Abstract
β-l-Thymidine (l-dT) and β-l-2′-deoxycytidine (l-dC) are potent and highly specific inhibitors of hepatitis B virus (HBV) replication both in vivo and in vitro (50% effective concentrations, 0.19 to 0.24 μM in 2.2.15 cells). The intracellular metabolisms of l-dT and l-dC were investigated in HepG2 cells and primary cultured human hepatocytes. l-dT and l-dC were extensively phosphorylated in both cell types, with the 5′-triphosphate derivative being the predominant metabolite. In HepG2 cells, the 5′-triphosphate levels were 27.7 ± 12.1 and 72.4 ± 1.8 pmol/106 cells for l-dT and l-dC, respectively. In primary human hepatocytes, the 5′-triphosphate levels were 16.5 ± 9.8 and 90.1 ± 36.4 pmol/106 cells for l-dT and l-dC, respectively. Furthermore, a choline derivative of l-dCDP was detected at concentrations of 15.8 ± 1.8 and 25.6 ± 0.1 pmol/106 cells in human hepatocytes and HepG2 cells, respectively. In HepG2 cells exposed to l-dC, the 5′-monophosphate and 5′-triphosphate derivatives of β-l-2′-deoxyuridine (l-dUMP and l-dUTP, respectively) were also observed, reaching intracellular concentrations of 6.7 ± 0.4 and 18.2 ± 1.0 pmol/106 cells, respectively. In human hepatocytes, l-dUMP and l-dUTP were detected at concentrations of 5.7 ± 2.4 and 43.5 ± 26.8 pmol/106 cells, respectively. It is likely that deamination of l-dCMP by deoxycytidylate deaminase leads to the formation of l-dUMP, as the parent compound, l-dC, was not a substrate for deoxycytidine deaminase. The intracellular half-lives of l-dTTP, l-dCTP, and l-dUTP were at least 15 h, with intracellular concentrations of each metabolite remaining above their respective 50% inhibitory concentrations for the woodchuck hepatitis virus DNA polymerase for as long as 24 h after removal of the drug from cell cultures. Exposure of HepG2 cells to l-dT in combination with l-dC led to concentrations of the activated metabolites similar to those achieved with either agent alone. These results suggest that the potent anti-HBV activities of l-dT and l-dC are associated with their extensive phosphorylation.
Hepatitis B virus (HBV) is the major cause of acute and chronic hepatitis, leading to progressive development of necroinflammatory changes in the liver, which can result in cirrhosis and hepatocellular carcinoma (1, 7). Approximately 350 million people (5% of the world's population) are chronically infected with HBV, and 1 million of these patients die every year as a result of this infection (11). Although the development of an effective vaccine to prevent HBV infection has shown promising results and should lead to its eventual eradication, antiviral chemotherapy remains the only effective method to prevent the progression of the disease in chronic carriers (8). Initially, alpha interferon was used as therapy for chronic HBV infection; however, the majority of patients did not benefit, and side effects were significant in some patients (15). At present, β-l-2′,3′-dideoxy-3′-thiacytidine (lamivudine) is the only nucleoside analogue approved for use for the treatment of chronic hepatitis B; however, upon the cessation of treatment serum HBV DNA levels return to pretreatment levels. This rebound is also associated with the appearance of drug-resistant virus that is mutated at the active site of the viral reverse transcriptase (9). Therefore, the development of new antiretroviral agents active against HBV is needed.
Recently, β-l-thymidine (l-dT) and β-l-2′-deoxycytidine (l-dC) were shown to be potent and specific inhibitors of HBV replication both in vivo and in vitro (50% effective concentrations [EC50s], 0.19 to 0.24 μM in human hepatoma 2.2.15 cells) (2). In a phase I-II clinical trial, treatment with l-dT has also been demonstrated to cause marked reductions in HBV DNA levels in chronically infected patients (C. L. Lai, S. G. Lim, M. F. Yuen, D. M. Pow, and M. W. Myers, Abstr. Eur. Assoc. Study Liver, abstr. 500, 2001; S. G. Lim, C. L. Lai, Y. M. Lee, D. M. Pow, and M. W. Myers, Abstr. Dig. Dis. Week, abstr. 2908, 2001). In vitro studies indicated that at concentrations as high as 100 μM l-dT and l-dC did not exhibit cellular or mitochondrial toxicities and did not inhibit human cellular DNA polymerases α, β, and γ (J. P. Sommadossi, M. L. Bryant, G. Gosselin, R. F. Schinazi, and J. L. Imbach, Program Abstr. 3rd Int. Conf. Ther. Viral Hepatitis, abstr. 19, Antivir. Ther. 4(Suppl. 4):8, 1999). Preliminary studies demonstrated the intracellular presence of phosphorylate derivatives of l-dT and l-dC [L. Placidi et al., abstract from the 3rd International Conference on Therapy for Viral Hepatitis, Antivir. Ther. 4(Suppl. 4):48, abstr. A122, 1999]. The present study examines the intracellular metabolism and extent of phosphorylation of l-dT and l-dC in HepG2 cells and primary cultured hepatocytes isolated from human liver.
