Abstract
This work describes the discovery and characterization of a novel series of tricyclic natural product-derived metallo-β-lactamase inhibitors. Natural product screening of the Bacillus cereus II enzyme identified an extract from a strain of Chaetomium funicola with inhibitory activity against metallo-β-lactamases. SB236050, SB238569, and SB236049 were successfully extracted and purified from this extract. The most active of these compounds was SB238569, which possessed Ki values of 79, 17, and 3.4 μM for the Bacillus cereus II, Pseudomonas aeruginosa IMP-1, and Bacteroides fragilis CfiA metallo-β-lactamases, respectively, yet none of the compounds exhibited any inhibitory activity against the Stenotrophomonas maltophilia L-1 metallo-β-lactamase (50% inhibitory concentration > 1,000 μM). The lack of activity against angiotensin-converting enzyme and serine β-lactamases demonstrated the selective nature of these compounds. The crystal structure of SB236050 complexed in the active site of CfiA has been obtained to a resolution of 2.5 Å. SB236050 exhibits key polar interactions with Lys184, Asn193, and His162 and a stacking interaction with the indole ring of Trp49 in the flap, which is in the closed conformation over the active site groove. SB236050 and SB238569 also demonstrate good antibacterial synergy with meropenem. Eight micrograms of SB236050 per ml gave rise to an eightfold drop in the MIC of meropenem for two clinical isolates of B. fragilis producing CfiA, making these strains sensitive to meropenem (MIC ≤ 4 μg/ml). Consequently, this series of metallo-β-lactamase inhibitors exhibit the most promising antibacterial synergy activity so far observed against organisms producing metallo-β-lactamases.
Metallo-β-lactamases provide bacteria with an efficient and effective way of mediating resistance to β-lactam-based antibacterial agents. More significantly, they confer resistance to carbapenems. Therefore, if metallo-β-lactamases increase in prevalence, they could compromise the efficacy of this group of antibiotics to treat life-threatening hospital infections.
Metallo-β-lactamases have now been identified in a wide spectrum of clinically important pathogens, such as Klebsiella pneumoniae, Pseudomonas aeruginosa, Serratia marcescens, and Acinetobacter sp. (21; H. Ito, K. Senda, T. Yagi, K. Shibayama, M. Ohta, N. Kato, and Y. Arakawa, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-93, 1997). These enzymes also possess a proven capability to disseminate through bacterial populations, which is demonstrated by the spread of the IMP-1 metallo-β-lactamase in Japan (reviewed in reference 17). The spread of this enzyme is thought to be facilitated by its being encoded on an integron (1). More recently, clinical isolates producing metallo-β-lactamases have been identified in Europe (5, 12, 24).
One approach to combating this potential clinical problem is the use of a specific metallo-β-lactamase inhibitor in combination with a β-lactam antibiotic. In this regard a range of different non-β-lactam based molecules have been identified as specific inhibitors of these enzymes. A series of mercaptoacetic acid thiol ester compounds were identified as mechanism-based, irreversible inhibitors of metallo-β-lactamases (18). Walter et al. (23) described a series of trifluoromethyl alcohol and ketone inhibitors that were competitive inhibitors of a range of metallo-β-lactamases, and a group of biphenyl tetrazole compounds have been shown to be specific inhibitors of the Bacteroides fragilis CfiA-type metallo-β-lactamase (22). None of these compounds exhibited significant synergy with β-lactam antibiotics, nor did they exhibit potent broad-spectrum inhibition of metallo-β-lactamases. However, a recently discovered series of mercaptocarboxylates have been shown to exhibit both broad-spectrum metallo-β-lactamase inhibitory activity and antibacterial synergy with meropenem (17). In our quest to identify novel inhibitors of metallo-β-lactamases, we screened natural product extracts against the Bacillus cereus II enzyme and identified an extract from a strain of Chaetomium funicola with inhibitory activity against metallo-β-lactamases. SB236050, SB238569, and SB236049 were successfully extracted and purified from this extract, and we now report on the purification, extraction, and characterization of this novel series of metallo-β-lactamase inhibitors derived from a natural product.
MATERIALS AND METHODS
Bacterial strains and β-lactamases.
B. cereus II and P99 β-lactamase were purchased from Porton Products (Maidenhead, Berkshire, United Kingdom). The L-1 metallo-β-lactamase was purified as described previously from Stenotrophomonas maltophilia 511 (6). The CfiA enzyme was cloned and expressed from B. fragilis 262 (9) and purified as described previously (25). The L-1-like enzymes from S. maltophilia 37 and S. maltophilia GEL were obtained as described previously (19; D. J. Payne, P. Rowling, T. Khushi, C. Reading, and I. Dodd, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-60, 1994). The IMP-1 enzyme was purified from P. aeruginosa 101 as described by Laraki et al. (11). B. fragilis 288 and 460 are both CfiA producers (9; D. J. Payne, C. Betriu, T. Khushi, C. Hoyle, C. Reading, and D. Knowles, Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. A-75, 1995).
