Abstract
Expression libraries of a chlorhexidine-resistant Klebsiella pneumoniae strain were constructed and transformed into Escherichia coli XLOLR. Twenty chlorhexidine-resistant transformants were obtained after selection. All clones contained a novel 903-nucleotide locus. Its sequences were compatible with a cation efflux pump, and the locus was thus designated as cepA. Retransformation using cepA-containing plasmids conferred chlorhexidine resistance to both XLOLR and a chlorhexidine-sensitive K. pneumoniae strain. Therefore, CepA is associated with chlorhexidine resistance and may act as a cation efflux pump.
Chlorhexidine is an extensively used handwashing antiseptic (9, 12, 18). Some hospital-acquired gram-negative bacteria, including Klebsiella, Serratia, Pseudomonas, etc., are resistant to chlorhexidine (4, 6, 11, 21, 23). Among these bacteria, Klebsiella pneumoniae frequently causes pneumonia, urinary tract infection, wound infection, and bacteremia in hospitalized patients (5, 15). The mechanisms responsible for chlorhexidine resistance in gram-negative bacteria remain unclear. Therefore, we tried to isolate the gene(s) responsible for chlorhexidine resistance in K. pneumoniae by using λ-Zap II expression libraries.
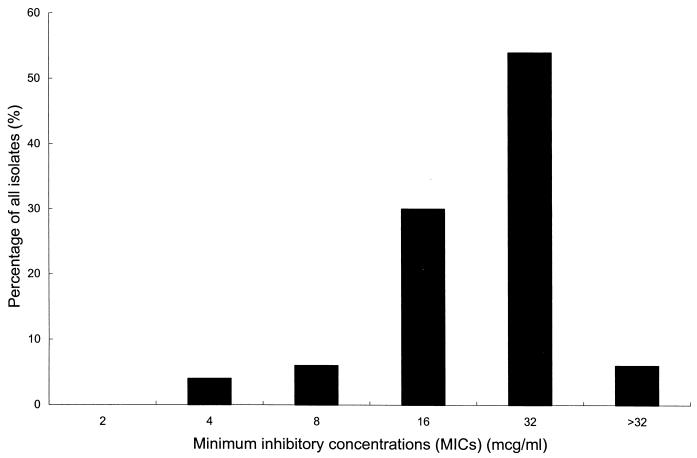
K. pneumoniae isolates were collected at National Taiwan University Hospital. Chlorhexidine MICs were determined by agar dilution techniques (13) by using chlorhexidine digluconate (Sigma, St. Louis, Mo.). Fifty randomly selected clinical K. pneumoniae isolates were tested for chlorhexidine MICs. Chlorhexidine had a MIC of ≥32 μg/ml for 30 (60%) isolates, a MIC of 16 μg/ml for 15 (30%) isolates, a MIC of 8 μg/ml for three (6%) isolates, and a MIC of 4 μg/ml for two (4%) isolates. The distribution of chlorhexidine MICs is shown in Fig. 1. Chlorhexidine had a MIC of 2 μg/ml for XLOLR and Escherichia coli ATCC 25922. A chlorhexidine-resistant strain (NTUH-2044; chlorhexidine MIC, 32 μg/ml) and a chlorhexidine-sensitive strain (NTUH-9770; chlorhexidine MIC, 4 μg/ml) were used (1). Details of the characteristics of the bacterial strains and plasmids used in this study are listed in Table 1.
FIG. 1.
Distribution of chlorhexidine MICs for 50 randomly selected clinical K. pneumoniae isolates.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| E. coli strains | ||
| XLOLR | Recipient of λ-ZAP II vector; chlorhexidine sensitive | Stratagene |
| S17-1/λpir | Donor of pUTKm1 Δtnp Δbla Δmini-Tn5 during conjugation; ampicillin sensitive | 7 |
| ATCC 25922 | Chlorhexidine sensitive and ampicillin sensitive | American Type Culture Collection |
| Plasmids | ||
| pBK-CMV | Phagemid vector resulting from in vivo excision of λ-ZAP II vector in E. coli XLOLR | Stratagene |
| pUTKm1 Δtnp Δbla Δmini-Tn5 | Suicide vector constructed from pUTKm1 by deleting tnp, bla, and mini-Tn5 transposon through restriction enzymatic digestion (SalI, ApaLI, and EcoRI, respectively) followed by ligation | This work |
| pCRII-TOPO | TA cloning vector | Invitrogen |
Genomic DNAs from NTUH-2044 were extracted and partially digested with Sau3AI (3, 19). Fragments of 3 to 5 kb in length were recovered. Construction of λ-ZAP II libraries and insertion of in vivo excision phages into pBK-CMV phagemids were carried out (Stratagene, La Jolla, Calif.) (3, 20).
