Abstract
It was previously found that certain nonnucleoside reverse transcriptase inhibitors (NNRTI) possess virucidal activity against human immunodeficiency virus type 1 (HIV-1), and it was suggested that the tight-binding mode of inhibition of reverse transcriptase might be important for this virucidal activity (Borkow et al., J. Virol. 71:3023-3030, 1997). To test this, we compared six different NNRTI, including three tight-binding NNRTI, namely UC781, efavirenz (EFV) (Sustiva), and 5-chloro-3-phenylsulfonylindole-2-carboxamide (CSIC), and three rapid-equilibrium NNRTI, delavirdine (DLV) (Rescriptor), nevirapine (NVP) (Viramune), and UC84, in a variety of virucidal tests. Incubation of isolated HIV-1 virions with UC781, EFV, or CSIC rapidly inactivated the virus, whereas DLV, NVP, and UC84 were ineffective in this respect. Exposure of H9+ cells chronically infected by HIV-1 to the tight-binding NNRTI abolished the infectivity of nascent virus subsequently produced by these cells following removal of extracellular drug, thereby preventing cell-to-cell virus transmission in the absence of exogenous drug. In contrast, cell-to-cell transmission of HIV was blocked by DLV, NVP, and UC84 only when the drug remained in the extracellular medium. Pretreatment of uninfected lymphocytoid cells with UC781, EFV, or CSIC, but not DLV, NVP, or UC84, protected these cells from subsequent HIV-1 infection in the absence of extracellular drug. The protective effect was dependent on both the dose of NNRTI and the viral load. The overall virucidal efficacy of the tight-binding NNRTI tested was CSIC > UC781 ≃ EFV. We conclude that the tight-binding mode of inhibition is an essential characteristic for virucidal NNRTI and that antiviral potency is an insufficient predictor for virucidal utility of NNRTI.
Since heterosexual contact is the primary mode for the transmission of the human immunodeficiency virus (HIV), strategies to prevent HIV transmission from an infected individual to a noninfected person are needed in order to minimize the spread of the virus. Vaccines would be most useful in this respect; unfortunately, despite enormous effort, anti-HIV vaccines are not yet available. Another strategy to minimize the sexual transmission of HIV is the use of topical antiviral vaginal and/or rectal virucides. The ideal topical virucide should have high potency against HIV, act directly on and inactivate the virus without the need for metabolic activation, be effective against a wide range of HIV strains, inactivate viral infectivity over a broad range of pH, prevent cell-to-cell transmission of HIV, provide a barrier to infection of uninfected cells, act rapidly, and be relatively inexpensive and readily formulated for topical use. Furthermore, the virucide must be effective at concentrations that do not affect the vaginal environment (pH, bacterial flora, etc.). Finally, the virucide components should have minimal toxicity against human cells and tissues and not be absorbed into the bloodstream on topical application, in order to minimize systemic exposure of healthy individuals to the virucidal drug.
A variety of compounds have been proposed for use as potential anti-HIV topical virucides (see reference 14 for an overview). These include detergents such as nonoxynol-9 (13, 17) that act by disrupting the virus membrane envelope and agents that inhibit virus binding and entry by blocking CD4 receptors and coreceptors, such as dextran sulfate and modified milk proteins (1, 11, 12). Some envelope-disrupting detergents, particularly nonoxynol-9, can be toxic to the vaginal epithelium, and there are concerns that these agents may in fact increase the risk of HIV transmission, due to local tissue trauma and inflammation. However, sodium dodecyl sulfate has been proposed as an effective and less-toxic substitute for nonoxynol-9 and may provide an important component in the formulation of an effective anti-HIV topical virucide (9, 15).
It was shown that the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 had potential for use as an anti-HIV-1 virucide (3). However, it was also noted that not all NNRTI have this potential, since nevirapine did not show virucidal properties. Nevirapine is a rapid-equilibrium inhibitor of HIV-1 reverse transcriptase (RT) (19), whereas UC781 is a tight-binding inhibitor of RT (2), the first NNRTI to be identified as having this property. Accordingly, we suggested that tight-binding inhibition might be an important criterion for the virucidal activity of NNRTI (3). However, the scarcity of NNRTI with this property prevented us from directly testing this hypothesis.
