, concentrations in plasma are sustained above the MEC for 24 h after the administration of a dosage of 2 mg/kg/day. Notably, the AUC0-24s for micafungin tend to be higher after the administration of dosages of 1 and 2 mg/kg/day compared to the AUC0-24s obtained in the model of disseminated candidiasis. Despite these slightly higher concentrations, the organism was still persistent when micafungin was administered at 2 mg/kg/day.
In summary, the studies described here demonstrate marked differences in the antifungal activities of micafungin against disseminated candidiasis due to C. albicans compared to the activities of the drug against invasive pulmonary aspergillosis due to A. fumigatus. Against disseminated candidiasis, the drug had concentration- and dosage-dependent effects on the microbiologic eradication of C. albicans from tissues. By comparison, treatment of rabbits with invasive aspergillosis with micafungin led to a concentration-dependent effect of hyphal fragmentation that paradoxically increased serum galactomannan levels and increased the residual fungal burden (in CFU per gram).
REFERENCES
- 1.Anaissie, E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14(Suppl. 1):S43-S53. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1999. FK 463. Drugs Res. Dev. 1:172-173. [DOI] [PubMed] [Google Scholar]
- 3.Cabib, E., J. Drgonova, and T. Drgon. 1998. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67:307-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2001. The interaction of human monocytes, monocyte-derived macrophages, and polymorphonuclear neutrophils with caspofungin (MK-0991), an echinocandin, for antifungal activity against Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 39:99-103. [DOI] [PubMed] [Google Scholar]
- 5.Chiou, C. C., A. H. Groll, and T. J. Walsh. 2000. New drugs and novel targets for treatment of invasive fungal infections in patients with cancer. Oncologist 5:120-135. [DOI] [PubMed] [Google Scholar]
- 6.Cole, G. T., K. R. Seshan, M. Phaneuf, and K. T. Lynn. 1991. Chlamydospore-like cells of Candida albicans in the gastrointestinal tract of infected, immunocompromised mice. Can. J. Microbiol. 37:637-646. [DOI] [PubMed] [Google Scholar]
- 7.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 8.Francis, P., and T. J. Walsh. 1992. The evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Rev. Infect. Dis. 15:1003-1018. [DOI] [PubMed] [Google Scholar]
- 9.Frosco, M. B., and J. F. Barret. 1998. Importance of antifungal drug-resistance: clinical significance and need for novel therapy. Expert Opin. Investig. Drugs 7:175-197. [DOI] [PubMed] [Google Scholar]
- 10.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 11.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed., p. 455-459. Marcel Dekker, Inc., New York, N.Y.
- 12.Groll, A., A. Glasmacher, J. Gudrun, G. Maschmeyer, and T. J. Walsh. Clinical pharmacology of antifungal agents. Infect. Dis. Clin. N. Am., in press. [DOI] [PubMed]
- 13.Groll, A. H., D. Mickiene, V. Petraitis, R. Petraitiene, K. H. Ibrahim, I. Bekersky, S. C. Piscitelli, I. Bekersky, and T. J. Walsh. 2001. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin-like lipopeptide FK463 in rabbits. Antimicrob. Agents Chemother. 45:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 15.Groll, A. H., and T. J. Walsh. 2000. FK-463. Curr. Opin. Antifungal Infective Investig. Drugs 2:405-412. [Google Scholar]
- 16.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klepser, M. E., E. J. Ernst, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1998. Evaluation of endpoints for antifungal susceptibility determinations with LY303366. Antimicrob. Agents Chemother. 42:1387-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, J. W., E. Navarro, P. Francis, E. McManus, R. Schaufele, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1994. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 38:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maesaki, S., M. A. Hossain, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 2000. Efficacy of FK463, a (1,3)-β-d-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob. Agents Chemother. 44:1728-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto, S., Y. Wakai, T. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikamo, H., Y. Sato, and T. Tamaya. 2000. In vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptide. J. Antimicrob. Chemother. 46:485-487. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.National Research Council, Committee on the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 26.Nguyen, M. H., J. E., Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 27.Pannuti, C., R. Gingrich, M. A. Pfaller, C. Kao, and R. P. Wenzel. 1992. Nosocomial pneumonia in patients having bone marrow transplant. Attributable mortality and risk factors. Cancer 69:2653-2662. [DOI] [PubMed] [Google Scholar]
- 28.Pannuti, C. S., R. D. Gingrich, M. A. Pfaller, and R. P. Wenzel. 1991. Nosocomial pneumonia in adult patients undergoing bone marrow transplantation: a 9-year study. J. Clin. Oncol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 29.Pittet, D., and R. P. Wenzel. 1995. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch. Intern. Med. 155:1177-1184. [DOI] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringden, O., F. Meunier, J. Tollemar, P. Ricci, S. Tura, E. Kuse, M. A. Viviani, N. C. Gorin, J. Klastersky, and P. Fenaux. 1991. Efficacy of amphotericin B encapsulated in liposome (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J. Antimicrob. Chemother. 28:73-82. [DOI] [PubMed] [Google Scholar]
- 32.Sarosi, G. A. 1990. Amphotericin B: still the ′gold standard’ for antifungal therapy. Postgrad. Med. 88:151-152, 155-161, 165-166. [DOI] [PubMed] [Google Scholar]
- 33.Stynen, D., A. Goris, J. Sarfati, and J. P. Latge. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomishima, M., H. Ohki, A. Yamada, H. Takasugi, K. Maki, S. Tawara, and H. Tanaka. 1999. FK463, a novel water-soluble echinocandin lipopeptide: synthesis and antifungal activity. J. Antibiot. (Tokyo) 52:674-676. [DOI] [PubMed] [Google Scholar]
- 37.Uchida, K., Y. Nishiyama, N. Yokota, and H. Yamaguchi. 2000. In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J. Antibiot. (Tokyo) 53:1175-1181. [DOI] [PubMed] [Google Scholar]
- 38.Wald, A., W. Leisenring, J. A. van Burik, and R. A. Bowden. 1997. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J. Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 39.Walsh, T. J., C. McEntee, and D. M. Dixon. 1987. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J. Clin. Microbiol. 25:931-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh, T. J., J. W. Hiemenz, and E. Anaissie. 1996. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect. Dis. Clin. N. Am. 10:365-400. [DOI] [PubMed] [Google Scholar]
- 41.Walsh, T. J., P. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab. Anim. Med. 38:467-470. [PubMed] [Google Scholar]
- 42.Walsh, T. J., J. W. Lee, P. Kelly, J. Bacher, J. Lecciones, V. Thomas, C. Lyman, D. Coleman, R. Gordee, and P. A. Pizzo. 1991. The antifungal effects of the nonlinear pharmacokinetics of cilofungin, a 1,3-β-glucan synthase inhibitor, during continuous versus intermittent infusion of cilofungin in treatment of experimental disseminated candidiasis. Antimicrob. Agents Chemother. 35:1321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaoka, K., T. Nakagawa, and T. Uno. 1978. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165-175. [DOI] [PubMed] [Google Scholar]