DNA sequencing was performed on an ABI PRISM 377 DNA sequencer. Primers (5′-AATTAACCCTCACTAAAGGG-3′ and 5′-GTAATACGACTCACTATAGGGC-3′) based on the phagemid vector were designed for sequencing inserts. Nucleotide sequences and deduced amino acid sequences were analyzed and compared with those listed in GenBank, the SWISS-PROT databases, and the BLAST network service at the National Center for Biotechnology Information.
An integrative vector from pUTKm1Δtnp Δmini-Tn5 (10), with E. coli lacZ in the SalI restriction site and K. pneumoniae cepA in the EcoRI restriction site, was transformed into E. coli ATCC 25922 by both conjugation and electroporation (3, 10).
For knockout experiments, the following procedures were performed. (i) A linear DNA, cat::cepA, was constructed by inserting a chloramphenicol acetyltransferase cassette (gift from D. E. Taylor) (24) into the AccI site within cepA. The DNA fragment containing cepA was amplified by PCR using primers PT-F1 (5′-CAACTCCTTCGCCTATCCCG-3′) and PT-R1 (5′-TCAGGTCAGACCAAACGGCG-3′) to anneal sites −72 and +958, respectively. (ii) The cat::cepA fragment was also inserted into the EcoRI site on suicide vector pUTKm1Δtnp Δbla Δmini-Tn5 (10). (iii) A 200-bp intragenic fragment (cepA nucleotides +97 to +297, amplified by PCR) in the same suicide vector carrying a chloramphenicol acetyltransferase cassette was also constructed. Linear DNA (cat::cepA) and suicide vectors for both constructs were transformed into NTUH-2044 by electroporation and conjugation (3, 10).
Screening of the phagemid expression library in XLOLR revealed 20 clones which grew on plates containing chlorhexidine (16 μg/ml). DNA sequencing of the 20 clones revealed three different inserts. However, all clones contained a 903-nucleotide locus (Fig. 2A). Plasmid carrying this locus was retransformed into XLOLR by 42°C heat shock. Retransformants again grew on plates containing chlorhexidine (16 μg/ml).
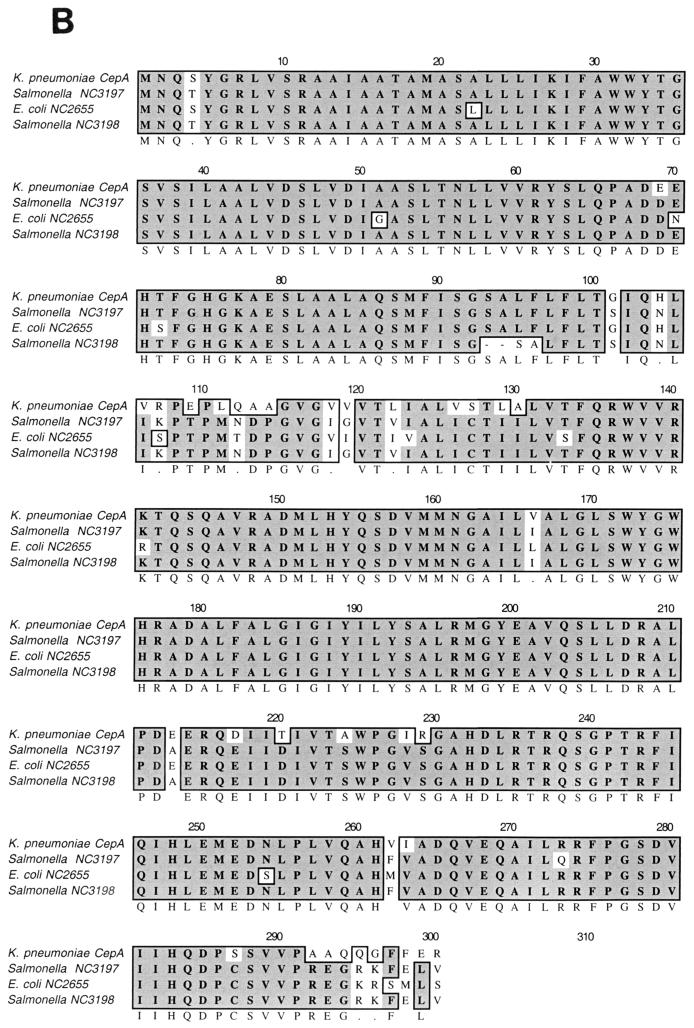
FIG. 2.