Recently, efavirenz (EFV) was shown to be a tight-binding inhibitor of HIV-1 RT (10), and now a third NNRTI with this property, namely, 5-chloro-3-phenylsulfonylindole-2-carboxamide (CSIC) has been identified (D. Motakis, N. Sluis-Cremer, and M. A. Parniak, submitted for publication). In the present study, we have compared the virucidal properties of six different NNRTI with similar antiviral potencies. Three of these, namely, EFV, CSIC, and UC781, are tight-binding NNRTI. The other three NNRTI studied, delavirdine (DLV), nevirapine (NVP), and UC84, are rapid-equilibrium inhibitor NNRTI. Our results show that only tight-binding NNRTI possess virucidal properties and therefore establish the tight-binding mode of inhibition as an essential property required of NNRTI for use in anti-HIV-1 topical virucides.
MATERIALS AND METHODS
Reagents.
The HIV-1IIIB laboratory strain was obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. The CD4+ MT2 cell line was obtained from the American Type Culture Collection. The NNRTI CSIC was prepared by custom synthesis (Dalton Laboratories, Toronto, Ontario, Canada), UC781 and UC84 were provided by W. G. Brouwer (Uniroyal Chemical Ltd. Research Laboratories, Guelph, Ontario, Canada), nevirapine was a gift from Boehringer-Ingelheim (Laval, Quebec, Canada), and DLV and EFV were provided by M. A. Wainberg of the Lady Davis Institute for Medical Research. H9 cells chronically infected with HIV-1 (H9+ cells) were developed in our laboratories by methods similar to those previously described (16). HIV-1 p24 antigen analysis kits were obtained from SAIC-Frederick (Frederick, Md.). RPMI 1640 cell culture medium and heat-inactivated fetal bovine serum (FBS) were obtained from Canadian Life Technologies (GIBCO, Toronto, Ontario, Canada). Purified recombinant RT was prepared by a rapid isolation method which was previously described (5).
Characterization of NNRTI inhibition of recombinant HIV-1 RT.
Inhibition studies used a fixed-time assay for HIV-1 RT RNA-dependent DNA polymerase activity (RDDP) as previously described (5-7). Stock solutions of NNRTI were prepared in dimethyl sulfoxide (DMSO). Reaction mixtures (total volume, 50 μl) contained 50 mM Tris-HCl (pH 7.8; 37°C), 60 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, 5 μg of poly(rC)-oligo(dG)12-18 per ml, 20 μM [3H]dGTP, and various concentrations of NNRTI. Reactions were initiated by the addition of 20 ng of p51/p66 RT. The maximum concentration of DMSO was 1% or less, and control experiments showed that this concentration of DMSO had no effect on RT activity. After addition of RT, the reaction mixtures were incubated at 37°C for 15 min and then quenched with 500 μl of ice-cold 10% trichloroacetic acid containing 20 mM sodium pyrophosphate. Quenched samples were left on ice for 15 min and then filtered through Whatman 934-AH glass fiber filters. The filters were washed with 10% trichloroacetic acid containing 20 mM sodium pyrophosphate and ethanol and dried, and the extent of radionucleotide incorporation was assessed by liquid scintillation spectrometry. The 50% inhibitory concentration for each NNRTI, which is the concentration of NNRTI that provides a 50% reduction in RT activity relative to the no-drug control, was calculated from the data by using the program CalcuSyn (BioSoft, Ferguson, Mo.).
Cell culture and virus replication.
MT-2 and H9+ lymphocytoid cells were cultured in RPMI 1640 containing 10% FBS. Stock solutions of NNRTI were prepared in DMSO. Working solutions of NNRTI were prepared by dilution of the DMSO stock solutions into media immediately before use. In all cases, the final concentrations of DMSO were 1% or less. Control experiments showed that these concentrations of DMSO had no effect on viral infectivity or on cell viability. HIV-1 infection of MT2 lymphocytoid cells was determined by microscopic observation of syncytium formation (all samples were assessed by two independent observers, and the results were averaged) and by analysis of extracellular p24 antigen levels (18, 22). As was previously shown, the two methods provide very similar assessments of the extent of HIV-1 infection (2). The 50% effective concentration for each NNRTI, which is the concentration of NNRTI that provides a 50% reduction in HIV-1 infectivity relative to the no drug control, was calculated from the data by using the program CalcuSyn (BioSoft). Cytotoxicity was determined by cell growth kinetics and by trypan blue exclusion, as previously described (8).
Incubation of isolated HIV-1 virions with NNRTI.