MATERIALS AND METHODS
Chemicals.
l-dT and l-dC were obtained from Novirio Pharmaceuticals, Cambridge, Mass. [3H]l-dT (6 Ci/mmol) and [3H]l-dC (17.5 Ci/mmol) were obtained from Moravek Biochemicals Inc. (Brea, Calif.). Tetrabutylammonium phosphate was obtained from Alltech (Deerfield, Ill.). All other chemicals were of the highest grade available.
Cell culture conditions and determination of intracellular metabolites.
HepG2 cells were obtained from the American Type Culture Collection (Manassas, Va.) and were grown in 225-cm2 tissue culture flasks in minimal essential medium supplemented with nonessential amino acids, 1% sodium pyruvate, 10% dialyzed fetal bovine serum, and 1% penicillin-streptomycin. The medium was renewed every 3 days, and the cells were subcultured once a week. For intracellular metabolism studies, confluent HepG2 cells were detached from the adherent monolayer by a 10-min exposure to 30 ml of trypsin-EDTA, followed by three consecutive washes with medium, and at confluency were seeded (2.5 × 106 cells per well) in a six-well plate. HepG2 cells were exposed to 1 or 10 μM [3H]l-dT or [3H]l-dC (1,000 and 500 dpm/pmol, respectively) for the specified time periods. The cells were maintained at 37°C under a 5% CO2 atmosphere. At selected times, the extracellular medium was removed and the cell layer was washed with ice-cold phosphate-buffered saline (PBS). Cells were removed from the wells by scraping in the presence of 60% methanol, and the cell-methanol mixture was incubated overnight at −20°C. l-dT or l-dC and their respective metabolites were extracted from the cells by incubation in 200 μl of cold methanol for 1 h on ice. The extracts were dried under a gentle filtered airflow and stored at −20°C until high-pressure liquid chromatography (HPLC) analysis. The method for calculating the concentration of phosphorylated metabolites (1 pmol/106 cells = 1 μM) was based on previous studies with H9 cells (5).
Isolation and culture of human hepatocytes.
Human livers were obtained through the University of Alabama at Birmingham Liver Center. All livers had normal histologies and tested negative for human immunodeficiency virus and HBV. The isolation and culture of human hepatocytes were performed as described previously (12). Briefly, the livers were washed in situ at 4°C with Eurocollins buffer (12) supplemented with heparin to remove blood from the vessels. Liver samples were then perfused with previously oxygenated calcium-free HEPES buffer (pH 7.4) and then with 0.05% (wt/vol) collagenase solution containing calcium under recirculation and continuous oxygenation conditions. After 15 to 20 min of perfusion, necessary for disruption of Glisson's capsule, hepatocytes were suspended in L15 medium (Gibco BRL, Life Technologies) containing 5% fetal calf serum. The freshly isolated cells were then washed three times and centrifuged at 40 × g at 4°C for 10 min in L15 medium supplemented with 10% fetal calf serum to remove debris and damaged cells. After the final wash, the cell number was determined by an erythrosin B exclusion test. Viable hepatocytes (>90%) were resuspended in William's medium (Gibco BRL, Life Technologies) containing 2 mM glutamine and antibiotics. The hepatocytes were seeded at a density of 0.75 × 106 cells/ml in six-well plates previously coated with rat tail collagen, and the plates were incubated in a 5% CO2 atmosphere at 37°C. The cells were allowed to attach overnight, and then the medium was replaced by the same medium (without fetal bovine serum) containing 10 μM hydrocortisone hemisuccinate, 10 mM sodium pyruvate, 10 ng of selenium per ml, 4 μg of glucagon per ml, 6.8 μM ethanolamine, and 10 μg of human transferrin per ml. After 24 h, this medium was renewed and drug metabolic assays were initiated. The hepatocytes were incubated with 10 μM [3H]l-dT (1,000 dpm/pmol) or [3H]l-dC (1,000 dpm/pmol). At selected times, the extracellular medium was removed and the cell layer was washed with cold PBS, followed by cell scraping in 60% methanol as described above. l-dT or l-dC and their respective metabolites were extracted from the cells by incubation overnight at −20°C in 60% methanol, followed by an additional extraction with 500 μl of cold methanol for 1 h on ice. Combined extracts were dried under a gentle filtered airflow and stored at −20°C until analysis by HPLC. Extract residues were resuspended in 250 μl of distilled water, and 200 μl was injected onto the HPLC system described below.