(A) Nucleotide sequences of cepA and flanking regions. The predicted promoter region is underlined. The start codon is marked by +1. (B) CepA amino acid sequence alignment with putative transport system permease protein in E. coli (accession number NC 002655) and putative transmembrane efflux protein in Salmonella enterica serovar Typhi (accession number NC 003198). A dark background indicates identical residue homologies, and the lighter background shows residues with similarities. Alignment was done using Macvector 6.5 sequence analysis software (Oxford Molecular Group). Comparison of the CepA sequences with those of putative cationic efflux pumps in E. coli and S. enterica serovar Typhi revealed 83 to 84% similarity.
Sequencing of this open reading frame (ORF) showed a 90% similarity to yiip, a putative transporter in E. coli K12 (2). The deduced amino acid sequence showed that it could be a cation efflux pump. Thus, this ORF was designated cepA (GenBank accession number AB073019). Proteins displaying similarity to the deduced amino acid sequence of cepA included several putative permease proteins in E. coli and two putative transmembrane efflux proteins in Salmonella enterica serovar Typhi (Fig. 2B). The adjacent ORFs upstream and downstream with respect to cepA had nucleotide sequences 87% similar to E. coli transcription factor cpxR and Enterobacter cloacae phosphofructokinase pfkA, respectively. The chromosome and plasmid were separated by pulsed field gel electrophoresis. Results of a nested PCR using cepA-specific primer pairs showed that cepA was present on the chromosome.
pBK-CMV plasmids carrying cepA were also transformed into NTUH-9770 by electroporation. Transformants had a fourfold increase in chlorhexidine MICs. Transformation with suicide vector lacZ-cepA-pUTKm1 Δtnp Δmini-Tn5 into ATCC 25922 yielded cepA single-integration recombinants (confirmed by PCR with different alignments of primer pairs) for which chlorhexidine showed a twofold increase in MIC.
No chloramphenicol-resistant transformants were obtained by transformation of the linear DNA cat::cepA or intragenic fragment in a suicide vector. Transformations with suicide plasmid vector cat::cepA-pUTKm1 Δtnp Δbla Δmini-Tn5 yielded chloramphenicol-resistant clones. However, PCR amplification with PT-F1 and PT-R1 yielded both 1-kb and 1.8-kb products (not shown), which corresponded to the sizes of wild-type cepA and cat::cepA, respectively. This result suggested that an integration, not a knockout, had occurred.
cepA was expressed in XLOLR transformants carrying cepA::pBK-CMV as previously described (19, 20). A pBK-CMV plasmid without cepA was used as a control. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis with Coomassie blue staining revealed a 33-kDa protein (the predicted size of CepA) carrying cepA::pBK-CMV in XLOLR but not in the control vector (data not shown).
Chlorhexidine is a cationic biguanide that kills bacteria by membrane damage followed by intracellular coagulation (8). Gram-negative bacteria are less susceptible to chlorhexidine than gram-positive bacteria (12, 18). Impermeability of the outer membrane to chlorhexidine has been implicated in Pseudomonas, Proteus, and Providencia (12, 18). Formation of biofilm prolongs survival of Serratia and Burkholderia on exposure to chlorhexidine (12, 18). A chlorhexidine-degrading enzyme has been discovered in Achromobacter xylosoxidans (14).
We demonstrated that transformation of cepA-containing phagemid into XLOLR resulted in significant elevation of chlorhexidine MICs. A single integration of cepA was sufficient to increase chlorhexidine MICs for E. coli twofold. Transformation of cepA-containing pBK-CMV into NTUH-9770 also resulted in a fourfold elevation of chlorhexidine MICs. These results suggest that cepA is associated with chlorhexidine resistance. Attempts in three different experiments failed to obtain knockout mutants. Therefore, cepA is probably essential. Our findings indicate that drug efflux might be an important mechanism of chlorhexidine resistance in K. pneumoniae and possibly in other related gram-negative bacteria. A similar mechanism is observed in staphylococci, in which export proteins, encoded by qacA and/or qacB, are also responsible for chlorhexidine resistance (17, 22).
Chlorhexidine is usually supplied in a 0.5 to 4% solution for handwashing (9). In a clinical environment, chlorhexidine concentrations 10- to 50-fold higher than MICs are required to produce 99.99% killing (4 log10 reduction) within 10 min at 20°C (8). Handwashing with chlorhexidine has failed to control nosocomial spread of methicillin-resistant Staphylococcus aureus with qacA and/or qacB-mediated resistance (MIC, 2 to 4 μg/ml) (16). Because of the higher levels of MICs required, chlorhexidine-resistant K. pneumoniae could be clinically more problematic than staphylococci.