Aliquots of isolated HIV-1 (corresponding to a total 50% tissue culture infective dose [TCID50] of 5.5 × 104) were incubated with various concentrations of NNRTI in a final volume of 200 μl of RPMI culture medium without FBS for 2 h at 37°C. Subsequently, the virus samples were diluted with drug-free medium to a volume of 15 ml and then concentrated to a volume of 0.5 ml by using Centriprep-100 centrifugal concentrators. The concentrated virus was diluted to 15 ml and again concentrated to 0.5 ml; this step was repeated three times in total. The calculated concentration of residual extravirion drug in samples exposed to the highest NNRTI concentration used (10 μM) is 0.005 nM, well below the effective antiviral range for any of the NNRTI employed in these studies. In addition, control experiments showed that these multiple dilution and concentration steps effectively removed all free extravirion drug. Identical aliquots of the drug-treated virus were added to MT2 cells for 2 h at 37°C, and then the cells were isolated by centrifugation, washed, and resuspended in culture medium without NNRTI. HIV-1 infection of these cells was assessed by microscopic examination of syncytium formation and measurement of HIV-1 p24 antigen levels in the culture supernatants.
Effect of NNRTI pretreatment on infectivity of HIV-1 produced from chronically infected H9+ cells.
H9+ cells (5 × 104) were incubated with various NNRTI concentrations in RPMI 1640 containing 10% FBS for 18 h at 37°C. The cells were isolated by low-speed centrifugation and extensively washed with medium to ensure removal of all extracellular drug and virus. The cells were resuspended in medium containing FBS and incubated for 24 h to allow nascent virus production. At this time, cell-free virus-containing culture supernatants were collected by centrifugation (1,000 × g; 10 min). Virus levels in the cell-free culture supernatants were quantified by measurement of p24 antigen levels. Equal amounts of virus, corresponding to 0.25 ng of p24, were added to 5 × 104 MT2 cells in a total volume of 200 μl. The extent of infection was measured after 4 days by microscopic evaluation of syncytium formation. To test the effect of NNRTI treatment on cell-to-cell transmission of virus, H9+ cells were treated with various concentrations of NNRTI for 18 h at 37°C, and then the cells were isolated by centrifugation. Extracellular NNRTI was removed by extensive washing of the cells, involving resuspension of the cells in 15 ml of drug-free RPMI medium followed by collection of the cells by centrifugation, a process that was repeated at least three times. The washed cells were finally suspended in culture medium containing 10% FBS. Aliquots of the H9+ cells (containing 103 cells) were then added to cultures containing 3 × 105 MT2 lymphocytoid cells, and the extent of infection of the latter cells was determined daily by microscopic examination for syncytium formation.
Effect of NNRTI pretreatment of cells on subsequent infection by HIV-1 in the absence of drug.
Uninfected MT2 cells were incubated with various concentrations of NNRTI for 18 h at 37°C, and then the NNRTI drug was removed by extensive washing of the cells following centrifugal isolation. Identical aliquots of HIV-1 (TCID50 = 1.5 × 10−3) were added to the drug-pretreated cells, and virus infectivity was assessed by daily microscopic assessment of syncytium formation. To test the effect of viral load on NNRTI lymphocyte protection, MT-2 cells (5 × 104) were pretreated with various concentrations of NNRTI as described above. These cells were then infected with different amounts of virus ranging from a final multiplicity of infection (MOI) of 0 to 0.18 (TCID50 = 7.1 × 103). HIV-1-induced cytopathic effects were determined microscopically 4 days postinfection.
RESULTS
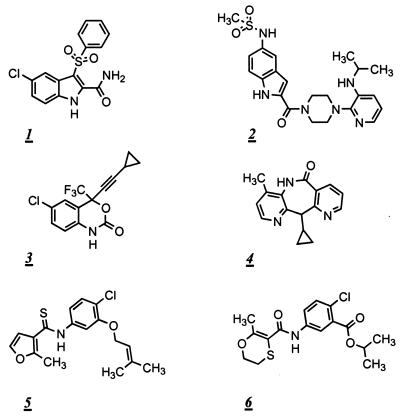
The inhibitory properties of the six NNRTI used in this study (shown in Fig. 1) are summarized in Table 1. All of the NNRTI selected, with the exception of UC84, have reasonably similar antiviral potencies. UC781 and UC84 have similar structures; thus, we used UC84 to test whether the virucidal activity of UC781 was related to the structural characteristics of the UC series of compounds.
FIG. 1.
Structures of the NNRTI used in the present studies: CSIC (1); DLV (2); EFV (3); NVP (4); UC781 (5); UC84 (6).
TABLE 1.