Tricyclic metallo-β-lactamase inhibitors.
C. funicola TCF 6040 was found to produce three novel tricyclic heterocycles that inhibit metallo-β-lactamases. The purification and characterization of these leads are described below.
Preinoculum preparation.
Six 250-ml flasks, each containing 30 ml of BGA1 medium (0.5% beef extract [Oxoid], 1.0% glycerol [Sigma], and 2% starch [Sigma]), were each seeded with four plugs of C. funicola TCF 6040 from a well-sporulated solid culture. The flask contents were incubated for 96 h at 28°C in an orbital shaker.
Inoculum preparation.
Six 2-liter flasks, each containing 400 ml of BGA1 medium, were each seeded with 30 ml of incubated preinoculum of C. funicola TCF 6040. The flasks were incubated for 48 h at 28°C in an orbital shaker.
Final fermentation.
Three Molecular BioReactors 42-liter fermentors, each containing 20 liters of BGA2 medium (0.5% beef extract [Oxoid], 1.0% glycerol [Sigma], and 4% collofilm dextrin) plus 0.02% SAG 471 silicon antifoam (Union Carbide) and 0.18% olive oil, were sterilized at 121°C for 45 min. After cooling to 28°C, each fermentor was inoculated with 800 ml of incubated inoculum. These fermentors were incubated at 28°C and maintained at 0.5 × 105 Pa of overpressure with an agitator speed of 300 rpm (tip speed, 1.35 m/s) and an airflow rate of 10 liters of air/min. The set point for partial O2 pressure was adjusted to 60% air saturation at 1.5 atm. This concentration of dissolved oxygen was maintained by a cascade system, which increased agitation from 300 rpm to the necessary level (maximum, 750 rpm) when partial O2 pressure dropped below 60%. The vessels were harvested after 6 days of incubation.
Isolation of SB236049, SB236050, and SB238569.
Sixty liters of broth was centrifuged (10 batches, 6 liters each, Beckman J6-MC centrifuge, TY JS 4.2 rotor, 4,000 rpm, 20 min, 10°C), and the supernatant was filtered through a sintered glass plate. The filtrate was loaded onto a column that was filled with Diaion HP-20 (Mitsubishi Chemical Co., Ltd., Tokyo, Japan) (15 liters, 18 by 60 cm, flow rate of 400 ml/min) and stabilized with water. The percolate was collected in three 20-liter fractions. The column was washed with 25 liters of water collected in one fraction, followed with 60 liters of water-methanol (2:1) collected in three fractions always at 400 ml/min. The active compounds were eluted with water-methanol (1:1) (120 liters) collected in 12 10-liter fractions at 400 ml/min, and the column was finally washed with 25 liters of methanol. The active fractions (50 liters) were concentrated in vacuo in a Büchi R-153 rotavapor (bath temperature 35°C) to eliminate the methanol and the aqueous residue (25 liters was then extracted with diethyl ether [9 liters, three times]). The organic phases were combined and evaporated in vacuo to dryness to give a yellow solid (3.7 g).
This solid was then extracted with 50 ml of chloroform to give dark yellow solid and liquid phases. The solid was further purified by extraction with 50 ml of tetrahydrofuran to give 200 mg of a solid that was again extracted with tetrahydrofuran, and 120 mg of SB236049 was filtered off as a yellow powder that was > 95% pure by nuclear magnetic resonance (NMR).
The mother liquors of SB236049 were evaporated to dryness and reextracted with 20 ml of tetrahydrofuran. A further SB236049 (35 mg) was filtered off, and the supernatant was treated with water to precipitate a yellow solid. This was redissolved in hot methanol, and a small amount of SB236049 was filtered off while hot. After cooling, 65 mg of SB238569 was filtered off as a yellow solid (>90% pure) by NMR.
The chloroform-soluble fraction was evaporated to dryness and was extracted with 50 ml of methanol to give a light yellow solid and a solution. The solid was reprecipitated with water from tetrahydrofuran to give a light yellow solid. This was again reprecipitated in the same way to give 220 mg of SB236050 as a light yellow solid (>95% pure) by NMR.
