Abstract
Strains of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 (DT104) and S. enterica serovar Agona (Agona) have been found to harbor Salmonella genomic island 1 (SGI1), a 43-kb genomic region that contains many of the drug resistance genes. Such strains are resistant to ampicillin (pse-1), chloramphenicol/florfenicol (floR), streptomycin/spectinomycin (aadA2), sulfonamides (sul1), and tetracycline [tet(G)] (commonly called the ACSSuT phenotype). All five resistance genes are found in a 13-kb multidrug resistance (MDR) region consisting of an unusual class I integron structure related to In4. We examined DT104 and Agona strains that exhibited other resistance phenotypes to determine if the resistance genes were associated with variant SGI1 MDR regions. All strains were found to harbor variant SGI1-like elements by using a combination of Southern hybridization, PCR mapping, and sequencing. Variant SGI1-like elements were found with MDR regions consisting of (i) an integron consisting of the SGI1 MDR region with the addition of a region containing a putative transposase gene (orf513) and dfrA10 located between duplicated qacEΔ1/sulI genes (SGI1-A; ACSSuTTm); (ii) an integron with either an aadA2 (SSu) or a pse-1 (ASu) cassette (SGI1-C and SGI1-B, respectively); (iii) an integron consisting of the SGI1-C MDR region plus an orf513/dfrA10 region as in SGI1-A (SGI1-D; ASSuTm; ampicillin resistance due to a TEM β-lactamase); and (iv) an integron related to that in SGI1 but which contains a 10-kb inversion between two copies of IS6100, one which is inserted in floR (SGI1-E; ASSuT). We hypothesize that the MDR of SGI1 is subject to recombinational events that lead to the various resistance phenotypes in the Salmonella strains in which it is found.
In the 1990s, the prevalence of multidrug-resistant (MDR) Salmonella enterica serovar Typhimurium phage type DT104 (hereafter called DT104) has increased dramatically in the United Kingdom (45, 46), the United States (18, 22), and Canada (29). Many countries have also documented outbreaks associated with MDR DT104 in poultry, beef, cheese, and swine (11, 13, 19, 26, 48). Although case-control studies have suggested that MDR DT104 is more virulent than sensitive strains of DT104 or other Salmonella serotypes (15, 49), another report suggested that the percentage of bacteremia was no higher than with other Salmonella serotypes (42).
Nonetheless, encouraging findings have come from the United Kingdom which have documented a 22% decrease in 1999 from 1996 levels of DT104 displaying the multidrug resistance phenotype (43). MDR DT104 strains have been described as resistant to a core group of antimicrobial agents, including resistance to ampicillin, chloramphenicol/florfenicol, spectinomycin/streptomycin, sulfonamides, and tetracycline (commonly abbreviated ACSSuT). In addition to ACSSuT strains, others have been identified which are resistant to fluoroquinolones, trimethoprim, and aminoglycosides (12, 17, 29, 44). The majority of ACSSuT DT104 isolates have a similar MDR region in their genomes comprised of the floR and tet(G) genes bracketed by the pse-1 and aadA2 gene cassettes of two class 1 integrons clustered in a 13-kb region (2, 6, 28, 36, 38).
A number of Salmonella enterica serovar Agona (hereafter referred to as Agona) strains harboring the same antimicrobial resistance region have been characterized, suggesting horizontal gene transfer of this region (10). The element containing the MDR region, Salmonella genomic island 1 (SGI1), has been cloned from the genome of a Canadian isolate and comprises a 43-kb region between thdF and a novel retron sequence (5). Recently, the entire SGI1 element has been sequenced and, in addition to the MDR region, contains at least 25 or more open reading frames (ORFs), including an integrase gene and an excisionase gene, some of which showed similarity to genes commonly found on conjugative plasmids (4).
Among the members of the family Enterobacteriaceae and pseudomonads, transfer of antibiotic resistance genes is due largely to broad-host-range plasmids that carry transposons (14). Many of the resistance genes in the transposons have been found to be mobile gene cassettes carried as part of integrons (16). Four classes of integrons have been described based on the similarities between their integrases, but the majority described belong to class 1, and this type has been found in multiple Enterobacteriaceae, including Escherichia coli, Citrobacter spp., Klebsiella spp., Enterobacter spp., Proteus spp., and Salmonella spp. (14, 20, 27, 40, 50).
Class 1 integrons contain a 5′ conserved segment (5′-CS) which consists of the intI1 gene, encoding the site-specific integrase, and the associated attI1 site, the primary site of recombination, and the 3′ conserved segment (3′-CS) of variable length but generally consisting of qacEΔ1, encoding low-level resistance to some antiseptics; the sul1 gene, encoding sulfonamide resistance; and orf5, a gene of unknown function (16). The gene cassettes, of which over 60 have been described (16, 35), consist of the coding region and the downstream 59-base element (59-be), which is responsible for recognition and mobilization of cassettes. The IntI1-catalyzed recombination between the attI1 and 59-be sites is the main reaction responsible for inserting gene cassettes into the integron (33).
Transposon Tn402 is a mobile class 1 integron that contains the 5′-CS and a transposition module consisting of four genes (tniA, -B, -Q, and -R) required for transposition (7). In addition, Tn402 is bound by inverted repeats of 25 bp, IRi at the integrase end and IRt at the tni end. Several class 1 integrons appear to have originated from a Tn402-like ancestor by incorporation of the common part of the 3′-CS, including qacEΔ1, sul1, and orf5. Most of these integrons, though still bound by IRi and IRt, have lost part or all of the tni module and are deemed defective transposon derivatives (7).