Determination of intracellular l-dTTP, l-dCTP, and l-dUTP half-lives.
HepG2 cells (2.5 × 106 cells per well) were incubated with either 10 μM [3H]l-dT or [3H]l-dC (500 dpm/pmol) for 24 h at 37°C in a 5% CO2 atmosphere. The cells were then washed three times with drug-free medium to remove extracellular drug and incubated for specific time intervals. At selected times, the cells were washed three times with ice-cold PBS and intracellular l-dT, l-dC, and their metabolites were extracted with methanol as described above. The samples were then stored at −20°C until HPLC analysis. The half-life was calculated from 0.693/κ, where κ is the terminal-phase slope of a plot of the intracellular triphosphate concentration versus time.
Effect of addition of 10 μM l-dT or l-dC on phosphorylation of [3H]l-dT or [3H]l-dC.
HepG2 cells (2.5 × 106 cells per well) were incubated in six-well plates at 37°C for 24 h with either [3H]l-dT and l-dC or [3H]l-dC and l-dT at a final concentration of 10 μM for each agent. After incubation, intracellular l-dT or l-dC and their respective metabolites were extracted and analyzed as described above.
HPLC analysis.
Cell extracts were reconstituted in 250 μl of distilled water prior to HPLC analysis. l-dT, l-dC, and their respective metabolites were separated by reverse-phase HPLC with a Phenomenex 5-μm C18 column (model 1050; Hewlett-Packard Co., Palo Alto, Calif.) by manual injection of 200 μl. The mobile phase consisted of 25 mM ammonium acetate with 5 mM tetrabutylammonium phosphate (pH 7.0) (buffer A) and methanol (buffer B). Elution was performed with a multistage linear gradient (0 to 5%) of buffer B for the first 5 min that was then leveled off at 5% until 10 min, with a constant flow of 0.8 ml/min. The elution flow was then increased to 1 ml/min; and the gradient of buffer B was increased from 5 to 15% at 20 min, reaching 20% at 35 min, and was kept stable from 35 to 45 min, raised to 30% at 50 min, and finally increased by 5% every 5 min until 60 min. Radioactivity was measured by use of a radiochromatography analyzer (500TR Radiometric Flo-One; Packard Instrument Company, Inc., Meriden, Conn.). l-dT, l-dC, and their respective metabolites were identified by use of a combination of authentic cold standards and enzyme digestion of whole-cell extracts with phosphodiesterase and alkaline phosphatase.
RESULTS
HPLC analysis of [3H]l-dT and [3H]l-dC and their respective intracellular metabolites.
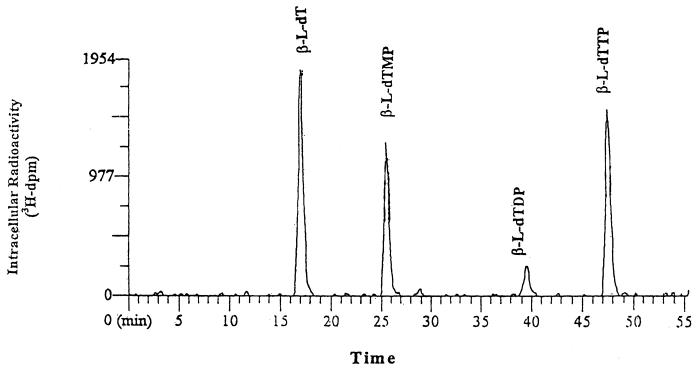
Figure 1 shows the HPLC radiochromatogram for intracellular extracts from primary human hepatocyte cultures following exposure to 10 μM [3H]l-dT for 24 h. l-dT was extensively phosphorylated, leading to the formation of l-dTMP, l-dTDP, and l-dTTP. No other metabolites were detected. Under these chromatographic conditions, the retention times of the parent drug, l-dT, and its phosphorylated metabolites, l-dTMP, l-dTDP, and l-dTTP, were 17, 26, 39, and 48 min, respectively.
FIG. 1.