In conclusion, cepA is associated with chlorhexidine resistance in K. pneumoniae. CepA protein may act as a cation efflux pump.
Nucleotide sequence accession number.
The sequence for ORF cepA has been listed in GenBank with the accession number AB073019.
Acknowledgments
This study was supported by grant NTUH-90-S10 from National Taiwan University Hospital and grant NSC-90-2320-B-002-151 from National Science Council, Taiwan.
REFERENCES
- 1.Abbott, S. 1999. Klebsiella, Enterobacter, Citrobacter, and Serratia, p. 475-482. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 2.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Chang, K. C., S. W. Ho, J. C. Yang, and J. T. Wang. 1997. Isolation of a genetic locus associated with metronidazole resistance in Helicobacter pylori. Biochem. Biophys. Res. Commun. 236:785-788. [DOI] [PubMed] [Google Scholar]
- 4.Dance, D. A., A. D. Pearson, D. V. Seal, and J. A. Lowes. 1987. A hospital outbreak caused by a chlorhexidine- and antibiotic-resistant Proteus mirabilis. J. Hosp. Infect. 10:10-16. [DOI] [PubMed] [Google Scholar]
- 5.Eisenetein, B. I., and D. F. Zaleznik. 2000. Enterobacteriaceae, p. 2294-2309. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, New York, N. Y.
- 6.Hammond, S. A., J. R. Morgan, and A. D. Russell. 1987. Comparative susceptibility of hospital isolates of gram-negative bacteria to antiseptics and disinfectants. J. Hosp. Infect. 9:255-264. [DOI] [PubMed] [Google Scholar]
- 7.Herrero, M., V. Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing nonantibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugo, W. B. 1992. Disinfectant mechanism, p. 187-210. In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principle and practice of disinfection, preservation, and sterilization, 2nd ed. Blackwell Scientific Publications, Oxford, England.
- 9.Larson, E. L., and APIC Guideline Committee. 1995. APIC guidelines for handwashing and hand antisepsis in healthcare settings. Am. J. Infect. Control 23:251-269. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo, V., M. Herrero, U. Jacubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAllister, T. A., C. E. Lucas, H. Mocan, R. H. Liddell, B. E. Gibson, I. M. Hann, and D. J. Platt. 1989. Serratia marcescens outbreak in a paediatric oncology unit traced to contaminated chlorhexidine. Scott. Med. J. 34:525-528. [DOI] [PubMed] [Google Scholar]
- 12.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Ogase, H., I. Nagai, K. Kameda, S. Kume, and S. Ono. 1992. Identification and quantitative analysis of degradation products of chlorhexidine with chlorhexidine-resistant bacteria with three-dimensional high performance liquid chromatography. J. Appl. Bacteriol. 73:71-78. [Google Scholar]
- 15.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reboli, A. C., J. F. John, Jr., and A. H. Levkoff. 1989. Epidemic methicillin-gentamicin-resistant Staphylococcus aureus in a neonatal intensive care unit. Am. J. Dis. Child. 143:34-39. [DOI] [PubMed] [Google Scholar]
- 17.Rouch, D. A., D. S. Cram, D. DiBerardino, T. G. Littlejohn, and R. A. Skurray. 1990. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol. Microbiol. 4:2051-2062. [DOI] [PubMed] [Google Scholar]
- 18.Russell, A. D., and M. J. Day. 1993. Antibacterial activity of chlorhexidine. J. Hosp. Infect. 25:229-238. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Short, J. M., J. M. Fernandez, J. A. Sorge, and W. D. Huse. 1988. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 16:7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stickler, D. J., B. Thomas, and J. C. Chawla. 1981. Antiseptic and antibiotic resistance in gram-negative bacteria causing urinary tract infection in spinal cord injured patients. Paraplegia 19:50-58. [DOI] [PubMed] [Google Scholar]
- 22.Tennent, J. M., B. R. Lyon, M. Midgley, G. Jones, A. S. Purewal, and R. A. Skurray. 1989. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J. Gen. Microbiol. 135:1-10. [DOI] [PubMed] [Google Scholar]
- 23.Thomas, L., J. Y. Maillard, R. J. Lambert, and A. D. Russell. 2000. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a “residual” concentration. J. Hosp. Infect. 46:297-303. [DOI] [PubMed] [Google Scholar]
- 24.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]