Some of these integrons have been analyzed and shown to have an identical 5′-CS and 2-kb of the 3′-CS, after which this region diverges (21). One group, the In5 type, have lost parts of the 3′-CS and tni module, likely due to IS1326-mediated and/or IS1353-mediated deletions (7, 32). Another group, the In4 type, have a 3′-CS that includes a copy of IS6100 but no transposition genes (34). In In4 itself, the IS6100 element is flanked by short segments from the IRt end of Tn402, the outer one being 152 bp and the inner one 123 bp, in inverse orientation to one another. In other members of this group, only one or neither of the IRt ends may be present, and the IS6100 element does not contain the partial copy (34). Thus, the SGI1 MDR region has a structure similar to that of an In4 integron (4, 5).
Since a number of DT104 strains have been identified which exhibit different resistance phenotypes, e.g., SSu, ASu, and ASSuT (3, 12, 36), we have examined a number of Typhimurium and Agona strains for the presence of variant SGI1s. Furthermore, the MDR regions were characterized to determine their genetic structure, and a nomenclature system is suggested for SGI1 variants.
MATERIALS AND METHODS
Bacteria and media.
The Salmonella strains used in this study are listed in Table 1. All strains were grown at 37°C in brain heart infusion broth or Luria-Bertani (LB) medium. Stock cultures were stored at −70°C in Microbank vials (Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada).
TABLE 1.
Strains used in this study and Southern hybridization data with various probes
| Strain | Serovar | Resistance phenotypea | Genomic island name | Size(s) (kb) of XbaI fragment(s) hybridizingb with:
|
Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aadA2 | floR | tet(G) | pse-1 | qac/sul1 | tnpA/S044 | p1-9 | |||||
| 96-5227 | DT104 | ACSSuT | SGI1 | 11.7 | 11.7 | 11.7 | 11.7, 4.3 | 11.7, 4.3 | 4.3 | 9.0, 4.1 | 29 |
| 959SA97 | Agona | ACSSuT | SGI1 | 11.7 | 11.7 | 11.7 | 11.7, 4.3 | 11.7, 4.3 | 4.3 | 9.0, 4.1 | 10 |
| 1169SA97 | Agona | ACSSuTTm | SGI1-A | 11.7 | 11.7 | 11.7 | 11.7, 8.8 | 11.7, 8.8 | 8.8 | 9.0, 4.1 | 4 |
| S/960725 | DT104 | ASu | SGI1-B | — | — | — | 2.5, 4.3 | 4.3 | 4.3 | 9.0, 4.1 | 4 |
| 0047SA97 | Agona | SSu | SGI1-C | 6.6 | — | — | — | 6.6 | 6.6 | 9.0, 4.1 | This study |
| S/954435 | DT104 | SSu | SGI1-C | 6.6 | — | — | — | 6.6 | 6.6 | 9.0, 4.1 | 4 |
| 953SA98 | Agona | ASSuTmc | SGI1-D | ND | ND | ND | ND | ND | ND | ND | This study |
| S/960081 | DT104 | ASSuT | SGI1-E | 9.1 | 9.1, 7.9 | 9.1 | 9.1, 7.9 | 9.1 | 9.1, 7.9 | 9.0, 4.1 | 4 |
A, ampicillin; C, chloramphenicol (and flofenicol); S, spectinomycin and streptomycin; Su, sulfonamides; T, tetracycline; Tm, trimethoprim.
—, no hybridization signal observed; ND, not done.
Ampicillin resistance is due to the presence of a TEM β-lactamase.
Antimicrobial susceptibility testing.
Isolates were tested for susceptibility to various antimicrobial agents on Mueller-Hinton agar by the disk diffusion method as previously described (28). Disks containing: ampicillin at 10 μg, chloramphenicol at 30 μg, florfenicol at 30 μg, spectinomycin at 100 μg, streptomycin at 10 IU, sulfonamides at 200 μg, and tetracyclines at 30 IU were purchased from Sanofi Diagnostics Pasteur (Marnes-la-Coquette, France), except for disks with florfenicol, which were from Schering-Plough Santé Animale (Segré, France).
DNA methodology.