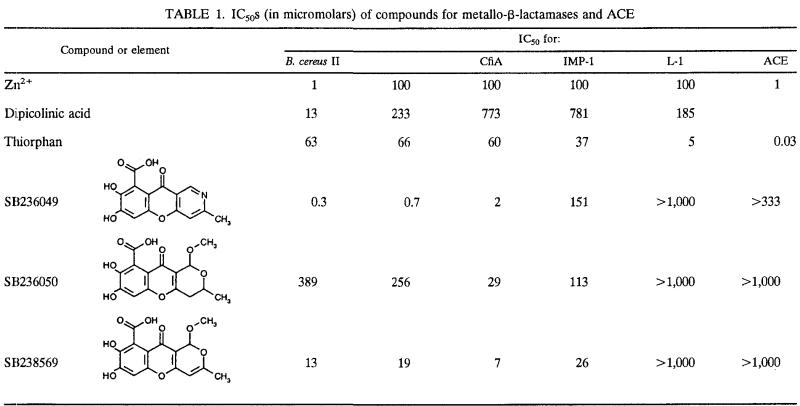
The structures of SB236049, SB236050, and SB238569 are shown in Table 1. Their physical and spectroscopic properties are given in Table 2.
TABLE 1.
IC50s (in micromolars) of compounds for metallo-β-lactamases and ACE
TABLE 2.
Spectroscopic data for compounds SB236049, SB236050, and SB238569
| Parametera | Data for:
|
||
|---|---|---|---|
| SB236049 | SB236050 | SB238569 | |
| EI-MS | M+, 287; Mol wt measured, 287.0439; Molecular formula, C14H9NO6 (requires 287.0430) | M+, 322; Mol wt measured, 322.0712; Molecular formula, C15H14O8 (requires 322.0689) | M+, 320; Mol wt measured, 320.0540; Molecular formula, C15H12O8 (requires 320.0532) |
| UV (H2O) | λmax = 223 and 289 nm | λmax = 220 and 289(sh), 312 nm | λmax = 214 and 232(sh), 330 nm |
| υmax (KBr) | 3,350, 1,666.40, 1,643.25, 1,613.36, 1,457.13, 1,378.06, 1,295.12, 1,192.90, 1,113.83, 1,038.61, 1,001.96, 869.84, 852.49, 839.95, 794.63, 752.19 | 3,350, 3,000, 1,627.82, 1,572.86, 1,482.21, 1,463.86, 1,398.31, 1,262.34, 1,090.68, 1,048.25, 967.24, 936.38, 916.13, 866.95, 792.70, 692.40, 682.76 | 3,500, 3,100, 2,950, 1,637.67, 1,593.30, 1,577.87, 1,556.65, 1,496.86, 1,467.92, 1,294.32, 1,195.94, 1,087.92, 981.83 |
| 1H NMR (d6-DMSO) | 12.80 (1H, broad s), 11.57 (1H, broad s), 9.33 (1H, broad s), 9.08 (1H, s), 7.44 (1H, s), 6.94 (1H, s), 2.59 (3H, s) | 6.84 (1H, s), 5.35 (1H, s), 4.21 (1H, m), 3.37 (3H, s), 2.67 (1H, dd, J4 Hz, 15 Hz), 2.56 (1H, dd, J 12 Hz, 15 Hz), 1.28 (3H, d, J 6 Hz) | 6.87 (1H, s), 6.17 (1H, s), 5.88 (1H, s), 3.43 (3H, s), 2.10 (3H, s) |
| 13C NMR (d6-DMSO) | 173.17 (C), 167.38 (C), 163.17 (C), 160.34 (C), 153.80 (C), 150.23 (C), 148.57 (CH), 140.66 (C), 120.76 (C), 114.74 (C), 111.06 (C), 110.62 (CH), 102.43 (CH), 24.29 (CH3) | 172.23 (C), 167.84 (C), 162.35 (C), 152.49 (C), 150.28 (C), 141.85 (C), 119.01 (C), 115.02 (C), 112.17 (C), 102.25 (CH), 94.03 (CH), 61.91 (CH), 55.07 (CH3), 33.26 (CH2), 20.51 (CH3) | 171.64 (C), 167.91 (C), 163.10 (C), 158.10 (C), 151.85 (C), 149.47 (C), 141.76 (C), 119.19 (C), 112.85 (C), 103.20 (C), 102.70 (CH), 7.02 (CH), 94.12 (CH), 54.89 (CH3), 20.06 (CH3) |
DMSO, dimethyl sulfoxide; EI-MS, electronic impact ionization mass spectrometry.
Determination of IC50 data.