Some properties of the NNRTI used in this study
| Drug | IC50 (nM)a | EC50 (nM)b | Type of inhibitionc |
|---|---|---|---|
| Clinical | |||
| Nevirapine | 200 ± 45 | 45 ± 10 | REI |
| Delavirdine | 5.4 ± 0.4 | 2.8 ± 1.4 | REI |
| Efavirenz | 7.7 ± 0.9 | 1.7 ± 0.2 | TBI |
| Experimental | |||
| UC84 | 117 ± 4 | 240 ± 70 | REI |
| UC781 | 1.7 ± 1.2 | 10 ± 0.2 | TBI |
| CSIC | 2.0 ± 0.2 | 1.2 ± 0.4 | TBI |
IC50 is the concentration of inhibitor that provides a 50% reduction in the RDDP activity of recombinant wild-type p51/p66 RT determined by measurement of this activity in the presence of various concentrations of inhibitor using [3H]dGTP and poly(rC)-oligo(dG)12-18 as described in Materials and Methods. Values are the means ± standard deviations of three independent measurements, each carried out in duplicate.
EC50 is the concentration of inhibitor that provides a 50% reduction in HIV-1 infection of MT2 cells, assessed as described in Materials and Methods. Values are the means ± standard deviations of three independent measurements, each carried out in triplicate.
Inhibition of HIV-1 RT in vitro: REI, rapid equilibrium inhibitor; TBI, tight-binding inhibitor.
Inactivation of isolated HIV-1 virions by NNRTI.
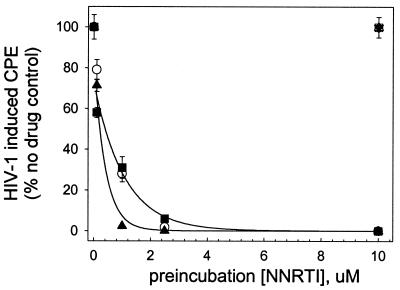
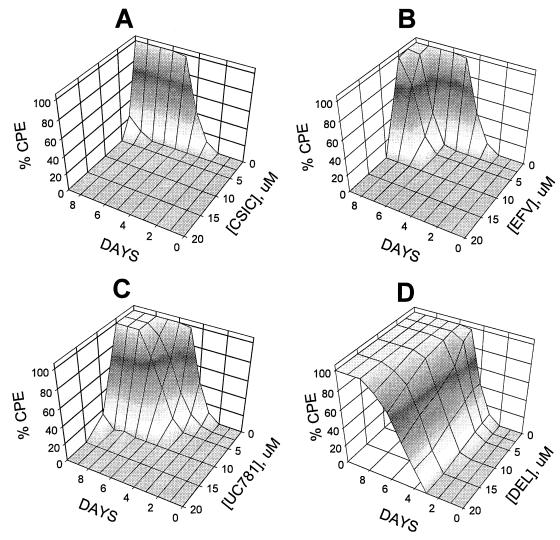
Isolated HIV-1 virions were incubated for 2 h with various concentrations of NNRTI, and then the virus was separated from free drug by multiple filtration and resuspension steps, as described in Materials and Methods. Figure 2 shows that each of CSIC, EFV, and UC781 attenuated HIV-1 infectivity in a dose-dependent manner, with complete attenuation of viral infectivity noted in virus exposed to concentrations of these NNRTI above 2.5 μM. CSIC seemed to be slightly more effective than either EFV or UC781 in this respect. In contrast, DLV, NVP, and UC84 were completely ineffective at inactivating the infectivity of isolated HIV-1 virions, even at 10 μM, the highest concentration used (Fig. 2).
FIG. 2.
Inactivation of isolated HIV-1 virus particles following exposure to different concentrations of NNRTI. Isolated HIV-1 particles were incubated with the indicated concentrations of various NNRTI for 2 h at 37°C. Excess drug was then removed by repeated dilution and concentration steps, as described in Materials and Methods. The exogenous drug-free virus was then used to infect MT-2 lymphocytes, and the extents of HIV-1-induced cytopathic effects were assessed 4 days postinfection. ▴, CSIC; ○, EFV; ▪, UC781; □, DLV; ▾, NVP; ▵, UC84. Data points are the means ± standard deviations of three separate determinations.
Inhibition of cell-to-cell transmission of HIV-1.