Genomic DNA was isolated as previously described (5). Primers used in the study are listed in Table 2. Standard PCRs and long PCR were carried out as previously described (4). Southern blotting was carried out by standard methods (37) with probes labeled and detected by ECL kits using the manufacturer's instructions (Amersham Pharmacia Biotech). Probes were made by PCR using the primer pairs listed in Table 2.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ to 3′) | Reference(s) | Description |
|---|---|---|---|
| U7-L12 | ACACCTTGAGCAGGGCAAG | 4, 5 | Left-junction PCR |
| LJ-R1 | AGTTCTAAAGGTTCGTAGTCG | 4, 5 | Left-junction PCR |
| 104-RJ | TGACGAGCTGAAGCGAATTG | 4, 5 | Right-junction PCR |
| C9-L2 | AGCAAGTGTGCGTAATTTGG | 4, 5 | Right-junction PCR |
| 104-D | ACCAGGGCAAAACTACACAG | 4, 5 | Right-junction PCR |
| aadA2-L | TGTTGGTTACTGTGGCCG | 29 | aadA2 probe |
| aadA2-R2 | TGCTTAGCTTCAAGTAAGACG | This study | aadA2 probe |
| StCM-L | CACGTTGAGCCTCTATATGG | This study | floR probe |
| St-CM-R | ATGCAGAAGTAGAACGCGAC | This study | floR probe |
| tetG-L | CAGCTTTCGGATTCTTACGG | 29 | tet(G) probe |
| tetG-R | GATTGGTGAGGCTCGTTAGC | 29 | tet(G) probe |
| pse-L | AATGGCAATCAGCGCTTCCC | 29 | pse-1 probe |
| pse-R2 | ACAATCGCATCATTTCGCTC | This study | pse-1 probe |
| QS-1 | ATGAAAGGCTGGCTTTTTCTTG | 4 | qac/sul1 probe |
| QS-2 | TGAGTGCATAACCACCAGCC | 4 | qac/sul1 probe |
| DB-T1 | TGCCACGCTCAATACCGAC | This study | IS6100/S044 probe |
| MDR-B | GAATCCGACAGCCAACGTTCC | This study | IS6100/S044 probe |
| MDR-7 | AACCGTGCATCTATCGAGC | This study | |
| F6 | TTGGAAACAGACGGCATGG | 10 |
Computer-aided analysis and annotation.
Homology searches were carried out using the Blast suite of programs (1), and open reading frames (ORFs) were detected with ORFinder via the World Wide Web interface of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih).
Nucleotide sequence accession number.
The complete nucleotide sequence of the region harboring the trimethoprim resistance determinant has been deposited in the GenBank database under accession number AY049746.
RESULTS
DT104 and Agona strains isolated in Scotland and Belgium displaying the ACSSuT, ACSSuTTm, ASu, SSu, ASSuTm, and ASSuT phenotypes were characterized at the genetic level in this study. The strains were shown to possess the SGI1 or a variation thereof by using a combination of Southern blotting and PCR (Table 1). All strains studied displayed a PCR product using primers U7-L12 and LJ-R1 (left junction) and 104-RJ and C9-L2 or 104-RJ and 104-D primers (right junction), indicating the presence of the left and right SGI1 junction regions (Fig. 1 ). This result demonstrated that the SGI1 was located between thdF and a novel cryptic retronphage in all of the DT104 variants studied and located between thdF and yidY in the Agona strains, in which the retronphage is absent (5). In addition, all strains displayed an 8.9-kb and a 4.1-kb fragment when Southern blots of XbaI-digested genomic DNA were probed with p1-9, a probe spanning ORFs S023 to S024, suggesting the majority of the SGI1 is present (Fig. 1; Table 1) (4).
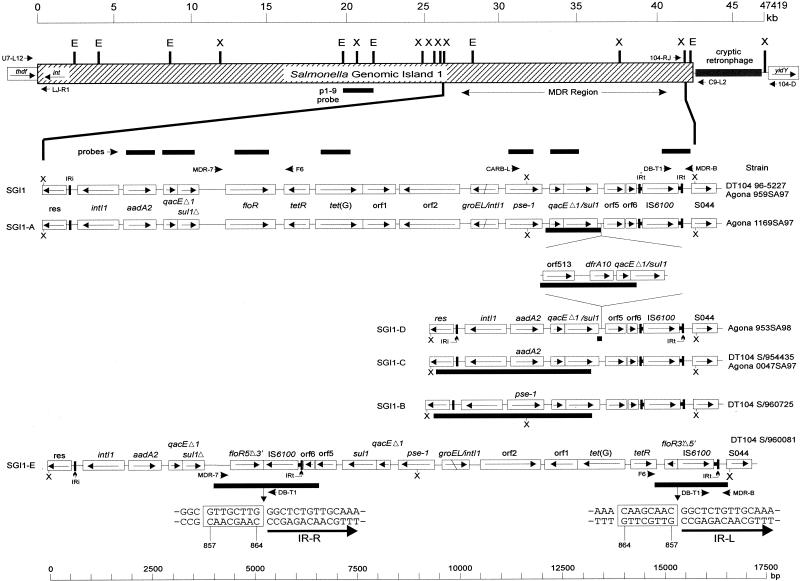
FIG. 1.
Genetic organization of the MDR regions of the various strains in this study based on Southern hybridization data, PCR mapping, and sequence analysis. A schematic of SGI1 is shown at the top, with the approximate locations of the primers used to detect the left and right junction regions indicated. The cryptic retronphage region is absent in Agona strains. Direction of transcription of genes is indicated by arrows. Locations of PCR products used as probes are indicated (thin black bars), as are the locations of some primers used in mapping. IRi and and IRt are 25-bp imperfect inverted repeats defining the ends of class 1 integrons. Regions sequenced are indicated under the various MDR regions by a thick black bar. The nucleotide sequence of the insertion point of the IS6100 element in the floR gene of S/960081 is shown; the 8-bp region duplicated upon insertion is boxed (coordinates are for the floR gene only). IR-L and IR-R refer to the inverted repeats that define one end of IS6100. The sequence of the orf513/dfrA10 region from Agona 1169SA97 has been assigned accession no. AY049746.
Thus, Agona 959SA97, previously shown to harbor the same MDR region as in ACSSuT DT104 (4), contains SGI1 (Fig. 1) (10). In the other strains studied, differences in SGI1 structure were revealed with Southern hybridization analysis of XbaI-digested genomic DNA using probes directed against genes found in the MDR region (Table 1). These variable MDR regions will be discussed in detail below.