HPLC radiochromatogram of intracellular extracts from primary human hepatocyte cultures exposed to 10 μM [3H]l-dT for 24 h.
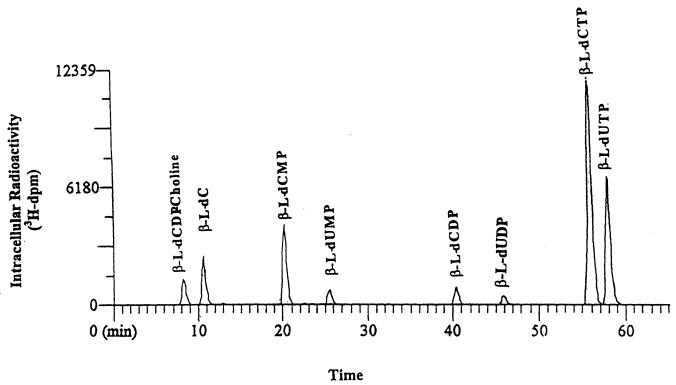
Figure 2 shows the HPLC radiochromatogram for intracellular extracts from primary human hepatocyte cultures exposed to 10 μM [3H]l-dC for 24 h. In addition to the parent drug and its 5′-phosphorylated metabolites, a choline derivative of l-dCDP (l-dCDP-choline) and the 5′-phosphorylated deaminated derivatives l-dUMP, l-dUDP, and l-dUTP were also detected. The retention times of the parent drug, l-dCDP-choline, l-dCMP, l-dCDP, l-dCTP, l-dUMP, l-dUDP, and l-dUTP were 11, 8, 20, 40, 56, 25, 46, and 58 min, respectively. Similar HPLC profiles were obtained with HepG2 cells.
FIG. 2.
HPLC radiochromatogram of intracellular extracts from primary human hepatocyte cultures exposed to 10 μM [3H]l-dC for 24 h.
Analysis of time course of accumulation of l-dT and its metabolites.
Table 1 shows the intracellular concentrations of l-dT metabolites in HepG2 cells exposed to 10 μM [3H]l-dT from 2 to 24 h. l-dT was extensively phosphorylated, with its 5′-triphosphate derivative (l-dTTP) being the predominant metabolite, reaching a concentration of 27.7 ± 12.1 pmol/106 cells by 24 h. The intracellular concentrations of the intracellular metabolites after 24 h of exposure of primary human hepatocytes to 10 μM [3H]l-dT are shown in Table 2. Similar to what was observed in HepG2 cells, l-dT was extensively phosphorylated, with l-dTTP reaching a maximum concentration of 16.5 ± 9.8 pmol/106 cells by 24 h.
TABLE 1.
Intracellular concentrations of l-dT 5′-phosphorylated metabolites in HepG2 cells after incubation with 10 μM [3H]l-dT
| Time of exposure (h) | Concn (pmol/106 cells)a
|
||
|---|---|---|---|
| l-dTMP | l-dTDP | l-dTTP | |
| 2 | 2.62 ± 1.31 | 1.42 ± 0.90 | 4.24 ± 3.31 |
| 6 | 4.38 ± 1.46 | 2.06 ± 0.87 | 10.8 ± 4.3 |
| 10 | 6.04 ± 2.66 | 2.07 ± 0.82 | 15.0 ± 8.3 |
| 24 | 8.09 ± 3.82 | 2.87 ± 0.99 | 27.7 ± 12.1 |
The values are means ± standard deviations of three independent experiments.
TABLE 2.
Intracellular concentrations of l-dT metabolites in primary cultured human hepatocytes cells after incubation with 10 μM [3H]l-dT
| Time of exposure (h) | Concn (pmol/106 cells)a
|
||
|---|---|---|---|
| l-dTMP | l-dTDP | l-dTTP | |
| 2 | 9.01 ± 5.76 | 1.25 ± 0.18 | 3.35 ± 1.78 |
| 6 | 14.2 ± 7.2 | 2.06 ± 0.92 | 10.9 ± 7.3 |
| 10 | 13.1 ± 5.4 | 2.36 ± 1.29 | 13.7 ± 6.8 |
| 24 | 15.2 ± 8.1 | 2.49 ± 0.64 | 16.5 ± 9.8 |
The values are means ± standard deviations of three independent experiments.
Analysis of time course of accumulation of l-dC and its metabolites.