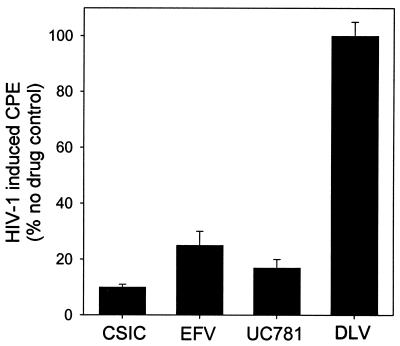
Two types of experiment were carried out. In the first, H9+ cells were incubated with NNRTI and then washed to remove cell-free virus and extracellular drug. The nascent virus produced from these cells over the subsequent 24-h period was isolated, and identical viral inocula (based on p24 antigen measurements) were used to infect MT2 lymphocytoid cells. None of the NNRTI tested affected the amount of HIV-1 virions produced by these cells, either during NNRTI exposure or following removal of exogenous drug, as assessed by p24 antigen levels in the culture supernatants (data not shown). However, as seen in Fig. 3, the virus produced by H9+ cells pretreated with CSIC, EFV, or UC781 was markedly attenuated in infectivity, whereas the virus produced from cells pretreated with DLV was essentially as infectious as that produced by untreated H9+ control cells. Treatment with NVP and UC84 was also ineffective in attenuating the infectivity of the nascent virus subsequently produced by the H9+ cells (data not shown). In the second type of experiment, H9+ cells were incubated with NNRTI and washed as above to remove exogenous drug and virus. The H9+ cells were then cocultured with uninfected MT2 cells (at a 1:300 ratio of H9+ to MT2 cells), and the extent of HIV-1 infection was assessed by microscopic examination of syncytium formation. As shown in Fig. 4, infection of MT2 cells by cell-associated virus produced by H9+ cells that had been pretreated with CSIC, EFV, or UC781 was reduced in a dose-dependent manner. In contrast, cell-to-cell transmission of HIV was unaffected by pretreatment of the H9+ cells with DLV (Fig. 4), NVP, or UC84 (data not shown).
FIG. 3.
Effects of NNRTI treatment of chronically infected H9+ cells on infectivity of HIV-1 virions subsequently produced in the absence of extracellular drug. H9+ cells were incubated with 20 μM of the indicated NNRTI for 18 h at 37°C, and then the cells were washed free of exogenous drug. The H9+ cells were then cultured for 24 h in the absence of drug, and the virus produced during that period was separated from the cells by centrifugation, as described in Materials and Methods. Equal amounts of virus, corresponding to 0.25 ng of p24, were added to 5 × 104 MT2 cells in a total volume of 200 μl, and the extent of infection of these cells was determined after 4 days by microscopic evaluation of syncytium formation. The data presented are the means ± standard deviations of three separate determinations.
FIG. 4.
Effects of NNRTI treatment of chronically infected H9+ cells on subsequent cell-to-cell transmission of HIV-1 in the absence of extracellular drug. H9+ chronically infected cells were incubated with the indicated concentrations of CSIC (▴), EFV (▪), UC781 (○), or DLV (□) for 18 h at 37°C, and then the cells were separated from extracellular drug and virus as described in Materials and Methods. The isolated H9+ cells were then cocultured with uninfected MT2 cells (1:300 ratio of H9+ to MT2 cells), and the extent of infection of the latter was determined 4 days later by microscopic assessment of HIV-1-induced syncytium formation.
Protection of uninfected cells from subsequent HIV-1 infection.
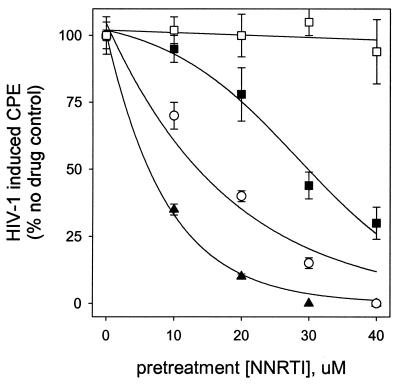
One of the most striking properties of UC781 is its ability to establish a protective barrier in cells that renders these UC781-treated cells refractory to subsequent HIV-1 infection in the absence of exogenous drug (3). As shown in Fig. 5, pretreatment of cells with CSIC and EFV also established a barrier to HIV-1 infection, whereas pretreatment with DLV (Fig. 5D), NVP, or UC84 (data not shown) did not. The extent of the protection afforded by CSIC, EFV, and UC781 was concentration dependent. Interestingly, CSIC was more effective than either EFV or UC781 in this respect, providing significant protection at lower doses and for longer periods of time. Indeed, cells treated with concentrations of CSIC above 10 μM showed no signs of infection even 30 days after exposure to virus (data not shown).
FIG. 5.
Effects of pretreatment of uninfected MT2 cells with various concentrations of NNRTI on their subsequent infection by HIV-1 in the absence of extracellular drug. Uninfected MT2 cells were incubated with the indicated concentrations of NNRTI for 18 h and then washed to remove exogenous drug. The cells were then exposed to identical inocula of HIV-1 (MOI, 7.5 × 10−3). HIV-1-induced cytopathic effects were assessed daily by microscopic examination of syncytium formation as described in Materials and Methods. (A) CSIC; (B) EFV; (C) UC781; (D) DLV.