MDR region coding for ACSSuTTm.
Agona 1169SA97 probed with aadA2, floR, or tet(G) showed the same sizes of hybridizing bands as for Agona 959SA97; however, when probed with pse-1, qac/sul1, or IS6100/S044, an 8.8-kb fragment hybridized with the probe, which was approximately 4.5 kb larger than the expected 4.3-kb XbaI fragment (Table 1). PCR of this region with primers pse-L and MDR-B produced a major band of 8.9 kb and a minor band of 4.4 kb (data not shown). Sequence analysis of the purified 8.9-kb product revealed a 2,868-bp region inserted between copies of the qacEΔ1 and sul1 genes that contains two other genes, dfrA10, encoding resistance to trimethoprim, and orf513, a putative transposase (Fig. 1). This structure is identical to one found in In7 (31).
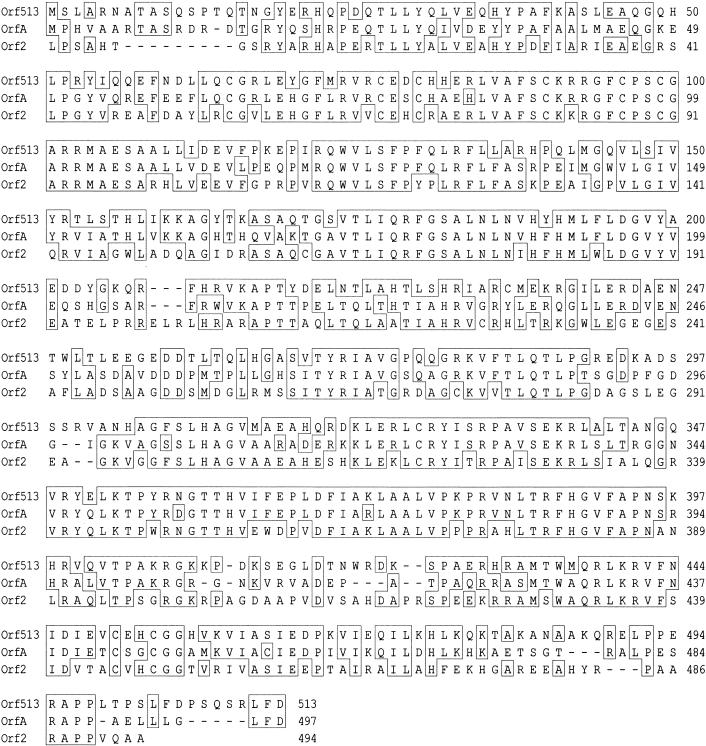
A 2,154-bp segment of this region, encompassing orf513, is also found upstream of the cat gene in In6 (31, 42), and the ampC and ampR genes in the integron in pSAL (47). The Orf513 protein is 65% identical to the OrfA protein, whose gene and a truncated version of it surround a floR gene from a plasmid found in E. coli isolated from cattle (9), and 55% identical to the Orf2 protein found in the SGI1 MDR region (4). An alignment of these proteins is shown in Fig. 2. All these proteins have been annotated as putative transposases or transposase-like, presumably due to homology to known transposases. Interestingly, all members of this group of proteins are found associated with resistance genes.
FIG. 2.
Alignment of Orf513, OrfA, and Orf2 proteins. Identical residues in two or three of the proteins are boxed. Coordinates are at the right end of each line.
The size of a PCR product using the pse-L/MDR-B primer set is 4.4 kb with DNA from DT104 96-5227, the same size as a minor band amplified when using 1169SA97 DNA. Restriction enzyme analysis of these two amplicons revealed identical patterns (data not shown). Thus, it appears that a small subpopulation of Agona 1169SA97 cells may exist in which the orf513/dfrA10 region is deleted. We propose to name the genomic island found in Agona 1169SA97 SGI1-A.
MDR regions coding for ASu, SSu, and ASSuTm.
DT104 strain S/960725, which was resistant to ampicillin and sulfonamides (ASu), was shown to contain SGI1 sequences upstream of the MDR region by PCR and Southern hybridization (Table 1). Southern blots of XbaI-digested S/960725 chromosomal DNA probed with either aadA2, floR, or tet(G) did not produce a hybridization signal, suggesting that these genes were absent in this strain. The hybridization signals generated when qac/sul1 or pse-1 (contains a XbaI site) were used to probe the blot suggested a single integron was present in this MDR region (Table 1). Indeed, PCR and sequence analysis of this region in DT104 S/960725 revealed that a single complete pse-1-containing integron was present between the res and IS6100 sequences (Fig. 1). We propose to name the genomic island found in DT104 S/960725 SGI1-B.
Two strains studied, Agona 0047SA97 and DT104 S/954435, displayed resistance to only two antimicrobials tested, streptomycin and sulfonamides (SSu). Southern blots of XbaI-digested genomic DNA did not reveal any sequences homologous to floR, tet(G), or pse-1, suggesting these sequences were absent in the strains (Table 1). Further probing of DT104 S/954435 and Agona 0047SA97 with aadA2, qac/sul1, or IS6100/S044 showed hybridization products of 6.6 kb, suggesting only one copy of qac/sul1 was present and it was localized to the same XbaI fragment that contains the aadA2 gene (Table 1). These results suggested a single integron may be present in the MDR region of these strains, and PCR and sequence analysis of this region in DT104 S/954435 confirmed that a single complete aadA2-containing integron was present between res and IS6100 (Fig. 1). We propose to name the genomic island found in DT104 S/954435 and Agona 0047SA97 SGI1-C.