Table 3 shows the intracellular metabolite formation profile of HepG2 cells exposed to 10 μM [3H]l-dC for up to 24 h. The 5′-phosphorylated metabolites of l-dC reached high intracellular concentrations. l-dCTP was the predominant metabolite, reaching 72.4 ± 1.8 pmol/106 cells at 24 h. In addition, l-dUTP achieved concentrations of 18.2 ± 1.0 pmol/106 cells at 24 h. The levels of l-dCDP-choline and l-dCMP were also significant, attaining maximum concentrations of 25.6 ± 0.1 and 23.2 ± 0.8 pmol/106 cells, respectively, at 24 h.
TABLE 3.
Intracellular concentrations of l-dC metabolites in HepG2 cells after incubation with 10 μM [3H]l-dC
| Time of exposure (h) | Concn (pmol/106 cells)a
|
||||||
|---|---|---|---|---|---|---|---|
| l-dCDP-choline | l-dCMP | l-dUMP | l-dCDP | l-dUDP | l-dCTP | l-dUTP | |
| 2 | 2.63 ± 0.03 | 3.21 ± 0.27 | 0.38 ± 0.05 | 0.48 ± 0.08 | NDb | 4.28 ± 0.30 | 0.26 ± 0.02 |
| 6 | 8.02 ± 0.71 | 4.39 ± 2.68 | 1.67 ± 0.57 | 2.24 ± 0.37 | ND | 19.0 ± 1.5 | 1.84 ± 0.18 |
| 10 | 14.0 ± 0.6 | 8.47 ± 0.17 | 2.94 ± 0.25 | 4.05 ± 0.22 | 0.95 ± 0.06 | 33.3 ± 0.8 | 4.93 ± 0.03 |
| 24 | 25.6 ± 0.1 | 23.2 ± 0.8 | 6.73 ± 0.41 | 10.2 ± 1.9 | 2.69 ± 0.45 | 72.4 ± 1.8 | 18.2 ± 1.0 |
The values are means ± standard deviations of three independent experiments.
ND, not detected.
Table 4 shows the intracellular concentration of l-dC metabolites in primary human hepatocytes after 2 to 24 h of exposure to 10 μM [3H]l-dC. Similar to what was observed in HepG2 cells, l-dC was extensively phosphorylated. Both l-dCTP and l-dUTP were the predominant metabolites, reaching concentrations of 90.1 ± 36.4 and 43.5 ± 26.8 pmol/106 cells, respectively, at 24 h. In addition, l-dCDP-choline and l-dCMP accounted for 15.8 ± 1.8 and 27.6 ± 15.2 pmol/106 cells, respectively, at 24 h.
TABLE 4.
Intracellular concentrations of l-dC metabolites in primary cultured human hepatocytes cells after incubation with 10 μM [3H]l-dC
| Time of exposure (h) | Concn (pmol/106 cells)a
|
||||||
|---|---|---|---|---|---|---|---|
| l-dCDP-choline | l-dCMP | l-dUMP | l-dCDP | l-dUDP | l-dCTP | l-dUTP | |
| 2 | 1.45 ± 0.52 | 14.3 ± 8.4 | 0.40 ± 0.07 | 1.46 ± 0.34 | NDb | 7.11 ± 3.46 | 0.66 ± 0.24 |
| 6 | 6.97 ± 1.02 | 19.3 ± 9.5 | 1.02 ± 0.48 | 3.92 ± 1.18 | 0.49 | 33.6 ± 7.2 | 3.77 ± 1.25 |
| 10 | 10.5 ± 2.9 | 21.6 ± 11.9 | 1.14 ± 0.17 | 5.43 ± 1.46 | 0.86 ± 0.29 | 45.1 ± 13.8 | 8.89 ± 4.14 |
| 24 | 15.8 ± 1.8 | 27.6 ± 15.2 | 5.74 ± 2.42 | 7.19 ± 2.28 | 3.93 ± 1.61 | 90.1 ± 36.4 | 43.5 ± 26.8 |
The values are means ± standard deviations of three independent experiments.
ND, not detected.
Determination of intracellular l-dTTP, l-dCTP, and l-dUTP half-lives.