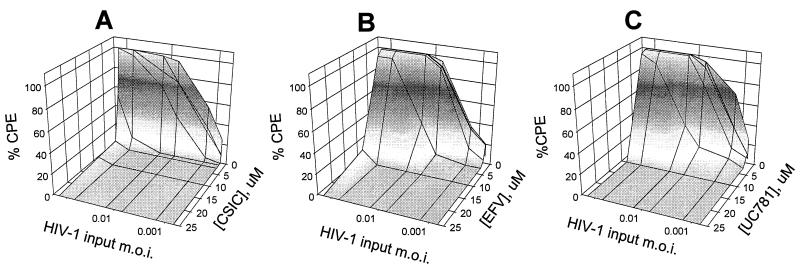
We also examined the effects of viral load on the protective barrier established by cell pretreatment with different concentrations of the tight-binding NNRTI (Fig. 6). Each of CSIC, EFV, and UC781 was very effective over the range of virus inocula used, especially at concentrations above 10 μM. Cells pretreated with CSIC were again the most refractory to infection at the highest viral load. None of the cells pretreated with the rapid-equilibrium NNRTI DLV, NVP, or UC84 were protected from infection, even at the lowest dose of virus used (data not shown).
FIG. 6.
Effects of viral load on infection of MT2 cells pretreated with various concentrations of tight-binding NNRTI. Uninfected MT2 cells were incubated with the indicated concentrations of NNRTI for 18 h and then washed to remove exogenous drug. The cells were then inoculated with the indicated amounts of HIV-1 (MOI, 0.0007 to 0.18). HIV-1-induced cytopathic effects were assessed 4 days postinfection by microscopic examination of syncytium formation as described in Materials and Methods. (A) CSIC; (B) EFV; (C) UC781.
DISCUSSION
We previously showed that UC781, a tight-binding NNRTI, had potential utility for virucidal applications. Brief exposure to UC781 inactivated isolated HIV-1 virions, incubation with UC781 of cells chronically infected with HIV-1 led to the subsequent production of nascent virus with attenuated infectivity and prevented cell-to-cell transmission of the virus, and finally, exposure of uninfected cells to UC781 established a chemical barrier that prevented subsequent virus infection in the absence of extracellular drug (3). We also noted that another NNRTI, nevirapine, was unable to provide any of these virucidal characteristics. Nevirapine is a rapid-equilibrium inhibitor of HIV-1 RT, i.e., the attainment of equilibrium between enzyme, inhibitor, and the enzyme-inhibitor complex is rapid and requires a large excess of inhibitor over enzyme (19). Unlike nevirapine, UC781 is a tight-binding inhibitor of HIV-1 RT (2). Tight-binding inhibitors exhibit unique properties in their interactions with enzymes, which distinguish them from rapid-equilibrium inhibitors (20, 21). UC781 binds rapidly to RT, but once bound, dissociates only very slowly. In the case of UC781, this dissociation from purified recombinant RT takes up to 1 h in the absence of exogenous drug (2). Thus, RT remains inhibited for prolonged periods of time after binding, even in the absence of significant levels of unbound inhibitor, and the total concentration of inhibitor needed to inhibit the enzyme is similar to the concentration of enzyme (2).
NNRTI are in general hydrophobic molecules and should therefore readily traverse membranes such as the HIV envelope. However, this hydrophobic nature would allow equally facile influx and efflux of the NNRTI from the virion, in the absence of other factors. Penetration of the hydrophobic UC781 through the virion membrane envelope and capsid core would allow binding to RT. The tight-binding nature of this binding would minimize dissociation of the NNRTI from RT, thereby trapping the NNRTI within the virion particle and imparting a prolonged inhibition of RT activity. We therefore suspected that the tight-binding mode of inhibition might be an important parameter for the virucidal activity of NNRTI such as UC781, but in a previous report (3) this could not be tested directly due to the scarcity of NNRTI with this property. Two additional tight-binding NNRTI have now been identified.These are 5-chloro-3-phenylsulfonylindole-2-carboxamide(CSIC) (Motakis et al., submitted) and the clinically used efavirenz (10). Our comparison of the tight-binding NNRTI CSIC, EFV, and UC781 with the rapid-equilibrium inhibitors DLV, NVP, and UC84 in a variety of virucidal tests now firmly establishes the tight-binding mode of inhibition as an essential parameter that must be met by NNRTI for use in virucidal applications. Each of the six NNRTI employed in the present studies had similar antiviral potencies; thus, antiviral potency alone is an insufficient predictor for virucide potential.