One strain, Agona 953SA98, was resistant to ampicillin, streptomycin, sulfonamides, and trimethoprim (ASSuTm). PCR analysis showed sequences from an aadA2-containing integron were present, but reactions with primers from pse-1 were negative. However, PCR analysis with TEM-specific primers were positive, revealing that ampicillin resistance in this strain is due to a TEM-1-related β-lactamase (data not shown). Further PCR and sequence analysis showed that the trimethoprim resistance in Agona 953SA98 was due the presence of the same orf513/dfrA10 region as found in Agona 1169SA97 (Fig. 1). We propose to name the genomic island found in Agona 953SA98 SGI1-D.
MDR region coding for ASSuT.
Strain DT104 S/960081 displayed a resistance phenotype of ASSuT, and initial PCR analysis of the MDR region suggested all of the genes were present and in the same order as in ACSSuT strains, including the floR gene. However, PCR using the StCM-L/StCM-R primer pair did not produce a product, suggesting that either a mutated primer binding site was present in floR or a deletion existed within floR that encompassed a primer-binding site. Southern hybridization results, however, suggested that either IS6100 or S044 was duplicated, as this probe hybridized with 7.9-kb and 9.1-kb XbaI fragments, as opposed to a single 4.3-kb fragment as in the ACSSuT strains (Table 1). The floR probe hybridized to the same two fragments, while the qac/sul1 probe hybridized only to the 9.1-kb fragment, suggesting a possible inversion had taken place within the MDR region which may have involved a portion of the floR gene and the IS6100-S044 region.
To test this hypothesis, PCRs using primer pair MDR-7/DB-T1 and primer pair F6/MDR-B were attempted, as both reactions were postulated to produce amplicons <2.0 kb in size if the inversion described above had occurred (Fig. 1). The MDR-7/DB-T1 reaction produced a 1.6-kb fragment, and the F6/MDR-B reaction produced a 1.9-kb fragment, both of which were sequenced. Analysis revealed that a second IS6100 element had interrupted floR, and an inversion of the region between the two IS6100 elements had taken place (Fig. 1 and Fig. 3). We propose to name the MDR region found in DT104 S/960081 SGI1-E.
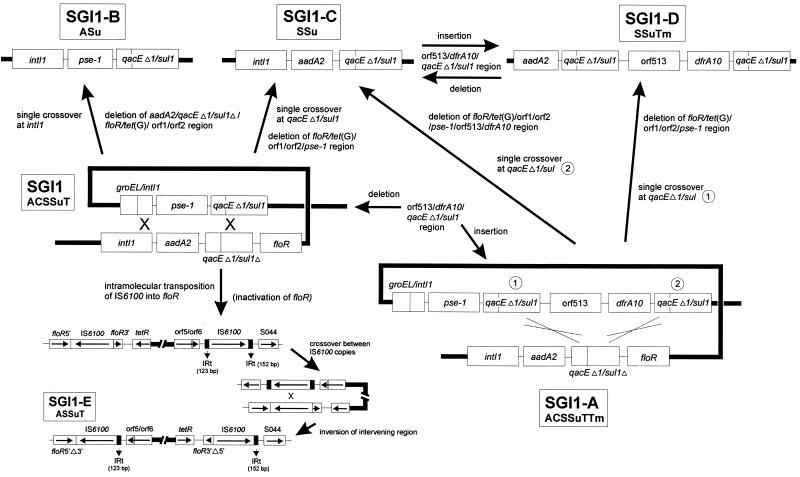
FIG. 3.
Schematic diagram of the generation of variant SGI1 MDR regions from SGI1. Mechanisms are discussed in the text.
DISCUSSION
Variant MDRs are In4-type integrons.
Original analysis of the DT104 SGI1 MDR region revealed an IS6100 element in a position identical to that in In4 except for the absence of the partial IS6100 copy (4, 5) (Fig. 1). Further analysis revealed the presence of IRi (positions 26637 to 26661 of the sequence with accession no. AF261825) between the res and intI1 genes of the aadA2 integron (Fig. 1). Thus, the entire MDR region of SGI1 (positions 26637 to 41698 of the sequence with accession no. AF261825) can be considered a single integron belonging to the In4 group (34). Additionally, this region is flanked by 5-bp direct repeats, ACTTG, strongly suggesting that it has been integrated by a transposition event. Hence, the MDR regions of the variant SGI1s described here can be described as In4-type integrons (34).
Generation of variant MDRs.
SGI1 (ACSSuT) has been found in DT104, DT120, and Agona serovars of S. enterica (4, 5, 10). In addition, we have detected SGI1 in a strain of S. enterica serovar Paratyphi B isolated from a tropical fish (25). In every case, SGI1 is located at the end of the thdF gene, suggesting site-specific insertion possibly involving the product of the int gene found at one end of the genomic island (Fig. 1) (4, 5). Thus, it seems likely that whatever the donor strain, SGI1 exists on a transferable element and is acquired by other serovars via horizontal transfer. As the cryptic retronphage downstream of SGI1 has been found only in Typhimurium strains, it seems unlikely that transfer is mediated by phage transduction, though the resistance genes have been shown to be transduced (39).