Twenty-five millimolar piperazine-N,N′-bis(2-ethanesulfonic acid) buffer (pH 7.0) was used for all kinetic measurements. All the 50% inhibitory concentrations (IC50s) were measured in microtiter format (20) using a concentration of reporter substrate (nitrocefin) that was 5.7 times the Km for the reporter substrate for each of the enzymes. This enables more accurate comparisons of IC50s for the different enzymes. IC50s for CfiA, IMP-1, L-1, and the L-1-like enzymes from S. maltophilia 37 and GEL were all performed in the presence of 100 μM ZnSO4, and the enzyme was added last. The IC50s for B. cereus II were determined at both 1 and 100 μM ZnSO4, following a 5-min preincubation of enzyme and inhibitor. These assay conditions were used to identify whether the compounds had metal ion-chelating activity. Inhibitors that have higher potency at low concentrations of ZnSO4 may be inactivating the enzyme by nonspecific chelation of the active site Zn2+. All kinetic measurements for CfiA, B. cereus II, and IMP-1 were performed with 100 μM ZnSO4. IC50s for P99 were determined in the absence of ZnSO4 following a 5-min preincubation of enzyme and inhibitor.
The inhibitory potency of the compounds was also measured against another metalloprotease, angiotensin-converting enzyme (ACE), to give an indication of the potential selectivity of these compounds. Furylacryloylphenyl-alanylglycylglycine (FAPGG; Sigma) was used as the substrate for ACE, and its cleavage was measured at 340 nm (7). A microtiter plate assay was configured, the compounds were preincubated with ACE enzyme (Sigma) for 5 min, and the substrate was added last. The final concentrations of ACE and FAPGG in the assay were 0.035 U/ml and 668 μM, respectively.
Determination of kinetic data.
Ki values for SB236049, SB236050, and SB238569 were determined for the B. cereus II, IMP-1, and CfiA metallo-β-lactamases by varying both substrate and inhibitor concentrations. The rates thus obtained were fitted to
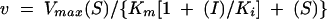 |
The Ki determinations were performed in the presence of 100 μM ZnSO4. All data were fitted using Grafit 3 (13).
Crystallography.
Cocrystals of the CfiA enzyme with the tricyclic inhibitor SB236050 were grown from 4 μl of sitting drops prepared by mixing 2 μl of protein solution (at a concentration of ∼14 mg/ml in 20 mM HEPES, pH 7.5, and to which solid inhibitor was added) with 2 μl of reservoir solution. The drops were equilibrated at room temperature against 500 μl of 32% PEG 1000, 0.1 M morpholineethanesulfonic acid, 10 μM ZnCl2, pH 6.0. The crystals belong to the space group P1 with the following unit cell parameters: a = 41.8 Å, b = 44.2 Å, c = 58.5 Å, α = 92.8°, β = 95.3°, and γ = 98.0°, with two independent molecules in the asymmetric unit and with an estimated 40% solvent content. The diffraction intensities from a single crystal mounted on a glass capillary tube were measured at room temperature using a Siemens X-1000 multiwire area detector mounted on a four-circle goniostat and CuKα radiation supplied by a Siemens rotating anode X-ray generator (50 kV, 100 mA). The diffraction data were integrated, reduced, and scaled using the program package XENGEN (8).
Determination of antibacterial synergy data.
Antibacterial synergy activity with meropenem was measured using a modified NCCLS broth microdilution method (15, 16). For this assay, the inoculum was prepared from a frozen stock containing a predetermined number of CFU (106) obtained from a 24-h broth culture. The inhibitors were tested in both a susceptibility profile, to determine if the inhibitor had antibacterial activity alone, and in a synergy profile with meropenem. The meropenem MIC for each organism was determined in the presence of both 8 and 32 μg of each inhibitor per ml. The contents of plates with aerobic organisms were incubated at 35°C for 24 h. The contents of plates with anaerobic organisms were incubated under anaerobic conditions at 35°C for 48 h.
RESULTS
Overall the IC50s illustrate that SB236049 was the most active of the three compounds, with IC50s of ≤2 μM for both CfiA and B. cereus II metallo-β-lactamases (Table 1). None of these compounds had any detectable activity against L-1 or the L-1-like enzymes from S. maltophilia 37 and GEL. In addition, no activity was detected against the serine active site enzyme, P99. Dipicolinic acid, a known metal ion chelator (Y. Yang, D. M Roll, M. J. Wildey, M. Lee, M. Greenstein, W. M. Maiesse, and K. Bush, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-56, 1994), had >15 times more inhibitory activity against B. cereus II at low Zn2+ than at 100 μM Zn2+. None of the tricyclic compounds exhibited this differential inhibitory activity at different levels of Zn2+. None of the compounds exhibited any activity against ACE at ≥333 μM.
Mechanism of inhibition of metallo-β-lactamases by tricyclic compounds.
Further analysis of the inhibition of metallo-β-lactamases by these compounds illustrated that they were competitive inhibitors of the CfiA, B. cereus II, and IMP-1 enzymes. Again, the CfiA enzyme appeared to be the most susceptible enzyme to inhibition by the compounds. SB238569 had the highest affinity of all the compounds tested, with a Ki of 3.4 μM for the CfiA enzyme (Table 3).