Our present studies also show that CSIC possesses virucidal properties equivalent to or, in some cases, better than either UC781 or EFV. While each of CSIC, EFV, and UC781 inactivated isolated HIV-1 virions with approximately equal potency and each of these NNRTI was similarly effective at preventing cell-to-cell transmission of virus, CSIC was superior to either EFV or UC781 at establishing a chemical barrier in uninfected cells, a barrier that rendered these cells refractory to subsequent HIV-1 infection in the absence of extracellular drug.
The prolonged chemical barrier to infection established in cells by tight-binding NNRTI implies that the drugs are sequestered in some cellular compartment(s) that enables access to HIV during subsequent virus exposure. It has been proposed that this compartment may be the cell plasma membrane (3). NNRTI such as CSIC, EFV, and UC781 are hydrophobic molecules that readily enter the lipid bilayer of the cell plasma membrane. Fusion of incoming virus with the drug-treated cell membrane might allow diffusion of the membrane-resident NNRTI into the hydrophobic capsid core of the virus, thereby allowing binding to RT within. When the NNRTI is tight-binding, reverse transcription would be inhibited for prolonged periods following fusion and entry, thereby preventing the cell from becoming infected. The membrane compartment hypothesis is also consistent with the attenuated infectivity of virions produced from UC781-treated H9+ cells. Nascent budding virions use cell plasma membrane as envelope. NNRTI within this envelope could then bind to RT after diffusion through the matrix and capsid shells. The resulting inhibition would prevent reverse transcription (endogenous and intracellular) and thereby attenuate viral infectivity. All NNRTI are hydrophobic, but as discussed above only tight-binding NNRTI are able to inhibit RT at concentrations similar to the concentration of enzyme.
The apparent superiority of CSIC over EFV or UC781 at establishing a barrier to infection may be related to the concentrations of the drug attainable in the specific subcellular compartment(s) (i.e., the plasma membrane) to which HIV has access. Clinically used NNRTI such as DLV, NVP, and the tight-binding EFV are orally bioavailable, indicating that they are able to transit cells, and thus they would not remain entirely within the plasma membrane lipid bilayer. UC781 has also been shown to possess oral bioavailability (4), but to a more limited extent than EFV. In contrast, CSIC has little or no oral bioavailability and may thus accumulate to a greater extent in that compartment, providing the maximal protection against HIV infection. Studies to identify the subcellular compartment in which virucidal NNRTI reside are currently in progress.
In the absence of an effective vaccine, topical virucides represent a potentially cost-effective means to minimize the sexual transmission of HIV. The ideal topical virucide should have high potency against HIV, act directly on and inactivate the virus without the need for metabolic activation, be effective against a wide range of HIV strains, prevent cell-to-cell transmission of HIV, provide a barrier to infection of uninfected cells, act rapidly, be relatively inexpensive, readily formulated for topical use, effective at concentrations that do not affect the vaginal environment, and not be absorbed into the bloodstream on topical application, in order to minimize systemic exposure of healthy individuals to the virucidal drug. As shown in the present studies, tight-binding NNRTI fulfill many of these characteristics. In addition, UC781 and CSIC are relatively nontoxic (50% cytotoxic concentration > 100 μM) (M. A. Parniak, unpublished observations). Tight-binding NNRTI may therefore represent attractive candidates for inclusion in anti-HIV-1 topical virucide formulations.
Acknowledgments
This work was supported by a grant (to M.A.P.) from amfAR, the American Foundation for AIDS Research. During the course of this work, M.A.P. was a Senior Scientist of the Medical Research Council of Canada and an International Research Scholar of the Howard Hughes Medical Institute. D.M. was the recipient of a Predoctoral Studentship from the Medical Research Council of Canada.