However SGI1 is acquired, the following questions arise: what is the origin of the SGI1 MDR region and how are the variants generated? It seems reasonable to assume that the SGI1 MDR region evolved from insertions into an MDR region consisting of a single class 1 integron, i.e., SGI1-B (ASu) or SGI1-C (SSu). One scenario is that either SGI1-B or SGI1-C acquired another integron that included the floR/tet(G)/orf1/orf2/groEL region (flo/tet) as part of its 3′-CS, or acquired a second integron first, with subsequent integration of the flo/tet region between the two integrons. In either scenario, truncation of the sul1 and intI1 genes, as found in SGI1, would most likely have occurred during integration of the flo/tet region into the integron. The origin of the flo/tet region itself is unknown, and this combination of genes exists only in SGI1, though segments similar to parts of this region are found in various plasmids.
Homologs of floR (>95% identity) and the upstream sequence including the 99-bp direct repeat in SGI1 that brackets the floR gene (4, 6) have been found in plasmids from Klebsiella pneumoniae, E. coli, and Photobacterium damselae (8, 23). In the P. damselae and E. coli plasmids, the floR genes are associated with a putative transposase gene (orfA) whose product has homology to the product of orf513 (Fig. 2) and to a lesser extent to the product of orf2 of SGI1 (see above and Fig. 2). In the K. pneumoniae plasmid R55, the floR gene is followed by a gene whose product has 67% identity to the LysR-like Orf1 protein from the MDR region of SGI1 and a gene whose product has a C-terminal end identical to that of OrfA (8). Thus, all floR variants characterized to date are associated with putative transposase genes. As well, a 361-bp region highly similar to the region upstream of floR including the direct repeat sequence is found just upstream of a tetR/tet(G) region from Pseudomonas plasmid pPSTG1 (41). Thus, it can be speculated that the direct repeat sequence may play a role in the acquisition and/or mobility of genes in elements where it is found.
However the SGI1 MDR region originated, various mechanisms could account for the generation of the variant SGI-like elements. It may be that each type of MDR region has been assembled on another element (i.e., a resident plasmid) and then integrated into the genomic island in an insertional hotspot between the res gene and S044 in a strain already carrying the SGI1 progenitor in its genome. A similar explanation is that each type of SGI1-like element could have been assembled outside of the genome and then acquired by site-specific integration into the end of the thdF gene in a previously antibiotic-sensitive strain. However, the generation of the variant MDR regions from an SGI MDR region can most easily be postulated to have occurred by recombination between homologous regions (Fig. 3).
The structure of SGI1-A can be explained by the insertion of the orf513/dfrA10 region via homologous recombination between the qacEΔ1 and sul1 regions, as has been suggested for In7 (42). This hypothesis is partially supported by the findings that deletion of the orf513/dfrA10 region from SGI1-A can be detected by PCR, indicating this region may not be stable and lost without selective pressure. The simplest MDR regions are those containing single intact class 1 integrons, as was found in SGI1-B and SGI1-C (Fig. 1). It is important to note that these integrons are different from the two which exist in the SGI1 MDR region in that intact intI1 and sul1 genes are found in SGI1-B and SGI-C (Fig. 1) (4, 5). The generation of both single intact integrons in SGI1-B and SGI1-C from SGI can be explained by a single crossover between the copies of the intI1 sequences or qacEΔ1/sul1 sequences, respectively, with concomitant loss of the intervening DNA (Fig. 3). Similarly, SGI1-C and SGI1-D could be generated from SGI1-A by crossover between the different qacEΔ1/sul1 copies (Fig. 3).
A single insertional event of the orf513/dfrA10 element into SGI1-C would give rise to SGI1-D (Fig. 3). The origin of the orf513/dfrA10 region is unknown, though the putative transposase Orf513 may be involved in its mobility and in the acquisition of different associated resistance genes (i.e., as in In6, In7, and pSAL). The SGI1-E variant likely arose from SGI1 after intramolecular transposition of IS6100 occurred (Fig. 3). The second copy inserted into floR in the opposite orientation to that of the other IS6100 element, generating small direct repeats of the host target sequence (shown in Fig. 1), as has been observed with the insertion of many insertion sequence elements (24). Subsequently, a single crossover event between the two IS6100 elements led to the inversion of the intervening region and the observed SGI1-E MDR region. Support for this hypothesis is provided for by the fact that each copy of IS6100 in the SGI1-E MDR region is associated with only one of the IRt sequences that are found surrounding the single IS6100 element in SGI1, the 123-bp region is found adjacent to the 5′ floR region, and the 152-bp region is found adjacent to the 3′ floR region (Fig. 1 and 3). A reverse scenario to produce the MDR region of SGI1, though possible, is unlikely to have occurred, as regeneration of an intact floR would necessitate deletion of one of the short direct repeats, and a copy of IS6100 would also have to have been deleted.
Conclusions.
This report describes the characterization of the MDR regions from a number of different DT104 and Agona strains which display antimicrobial resistance patterns other than the classical ACSSuT phenotype often described in strains involved in outbreaks. It will be interesting to see if strains containing other variant SGI1 elements coding for other resistance phenotypes can be isolated, e.g., ASSu, ASuTm, ACSu, CSSu, ACSuTm, and CSSuTm. Also, do MDR regions exist without at least one integron, i.e., CT, CTTm, C, T, CTm, or TTm? Knowing the relationship of the drug resistance phenotype to the type of SGI1 present may have important epidemiological implications if variants are isolated during the course of an outbreak situation involving an MDR Salmonella serovar containing an SGI1-like element. Investigators should also be aware that strains may exist with identical resistance phenotypes where a resistance may be due to different genes, such as ampicillin resistance due to the presence of a pse-1-containing MDR region in one strain but due to the presence of a TEM β-lactamase in the other strain.