TABLE 3.
Ki values (in micromolars) of SB236049, SB236050, and SB238569 for B. cereus II, CfiA, and IMP-1 metallo-β-lactamases
| Extract |
Ki (μM)
|
||
|---|---|---|---|
| B. cereus II | CfiA | IMP-1 | |
| SB236049 | NTa | 15 | NT |
| SB236050 | 88 | 10 | 32 |
| SB238569 | 79 | 3.4 | 17 |
NT, not determined.
Binding of the inhibitor SB236050 to the active site of the CfiA metallo-β-lactamase enzyme .
The crystal structure of the inhibitor complex was determined by molecular replacement with the program package AMoRe (14) using the B. fragilis native crystal structure coordinates (3) (Protein Data Bank accession code, 1ZNB) as a search model after removing metals and solvent from the coordinate set. The program X-PLORv3.851 (2) was used to refine the structure. The first round of refinement included a simulated annealing step from 3,000 to 300 K with slow cooling followed by positional refinement using tight noncrystallographic restraints. The structure of the inhibitor was built and minimized using the program QUANTA (Molecular Simulations Corp.). The bond and angle parameters used for the refinement of the inhibitor in the complex were generated from the idealized structure of the inhibitor using XPLO2D (10). Eight rounds of positional and restrained B-factor refinement and manual intervention produced the final model in which Rfree = 0.232 and R = 0.141 (R = Σh ‖Fo| − |Fc‖/Σh|Fo|, where |Fo| and |Fc| were the observed and calculated structure factor amplitudes, respectively) for 10,526 reflections for which the equation F > 2σF includes all the data to 2.5 Å of resolution (Table 4). The model includes 3,538 nonhydrogen protein atoms, 40 water molecules, 4 Zn2+ atoms, 2 Na+ atoms, and 46 atoms corresponding to the two molecules in the asymmetric unit. Figure 1 shows the electron density in the active site of the metallo-β-lactamase into which the inhibitor was modeled. The atomic coordinates have been deposited in the Research Collaboratory for Structural Bioinformatics database with Protein Data Bank accession code 1KR3.
TABLE 4.
Refinement statistics
| Parameter | Value for CfiA + SB236050 |
|---|---|
| Space group | P1 |
| Resolution range (Å) | 50-2.5 |
| No. of unique reflections, F > 2σ F | 10,526 |
| Competeness (%) | |
| Overall | 73.7 |
| High resolution shell | 58.0 (2.54-2.50) |
| R | 0.141 |
| Rfree (10% data) | 0.232 |
| No. of atoms in asymmetric unit | |
| Protein | 3,538 |
| Solvent | 40 |
| Zn2+ | 2 Zn2+/2 Na+ |
| Root mean square deviation from ideal geometry | |
| Bond length (Å) | 0.02 |
| Bond angle (°) | 2.0 |
FIG. 1.
Electron density map calculated using Fobs-Fcalc coefficients and phases calculated from the refined model. The electron density map around the inhibitor was contoured at the 3σ level.
The inhibitor lies flat across the active site sandwiched between the floor of the active site and the GWG motif (single-letter code for amino acid residues 48 to 50), which forms a flap. The inhibitor is bound near the dinuclear zinc metal center, and the flap is in the closed conformation. The ring A of the inhibitor is located closer to the metal center, and the contacts with the protein are made by substituents in rings A and B (Fig. 2).
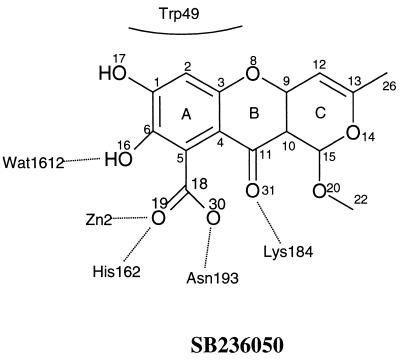
FIG. 2.
Binding of the inhibitor SB236050 to the active site of the CfiA metallo-β-lactamase enzyme.
Of the two molecules in the asymmetric unit, molecule B is the better ordered; therefore, the description of the interactions between enzyme and inhibitor is based on this molecule. The tricyclic molecule is oriented such that O-16 is lying close to the bridging water, Wat1612. This water molecule is located between the two zinc metals, Zn1 and Zn2. In the native B. fragilis structure a water molecule that interacts with the side chain of Asn193 occupies the position of O-16 (Fig. 2). Zn1 is coordinated to His99, His101, His162, and Wat1612. Zn2 is coordinated to Wat1612, Asp103, Cys181, His223, and O-19 of the inhibitor (Fig. 3). The inhibitor's carboxyl oxygen O-19 has displaced the water molecule ligand to Zn2 present in the native B. fragilis structure to become the fifth ligand to Zn2. O-30 interacts (2.7 Å) with a water molecule, Wat2017, located between His162ND and Ile191O. This water serves to orient and fix in position the imidazole ring of His162 for metal binding. In addition, O-30 interacts with the main chain amide nitrogen of Asn193 (3.2 Å). Lys184, a key residue involved in inhibitor binding, is interacting with the carbonyl oxygen O-31 (2.9 Å) of SB236050. Rings A and B of the inhibitor formed a stacking interaction with the indole ring of Trp49 in the flap, which is in the closed conformation over the active site groove (Fig. 3).