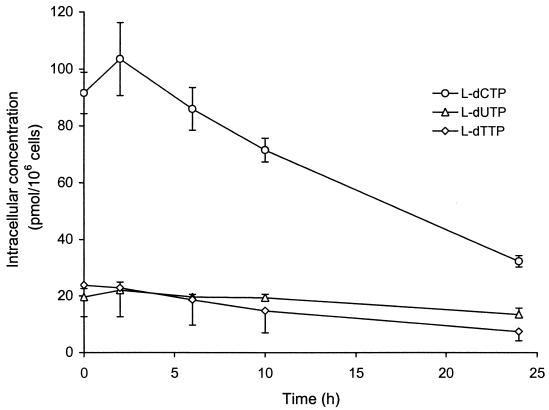
HepG2 cells were exposed to 10 μM l-dT or l-dC for 24 h. The rates of decay of intracellular phosphorylated metabolites were measured following the removal of drug from cell cultures (Fig. 3). The intracellular half-lives of l-dTTP, l-dCTP, and l-dUTP in HepG2 cells were at least 15 h. The concentrations of the active metabolites 24 h after removal of the respective parent drug from the cells measured 7.43 ± 3.26, 32.2 ± 2.0, and 13.5 ± 2.2 pmol/106 cells for l-dTTP, l-dCTP, and l-dUTP, respectively.
FIG. 3.
Rate of decay of 5′-phosphorylated metabolites of l-dC, l-dT, and l-dU in HepG2 cells after exposure to 10 μM radiolabeled drug for 24 h.
Effect of combination of l-dT and l-dC on concentrations of their respective 5′-triphosphate derivatives in HepG2 cells.
Tables 5 and 6 show the effect of l-dT (10 μM) on the phosphorylation of [3H]l-dC (10 μM) and the effect of l-dC (10 μM) on the phophorylation of [3H]l-dT (10 μM) in HepG2 cells, respectively. Exposure of HepG2 cells to l-dT and l-dC in combination led to concentrations of their respective activated metabolites that were similar to those concentrations achieved with either agent alone.
TABLE 5.
Effect of 10 μM l-dT on phosphorylation of 10 μM [3H]l-dC in HepG2 cells
| Incubation condition (24 h) | Concn (pmol/106 cells)a
|
||||||
|---|---|---|---|---|---|---|---|
| l-dCDP-choline | l-dCMP | l-dUMP | l-dCDP | l-dUDP | l-dCTP | l-dUTP | |
| Control | 20.7 ± 4.7 | 9.74 ± 1.70 | 4.69 ± 1.07 | 4.51 ± 3.15 | 1.20 ± 0.19 | 39.0 ± 0.6 | 7.65 ± 1.10 |
| 10 μM l-dT | 20.9 ± 4.7 | 10.5 ± 0.2 | 4.99 ± 2.07 | 4.01 ± 1.30 | 1.34 ± 0.41 | 43.2 ± 2.0 | 7.86 ± 2.08 |
The values are means ± standard deviations of three independent experiments.
TABLE 6.
Effect of 10 μM l-dC on phosphorylation of 10 μM [3H]l-dT in HepG2 cells
| Incubation condition (24 h) | Concn (pmol/106 cells)a
|
||
|---|---|---|---|
| l-dTMP | l-dTDP | l-dTTP | |
| Control | 2.94 ± 0.53 | 1.37 ± 0.68 | 10.6 ± 1.1 |
| 10 μM l-dC | 3.16 ± 0.76 | 1.63 ± 1.06 | 11.3 ± 1.9 |
The values are means ± standard deviations of three independent experiments.
DISCUSSION
The discovery of the potent antiviral activities of nucleoside analogues in the β-l configuration has attracted particular attention in recent years due to their increased selectivities compared with those of the corresponding β-d enantiomers (6, 10). In addition, the recent approval of lamivudine for the treatment of chronic hepatitis B has prompted further investigations with novel nucleosides and nucleoside analogues in the β-l configuration with activities against HBV. l-dT and l-dC have been shown to inhibit HBV replication in 2.2.15 cells with EC50s of 0.19 and 0.24 μM, respectively (2). These β-l-nucleosides have in common a hydroxyl group in the 3′ position on the sugar moiety that renders the compound inactive against other viruses such as human immunodeficiency virus, herpes simplex virus, respiratory syncytial virus, varicella-zoster virus, human cytomegalovirus, Epstein-Barr virus, measles virus, adenovirus, rhinovirus, influenza virus, and parainfluenza virus (2). Preliminary studies have demonstrated that their respective 5′-triphosphate derivatives, l-dTTP and l-dCTP, are potent inhibitors of woodchuck hepatitis virus DNA polymerase, with 50% inhibitory concentrations of 0.24 and 1.82 μM, respectively, whereas neither was a substrate for human DNA polymerase α, β, or γ (2). In addition, l-dT has demonstrated marked reductions in HBV DNA levels in a phase I-II clinical trial (Lai et al., Abstr. Eur. Assoc. Study Liver, abstr. 500, 2001; Lim et al., Abstr. Dig. Dis. Week, abstr. 2908, 2001).