In this report, we have initiated a nomenclature system to identify variants of the SGI1 regions in Salmonella strains. We encourage investigators to register any additional variants with us in order to maintain an ordered nomenclature system for SGI1 variants. Please contact the corresponding author with relevant information regarding novel SGI1 variants.
Acknowledgments
We thank S. Rankin (Scotland) and H. Imberechts (Belgium) for providing isolates and Tim Du and Romeo Hizon for excellent technical assistance.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcangioli, M. A., S. Leroy-Setrin, J. L. Martel, and E. Chaslus-Dancla. 1999. A new chloramphenicol and florfenicol resistance gene flanked by two integron structures in Salmonella typhimurium DT104. FEMS Microbiol. Lett. 174:327-332. [DOI] [PubMed] [Google Scholar]
- 3.Baggesen, D. L., D. Sandvang, and F. M. Aarestrup. 2000. Characterization of Salmonella enterica serovar Typhimurium DT104 isolates from Denmark and comparison with isolates from Europe and the United States. J. Clin. Microbiol. 38:1581-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, D. A., G. A. Peters, A. Cloeckaert, K. S. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. R. Mulvey. 2001. Complete nucleotide sequence of a 43-kb genomic island associated with the multidrug resistance region of Salmonella enterica Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 83:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd, D. A., G. A. Peters, L.-K. Ng., and M. R. Mulvey. 2000. Partial characterization of a genomic island associated with the multidrug resistance region of Salmonella enterica Typhimurium DT104. FEMS Microbiol. Lett. 189:285-291. [DOI] [PubMed] [Google Scholar]
- 6.Briggs, C. E., and P. M. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, H. J., H. W. Stokes, and R. M. Hall. 1996. The integrons In0, In2, and In5 are defective transposon derivatives. J. Bacteriol. 178:4429-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloeckaert, A., S. Baucheron, and E. Chaslus-Dancla. 2001. Nonenzymatic chloramphenicol resistance mediated by IncC plasmid R55 is encoded by a floR gene variant. Antimicrob. Agents Chemother. 45:2381-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloeckaert, A., S. Baucheron, G. Flaujac, S. Schwarz, C. Kehrenberg, J.-L. Martel, and E. Chaslus-Dancla. 2000. Plasmid-mediated florfenicol resistance encoded by the floR gene in Escherichia coli isolated from cattle. Antimicrob. Agents Chemother. 44:2858-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloeckaert, A., K. S. Boumedine, G. Flaujac, H. Imberechts, I. D'Hooghe, and E. Chaslus-Dancla. 2000. Occurrence of a Salmonella enterica serovar Typhimurium DT104-like antibiotic resistance gene cluster including the floR gene in S. enterica serovar Agona. Antimicrob. Agents Chemother. 44:1359-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cody, S. H., S. L. Abbott, A. A. Marfin, B. Schulz, P. Wagner, K. Robbins, J. C. Mohle-Boetani, and D. J. Vugia. 1999. Two outbreaks of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in Northern California. JAMA 281:1805-1810. [DOI] [PubMed] [Google Scholar]
- 12.Daly, M., and S. Fanning. 2000. Characterization and chromosomal mapping of antimicrobial resistance genes in Salmonella enterica serotype Typhimurium. Appl. Environ. Microbiol. 66:4842-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies, A., P. O'Neill, L. Towers, and M. Cooke. 1996. An outbreak of Salmonella typhimurium DT104 food poisoning associated with eating beef. Commun. Dis. Rep. 6:R159-R162. [PubMed] [Google Scholar]
- 14.Davies, J. E. 1997. Origins, acquisition, and dissemination of antibiotic resistance determinants, p. 15-27. In D. J. Chadwick and J. Goode (ed.), Antibiotic resistance: origins, evolution, selection and spread. John Wiley & Sons, Inc., New York, N.Y. [PubMed]
- 15.Evans, S., and R. Davies. 1996. Case control study of multiple-resistant Salmonella typhimurium DT104 infection of cattle in Great Britain. Vet. Rec. 139:557-558. [PubMed] [Google Scholar]
- 16.Fluit, A. C., and F. J. Schmitz. 1999. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur. J. Microbiol. Infect. Dis. 18:761-770. [DOI] [PubMed] [Google Scholar]
- 17.Frana, T. S., S. A. Carlson, and R. W. Griffith. 2001. Relative distribution and conservation of genes encoding aminoglycoside-modifying enzymes in Salmonella enterica serotype Typhimurium phage type DT104. Appl. Environ. Microbiol. 67:445-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 19.Grein, T., D. O'Flanagan, T. McCarthy, and D. Bauer. 1999. An outbreak of multidrug-resistant Salmonella typhimurium food poisoning at a wedding reception. Isr. Med. J. 92:238-241. [PubMed] [Google Scholar]