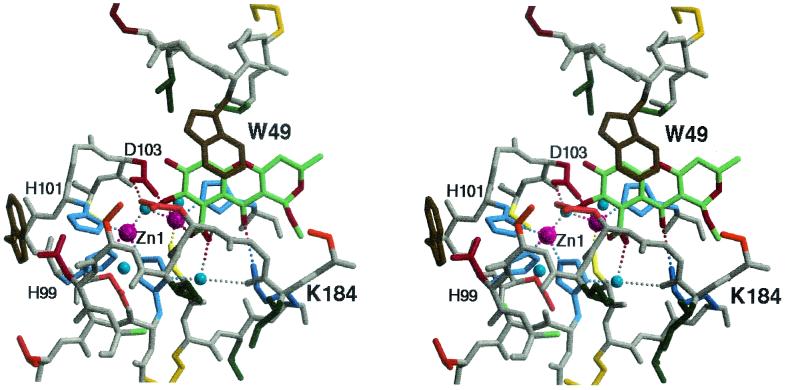
FIG. 3.
Binding of SB236050 in the active site of CfiA. Stereo view of the tricyclic inhibitor, SB236050, bound in the active site of the metallo β-lactamase from B. fragilis, CfiA. The inhibitor (carbon, green; oxygen, red; nitrogen, blue) lies flat at the floor of the active site. With the flap in the closed conformation, the inhibitor interacts with Trp49 by ring stacking. Polar interactions (represented by dotted lines) with the protein include interactions with the dinuclear zinc bridging water and with Lys184. The zinc ions are represented by purple spheres.
Antibacterial synergy of the tricyclic compounds with meropenem.
SB236050 and SB238569 were chosen as representative compounds for testing for antibacterial synergy with meropenem. Neither compound had any inherent antibacterial activity at 256 μg/ml. However, significant drops in MICs of meropenem for B. fragilis organisms producing CfiA were observed in the presence of 8 and 32 μg of each inhibitor per ml (Table 5). Eight micrograms of each of these derivatives per ml reduced the meropenem MIC of the B. fragilis 262 and the B. fragilis 460 strains to ≤4 μg/ml. B. fragilis 288 produced 10-fold more β-lactamase than strains 262 and 460, thus explaining the higher meropenem MIC observed with this organism. The meropenem MIC for B. fragilis 288 dropped eightfold in the presence of 8 μg of SB236050 per ml, but no effect on the MIC for the S. maltophilia strain was observed at 8 or 32 μg/ml. No synergy activity was observed against the P. aeruginosa strain producing the IMP-1 metallo-β-lactamase.
TABLE 5.
Antibacterial activity of meropenem in combination with metallo-β-lactamase inhibitors against strains producing metallo-β-lactamasesa
| Metallo-β-lactamase inhibitor | Concn (μg/ml) | Meropenem MICs (in μg/ml) when tested in combination with metallo-β-lactamase inhibitors at 32 and 8 μg/ml against the following organisms
|
|||
|---|---|---|---|---|---|
| B. fragilis 262 and B. fragilis 460 | B. fragilis 288 | S. maltophilia 511 | P. aeruginosa 101 | ||
| None | 16 | 256 | 128 | 512 | |
| SB236050 | 32 | 0.5 | 8 | 128 | 512 |
| SB236050 | 8 | 2 | 32 | 128 | 512 |
| SB238569 | 32 | 1 | 16 | 128 | 512 |
| SB238569 | 8 | 4 | 64 | 128 | 512 |
Neither compound possessed any antibacterial activity at 256 μg/ml.