REFERENCES
- 1.Baba, M., R. Pauwels, J. Balzarini, J. Arnout, J. Desmyter, and E. De Clercq. 1988. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 85:6132-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard, J., G. Borkow, and M. A. Parniak. 1997. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry 36:7786-7792. [DOI] [PubMed] [Google Scholar]
- 3.Borkow, G., J. Barnard, T.-M. Nguyen, A. Belmonte, M. A. Wainberg, and M. A. Parniak. 1997. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckheit, R. W., M. Hollingshead, S. F. Stinson, V. Fliakas-Boltz, L. A. Pallansch, J. Roberson, W. Decker, C. Elder, S. Borgel, C. Bonomi, R. Shores, T. Simford, L. Malspeis, and J. P. Bader. 1997. Efficacy, pharmacokinetics, and in vivo antiviral activity of UC781, a highly potent, orally bioavailable nonnucleoside reverse transcriptase inhibitor of HIV-1. AIDS Res. Hum. Retrovir. 13:789-796. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher, R. S., G. Holleschak, E. Nagy, D. Arion, G. Borkow, Z. Gu, M. A. Wainberg, and M. A. Parniak. 1996. Single-step purification of recombinant wild-type and mutant HIV-1 reverse transcriptase. Protein Expr. Purif. 7:27-32. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher, R. S., D. Arion, G. Borkow, M. A. Wainberg, G. I. Dmitrienko, and M. A. Parniak. 1995. Synergistic inhibition of HIV-1 reverse transcriptase DNA polymerase activity and virus replication in vitro by combinations of carboxanilide nonnucleoside compounds. Biochemistry 34:10106-10112. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher, R. S., K. Syed, S. Mithani, G. I. Dmitrienko, and M. A. Parniak. 1995. Carboxanilide derivative non-nucleoside inhibitors of HIV-1 reverse transcriptase interact with different mechanistic forms of the enzyme. Biochemistry 34:4346-4353. [DOI] [PubMed] [Google Scholar]
- 8.Hirabayashi, K., S. Iwata, M. Ito, S. Shigeta, T. Narui, T. Mori, and S. Shibata. 1989. Inhibitory effect of a lichen polysaccharide sulfate, GE-3-S, on the replication of human immunodeficiency virus (HIV) in vitro. Chem. Pharm. Bull. (Tokyo) 37:2410-2412. [DOI] [PubMed] [Google Scholar]
- 9.Krebs, F. C., S. R. Miller, D. Malamud, M. K. Howett, and B. Wigdahl. 1999. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir. Res. 43:157-173. [DOI] [PubMed] [Google Scholar]
- 10.Maga, G., D. Ubiali, R. Salvetti, M. Pregnolato, and S. Spadari. 2000. Selective interaction of the human immunodeficiency virus type 1 reverse transcriptase nonnucleoside inhibitor efavirenz and its thio-substituted analog with different enzyme-substrate complexes. Antimicrob. Agents Chemother. 44:1186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuya, H., D. J. Looney, S. Kuno, R. Ueno, F. Wong-Staal, and S. Broder. 1988. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science 240:646-649. [DOI] [PubMed] [Google Scholar]
- 12.Neurath, A. R., S. Jiang, N. Strick, K. Lin, Y. Y. Li, and A. K. Debnath. 1996. Bovine beta-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat. Med. 2:230-234. [DOI] [PubMed] [Google Scholar]
- 13.Niruthisard, S., R. E. Roddy, and S. Chutivongse. 1991. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 18:176-179. [DOI] [PubMed] [Google Scholar]
- 14.Pauwels, R., and E. De Clercq. 1996. Development of vaginal virucides for the prevention of heterosexual transmission of HIV. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:211-221. [DOI] [PubMed] [Google Scholar]
- 15.Piret, J., J. Lamontagne, J. Bestman-Smith, S. Roy, P. Gourde, A. Desormeaux, R. F. Omar, J. Juhasz, and M. G. Bergeron. 2000. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as virucides against herpes simplex and human immunodeficiency viruses. J. Clin. Microbiol. 38:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 17.Roddy, R. E., M. Cordero, C. Cordero, and J. A. Fortney. 1993. A dosing study of nonoxynol-9 and genital irritation. Int. J. STD AIDS 4:165-170. [DOI] [PubMed] [Google Scholar]
- 18.Rooke, R., M. Tremblay, and M. A. Wainberg. 1990. AZT (zidovudine) may act postintegrationally to inhibit generation of HIV-1 progeny virus in chronically infected cells. Virology 176:205-215. [DOI] [PubMed] [Google Scholar]
- 19.Spence, R. A., W. M. Kati, K. S. Anderson, and K. A. Johnson. 1995. Mechanism of inhibition of HIV-1 reverse transcriptase by nonnucleoside inhibitors. Science 267:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szedlacsek, S. E., and R. G. Duggleby. 1995. Kinetics of slow and tight-binding inhibitors. Methods Enzymol. 249:144-180. [DOI] [PubMed] [Google Scholar]
- 21.Williams, J. W., and J. F. Morrison. 1979. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 63:437-467. [DOI] [PubMed] [Google Scholar]
- 22.Yao, X.-J., M. A. Wainberg, and M. A. Parniak. 1992. Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology 187:56-62. [DOI] [PubMed] [Google Scholar]