In the HepG2 human hepatoma cell line and primary human hepatocytes, the 5′-triphosphate derivatives of l-dT and l-dC were the predominant metabolites detected in cell extracts. Interestingly, after exposure of HepG2 cells and primary human hepatocytes to l-dC, its 5′-phosphorylated deaminated metabolites were also observed. Similarly to what was shown with other β-l-nucleoside analogues (4, 13, 14), l-dC was not a substrate for the degradative enzyme deoxycytidine deaminase (EC 3.5.4.14), as demonstrated by the lack of inhibition of 3′,4′,5′,6′-tetrahydrouridine on the formation of 5′-phosphorylated l-dU derivatives (B. Hernandez-Santiago and J. P. Sommadossi, unpublished data). The lack of enzymatic deamination of β-l-cytidine nucleoside analogues suggests that deoxycytidine deaminase may be enantioselective, as previous studies have reported that lamivudine and β-l-2′,3′-dideoxy-5-fluoro-3′-thiacytidine (FTC) were not deaminated by partially purified deoxycytidine deaminase. Under the same conditions, their respective β-d enantiomers were rapidly deaminated to β-d-2′,3′-dideoxy-3′-thiauridine and β-d-2′,3′-dideoxy-5-fluoro-3′-thiauridine, respectively (3, 13, 14). When HepG2 cells were incubated with l-dC in the presence of deoxytetrahydrouridine, significant reductions in the concentrations of l-dUMP, l-dUDP, and l-dUTP were detected, demonstrating the involvement of deoxycytidylate deaminase (EC 3.5.4.12) in the formation of these 5′-phosphorylated metabolites from the deamination of l-dCMP (Hernandez-Santiago and Sommadossi, unpublished). In addition, l-dUTP is active against woodchuck hepatitis virus DNA polymerase (2). This metabolic pattern is of particular significance since the administration of one nucleoside yields two distinct pharmacologically active 5′-phosphorylated derivatives.
Moreover, results summarized in Tables 5 and 6 indicate that exposure of HepG2 cells to l-dT in combination with l-dC led to concentrations of their activated metabolites similar to those achieved with either agent alone, further warranting their use in combination chemotherapy. Indeed, in vitro and in vivo studies of the combination of l-dT and l-dC in the woodchuck model of chronic HBV infection indicate that the two drugs have a potent antiviral synergy when used in combination (E. G. Bridges, A. Juodawlkis, A. Faraj, B. Tennant, B. Korba, R. F. Schinazi, T. Barnett, G. Gosselin, J.-L. Imbach, C. Pierra, D. Dukhan, J.-P. Sommadossi, and M. Bryant, 13th Annu. Conf. Antivir. Res., Antivir. Res. 46:A62, 2000). The metabolic studies reported herein indicate that administration of l-dC to both cultured primary human hepatocytes and HepG2 cells led to the additional formation of the l-dCDP-choline metabolite. The formation of such a liponucleotide has previously been described with other cytidine analogues with the unnatural β-l configuration, such as l-ddC and its 5-fluorinated derivative l-FddC (10). This liponucleotide may act as an intracellular precursor for the active nucleoside 5′-triphosphate.
Pharmacokinetic studies with HepG2 cells demonstrated that l-dTTP, l-dCTP, and l-dUTP exhibited extended half-lives of 15 h. Importantly, the concentrations of these triphosphates remained greater than the in vitro 50% inhibitory concentration of the triphosphates for the woodchuck hepatitis virus DNA polymerase (2). These long intracellular half-lives probably reflect a low affinity of the 5′-phosphorylated metabolite of l-dT and l-dC for degradative enzymes. This characteristic may also contribute to the potent antiviral activities of these unnaturally configured β-l-nucleosides, as observed for lamivudine triphosphate and FTC triphosphate (3, 6).
In summary, the present study demonstrates that l-dT and l-dC are rapidly and extensively phosphorylated in HepG2 cells and primary human hepatocytes. The 5′-triphosphate derivatives of these two novel β-l-nucleoside enantiomers achieved high intracellular concentrations. Incubation of cells with l-dC led to the formation of l-dUTP through the deamination of l-dCMP to l-dUMP by deoxycytidylate deaminase, as the parent compound, l-dC, was not a substrate for deoxycytidine deaminase. Furthermore, l-dTTP, l-dCTP, and l-dUTP exhibited long intracellular half-lives, with their concentrations remaining above their EC50s for HBV in 2.2.15 cells. Lastly, neither l-dT nor l-dC interfered with the level of phosphorylation of the other compound, suggesting that combination antiviral therapy could be possible.