DISCUSSION
These compounds represent a novel class of metallo-β-lactamase inhibitors. The tricyclic compounds have significant inhibitory activity against IMP-1, CfiA, and B. cereus II. Comparison of the active sites of the key metallo-β-lactamases gives a possible insight to the spectrum of activity of these compounds. The CfiA/SB236050 structure demonstrates that the compound has key interactions with Lys184 and Asn194. However, in the L-1 enzyme at the position equivalent to Lys184, there is a serine and this serine does not superimpose with the lysine in CfiA. Furthermore, even though the Asn193 is conserved in the L-1 enzyme, it is in a very different position. These differences, along with other known variabilities between the active site of the L-1 and the other metallo-β-lactamases, are likely to significantly contribute to the inactivity of these compounds against L-1. It is also noted that these compounds are less active against the IMP-1 than against CfiA. A possible explanation as to the tighter binding of SB236049 and SB236050 to CfiA than to IMP-1 is suggested by the structural data. The superposition of the structures of IMP-1 + mercaptocarboxylate (1DD6 [4]) and the CfiA + tricyclic suggests that the tighter binding to CfiA by the inhibitors SB236049 and SB236050 may be related to how well the inhibitor fills the available space in the active site and interacts with protein atoms via van der Waals interactions. The loop preceding Lys184 in CfiA is longer than that in IMP-1, which provides a boundary and creates a shallow depression in which the inhibitor binds. In IMP-1, however, the loop is shorter and thus leaves a large portion of the inhibitor, including the methoxy group, exposed to solvent. This lack of potential interactions with the IMP-1 enzyme may be responsible for the lower inhibitory potency observed against this enzyme.
The compounds had similar IC50s for B. cereus II at high and low levels of Zn2+, suggesting that their mode of action was not via nonspecific chelation of the active site Zn2+. The compounds were also shown to be competitive inhibitors of metallo-β-lactamases, and their lack of activity against another metalloprotease (ACE) and a serine active site β-lactamase further suggested the specific nature of these compounds.
The tricyclic compounds have also shown good synergy with meropenem against clinical isolates of B. fragilis producing the CfiA metallo-β-lactamase. Eight micrograms of SB236050 and SB238569 per ml both reduced the MIC of strains 262 and 460 to sensitive (≤ 4 μg of meropenem per ml). Synergy was also observed with the B. fragilis 288, although the meropenem MIC achieved in combination with the inhibitors was much higher than for the 460 and 262 strains, due to the 288 strain producing a higher level of β-lactamase. Although the compounds were good inhibitors of the IMP-1 enzyme, neither 8 nor 32 μg of inhibitor per ml caused a drop in the meropenem MIC for P. aeruginosa 101, which is likely to be a result of poor penetration of the compound through the pseudomonal outer membrane. No drop in the meropenem MIC for S. maltophilia was observed with the addition of inhibitor at either 8 or 32 μg/ml. This was to be expected, as the compounds had no detectable activity against the L-1 metallo-β-lactamase produced by this strain.
The only other series of metallo-β-lactamase inhibitors reported to possess antibacterial synergy activity were the biphenyl tetrazoles (22) and the mercaptocarboxylates (17). A 2 mM concentration of the biphenyl tetrazoles was required to demonstrate antibacterial synergy with imipenem. Although the early examples of the mercaptocarboxylates exhibited synergy at 8 μg/ml against B. fragilis (CfiA), the level of potentiation of meropenem was less than that observed with these tricyclic compounds. Therefore, of all the metallo-β-lactamase inhibitors so far reported, the tricyclic compounds have exhibited the most promising antibacterial synergy activity with β-lactams against clinical isolates producing metallo-β-lactamases.
At this stage, the synergy activity demonstrated by this series of compounds is limited to B. fragilis and a metallo-β-lactamase inhibitor needs to be identified that exhibits a broader spectrum of antibacterial activity when combined with a carbapenem. To achieve this, it is probable that both the inhibitory potency and the penetration of the tricyclic compounds need to be improved. However, these compounds clearly demonstrate the feasibility of using a metallo-β-lactamase inhibitor-carbapenem combination to tackle this emerging resistance mechanism.
Acknowledgments
We are grateful to David Livermore for providing P. aeruginosa 101.
REFERENCES
- 1.Arakawa, Y., M. Murakami, K. Suzuki, H. Ito, R. Wacharotayankun, S. Ohsuka, N. Kato, and M. Ohta. 1995. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob. Agents Chemother. 39:1612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brünger, A. T. 1992. X-plor version 3.1: a system for X ray crystallography and NMR. Yale University Press, New Haven, Conn.