Acknowledgments
We thank Lee Anderson for assistance with preparation of the manuscript.
Footnotes
Dedicated to the memory of Martin L. Bryant.
REFERENCES
- 1.Beasley, R. P. 1988. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 61:1942-1956. [DOI] [PubMed] [Google Scholar]
- 2.Bryant, M. L., E. Bridges, L. Placidi, A. Faraj, A.-G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J.-L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, P. Marion, E. Cretton-Scott, R. F. Schinazi, and J.-P. Sommadossi. 2001. Antiviral l-nucleosides specific for hepatitis B virus infection. Antimicrob. Agents Chemother. 45:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, C. N., S. L. Doong, J. H. Zhou, J.W. Beach, L. S. Jeong, C. K. Chu, C.H. Tsai, and Y. C. Cheng. 1992. Deoxycytidine deaminase-resistant stereoisomer is the active form of (β)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J. Biol. Chem. 267:13938-13942. [PubMed] [Google Scholar]
- 4.Furman, P. A., M. Davis, D. C. Liotta, M. Paff, L. W. Frick, D. J. Nelson, R. E. Dornsife, J. A. Wurster, L. J. Wilson, J. A. Fyefe, J. V. Tuttle, W. H. Miller, L. Condreay, D. R. Averett, R. F. Schinazi, and G. R. Painter. 1992. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob. Agents Chemother. 36:2686-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman, P. A., J. A. Fyfe, M. H. St. Clair, K. Weinhold, J. L. Rideout, G. A. Freeman, S. N. Lehrman, D. P. Bolognesi, S. Broder, H. Mitsuya, and D. W. Barry. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furman, P. A., L. J. Wilson, J. E. Reardon, and G. R. Painter. 1995. The effect of absolute configuration on the anti-HIV and anti-HBV activity of nucleoside analogues. Antivir. Chem. Chemother. 6:345-355. [Google Scholar]
- 7.Hoofnagle, J. H., and A. M. Di Bisceglie. 1997. The treatment of chronic viral hepatitis. N. Engl. J. Med. 336:347-356. [DOI] [PubMed] [Google Scholar]
- 8.Hoofnagle, J. H. 1998. Therapy of viral hepatitis. Digestion 59:563-578. [DOI] [PubMed] [Google Scholar]
- 9.Ling, R., D. Mutimer, M. Ahmed, E. Boxall, E. Elias, G. Dusheiko, and T. Harrison. 1996. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology 24:711-713. [DOI] [PubMed] [Google Scholar]
- 10.Martin, L., A. Faraj, R. F. Schinazi, G. Gosselin, C. Mathe, J. L. Imbach, and J. P. Sommadossi. 1997. Effect of stereoisomerism on the cellular pharmacology of β-enantiomers of cytidine analogs in Hep-G2 cells. Biochem. Pharm. 53:75-87. [DOI] [PubMed] [Google Scholar]
- 11.Maynard, J. E. 1990. Hepatitis B: global importance and need for control. Vaccine 8(Suppl.):S18-S20. [DOI] [PubMed] [Google Scholar]
- 12.Nicolas F., G. DeSousa, P. Thomas, M. Placidi, G. Lorenzon, and R. Rahmani. 1995. Comparative metabolism of 3′-azido-3′-deoxythymidine in cultured hepatocytes from rats, dogs, monkeys and humans. Drug Metab. Dispos. 23:308-313. [PubMed] [Google Scholar]
- 13.Paff, M. T., D. R. Averett, K. L. Prus, W. H. Miller, and D. J. Nelson. 1994. Intracellular metabolism of (−)- and (+)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in Hep-G2 derivative 2.2.15 (subclone P5A) cells. Antimicrob. Agents Chemother. 38:1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shewach, D. S., D. C. Liotta, and R. F. Schinazi. 1993. Affinity of the antiviral enantiomers of oxathiolane cytosine nucleosides for human 2′-deoxycytidine kinase. Biochem. Pharmacol. 45:1540-1543. [DOI] [PubMed] [Google Scholar]
- 15.Wong, D. K., A. Cheng, K. O'Rourhe, C. D. Naylor, A. S. Delshy, and J. Heathcole. 1993. Effect of alpha-interferon treatment in patients with hepatitis Be antigen-positive chronic hepatitis B. Ann. Intern. Med. 119:312-323. [DOI] [PubMed] [Google Scholar]