- 3.Concha, N. O., B. A. Rasmussen, K. Bush, and O. Hertzberg. 1996. Crystal structure of a wide spectrum binuclear zinc β-lactamase form Bacteroides fragilis. Structure 4:823-836. [DOI] [PubMed] [Google Scholar]
- 4.Concha, N. O., C. A. Janson, B. C. Gasson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J.-M. Frere, D. J. Payne, J. H. Bateson, and S. S. Abdel-Meguid. 2000. The crystal structure of the IMP-1 metallo β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39:4288-4298. [DOI] [PubMed] [Google Scholar]
- 5.Cornaglia, G., M. I. Riccio, A. Mazzariol, L. Lauretti, R. Fontana, and G. M. Rossolini. 1999. Appearance of IMP-1 metallo-β-lactamase in Europe. Lancet 353:899-900. [DOI] [PubMed] [Google Scholar]
- 6.Felici, A., G. Amicosante, A. Oratore, R. Strom, P. Ledent, B. Joris, L. Fanuel, and J.-M. Frère. 1993. An overview of the kinetic parameters of Class B β-lactamases. Biochem. J. 291:151-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holquist, B., P. Bunning, and J. F. Riordan. 1979. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal. Biochem. 95:540-548. [DOI] [PubMed] [Google Scholar]
- 8.Howard, A. J., G. L. Gilliland, B. C. Finzel, T. Poulos, D. O. Ohlendorf, and F. R. Salemme. 1987. The use of an imaging proportional counter in macromolecular crystallography. The use of an imaging proportional counter in macromolecular crystallography. J. Appl. Crystallogr. 20:383-387. [Google Scholar]
- 9.Khushi, T., D. J. Payne, A. Fosberry, and C. Reading. 1995. Production of metal dependent β-lactamases by clinical strains of B. fragilis isolated before 1987. J. Antimicrob. Chemother. 37:345-350. [DOI] [PubMed] [Google Scholar]
- 10.Kleywegt, G. J. 1995. CCP4 newsletter on protein crystallography. Dictionaries Heteros 31:45-50. [Google Scholar]
- 11.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. Pauw, G. Amicosante, J. M. Frere, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leatherbarrow, R. J. 1992. Grafit 3.0. Erithacus Software Ltd., Staines, United Kingdom.
- 14.Navaza, J. 1994. AMoRe: an automated package for molecular replacement. Acta Crystallogr. 50:157-163. [Google Scholar]
- 15.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A5, vol. 20, no. 2. Approved standard, 5th ed. NCCLS, Wayne, Pa.
- 16.NCCLS. 1997. M11-A4, vol. 17, no. 22. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard, 4th ed. NCCLS, Wayne, Pa.
- 17.Payne, D. J., W. Du, and J. H. Bateson. 2000. β-Lactamase epidemiology and the utility of established and novel β-lactamase inhibitors. Expert Opin. Investig. Drugs 9:247-261. [DOI] [PubMed] [Google Scholar]
- 18.Payne, D. J., J. H. Bateson, B. C. Gasson, D. Proctor, T. Khushi, T. H. Farmer, D. Tolson, D. Bell, P. W. Skett, A. C. Marshall, R. Reid, L. Ghosez, Y. Combret, and J. Marchand-Brynaert. 1996. Inhibition of metallo-β-lactamases by a series of mercaptoacetic acid thiolester derivatives. Antimicrob. Agents Chemother. 41:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne, D. J., R. Cramp, J. H. Bateson, J. Neale, and D. Knowles. 1994. Rapid identification of serine and metallo-β-lactamases. Antimicrob. Agents Chemother. 38:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne, D. J., R. Cramp, D. Winstanley, and D. Knowles. 1994. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important β-lactamases. Antimicrob. Agents Chemother. 38:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senda, K., Y. Arakawa, K. Ichiyama, S. Nakashima, S. Ito, K. Ohsuka, N. Shimokata, M. Kato, and M. Ohta. 1996. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J. Clin. Microbiol. 34:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toney, J. H., P. M. D Fitzgerald, N. Grover-Sharma, S. H. Olson, W. J. May, J. G. Sundelof, D. E. Vanderwall, K. A. Cleary, S. K. Grant, J. K. Wu, J. W. Kozarich, D. L. Pompliano, and G. G. Hammond. 1998. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of Bacteroides fragilis metallo-β-lactamase. Chem. Biol. 5:185-196. [DOI] [PubMed] [Google Scholar]
- 23.Walter, M. W., A. Felici, M. Galleni, R. P. Soto, R. M. Adlington, J. E. Baldwin, J.-M. Frere, M. Gololobov, and C. J. Schofield. 1996. Trifluoromethyl alcohol and ketone inhibitors of metallo-β-lactamases. Bioorg. Med. Chem. Lett. 6:2455-2458. [Google Scholar]
- 24.Woodford, N., M.-F. I. Palepou, G. S. Babini, J. Bates, and D. M. Livermore. 1998. Carbapenemase-producing Pseudomonas aeruginosa in UK. Lancet 352:546-547. [DOI] [PubMed] [Google Scholar]
- 25.Yang, Y., B. A. Rasmussen, and K. Bush. 1992. Biochemical characterization of the metallo-β-lactamase CcrA from Bacteroides fragilis TAL3636. Antimicrob. Agents Chemother. 36:1155-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]