- 20.Guerra, B., S. Soto, S. Cal, and M. C. Mendoza. 2000. Antimicrobial resistance and spread of class 1 integrons among Salmonella serotypes. Antimicrob. Agents Chemother. 44:2166-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall, R. M., H. J. Brown, D. E. Brookes, and H. W. Stokes. 1994. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J. Bacteriol. 176:6286-6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosek, G., D. D. Leschinsky, S. Irons, and T. J. Safranek. 1997. Multidrug-resistant Salmonella serotype Typhimurium—United States, 1996. Morb. Mortal. Wkly. Rep. 46:308-310. [PubMed] [Google Scholar]
- 23.Kim, E., and T. Aoki. 1996. Sequence analysis of the florfenicol resistance gene encoded in the transferable R-plasmid of a fish pathogen, Pasteurella piscicida. Microbiol. Immunol. 40:665-669. [DOI] [PubMed] [Google Scholar]
- 24.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meunier, D., D. Boyd, M. R. Mulvey, S. Baucheron, C. Mammina, A. Nastasi, E. Chaslus-Dancla, and A. Cloeckaert. 2002. Identification of Salmonella enterica serovar Typhimurium DT104 antibiotic resistance genomic island I in serovar Paratyphi B. Emerg. Infect. Dis. 8:430-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Møbak, K., D. L. Baggesen, F. M. Aarestrup, J. M. Ebbesen, J. Engberg, K. Frydendahl, P. Gerner-Smidt, A. M. Petersen, and H. C. Wegener. 1999. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype Typhimurium DT104. N. Engl. J. Med. 341:1420-1425. [DOI] [PubMed] [Google Scholar]
- 27.Nastasi, A., and C. Mammina. 2001. Presence of class 1 integrons in multidrug-resistant, low-prevalence Salmonella serotypes, Italy. Emerg. Infect. Dis. 7:455-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.Ng, L.-K., M. R. Mulvey, I. Martin, G. A. Peters, and W. Johnson. 1999. Genetic characterization of antimicrobial resistance in Canadian isolates of Salmonella Typhimurium DT104. Antimicrob. Agents Chemother. 43:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng, L.-K., R. Khakhria, D. Woodward, and W. Johnson. 1997. National laboratory surveillance of enteric pathogens. Can. J. Infect. Dis. 8:133-136. [Google Scholar]
- 31.Parsons, Y., R. M. Hall, and H. W. Stokes. 1991. A new trimethoprim resistance gene, dhfrX, in the In7 integron of plasmid DGO100. Antimicrob. Agents Chemother. 35:2436-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partridge, S. R., H. J. Brown, H. W. Stokes, and R. M. Hall. 2001. Transposons Tn1696 and Tn21 and their integrons In4 and In2 have independent origins. Antimicrob. Agents Chemother. 45:1263-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge, S. R., G. D. Recchia, C. Scaramuzzi, C. M. Collis, H. W. Stokes, and R. M. Hall. 2000. Definition of the attI1 site of class 1 integrons. Microbiology 146:2855-2864. [DOI] [PubMed] [Google Scholar]
- 34.Partridge, S. R., G. D. Recchia, H. W. Stokes, and R. M. Hall. 2001. Family of class 1 integrons related to In4 from Tn1696. Antimicrob. Agents Chemother. 45:3014-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters, E. D. J., M. A. Leverstein-van Hall, A. T. A. Box, J. Verhoef, and A. C. Fluit. 2001. Novel gene cassettes and integrons. Antimicrob. Agents Chemother. 45:2961-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridley, A., and E. J. Threlfall. 1998. Molecular epidemiology of antibiotic resistance genes in multiresistant epidemic Salmonella typhimurium DT104. Microb. Drug Resist. 4:113-118. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Sandvang, D., F. M. Aarestrup, and L. B. Jensen. 1998. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol. Lett. 160:37-41. [DOI] [PubMed] [Google Scholar]
- 39.Schmieger, H., and P. Schicklmaier. 1999. Transduction of multiple drug resistance of Salmonella enterica serovar Typhimurium DT104. FEMS Microbiol. Lett. 170:251-256. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz. F.-J., D. Hafner, R. Geisel, O. Tollman, C. Kirschke, J. Verhoff, K. Köhrer, and A. C. Fluit. 2001. Increased prevalence of class 1 integrons in Escherichia coli. Klebsiella species, and Enterobacter species isolated over a 7-year period in a German hospital. J. Clin. Microbiol. 39:3724-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnabel, E. L., and A. L. Jones. 1999. Distribution of tetracycline resistance genes and transposons among phylloplane bacteria in Michigan apple orchards. Appl. Environ. Microbiol. 65:4898-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokes, H. W., C. Tomaras, Y. Parsons, and R. M. Hall. 1993. The partial 3′-conserved segment duplications in the integrons In6 from pSa and In7 from pDGO100 have a common origin. Plasmid 30:39-50. [DOI] [PubMed] [Google Scholar]
- 43.Threlfall, E. J., L. R. Ward, J. A. Skinner, and A. Graham. 2000. Antimicrobial drug resistance in nontyphoidal Salmonellas from humans in England and Wales in 1999: decrease in multiple resistance in Salmonella enterica serotypes Typhimurium, Virchow, and Hadar. Microb. Drug Res. 6:319-325. [DOI] [PubMed] [Google Scholar]
- 44.Threlfall, E. J., L. R. Ward, and B. Rowe. 1998. Multiresistant Salmonella typhimurium DT104 and salmonella bacteremia. Lancet 352:287-288. [DOI] [PubMed] [Google Scholar]
- 45.Threlfall, E. J., L. R. Ward, J. A. Skinner, and B. Rowe. 1997. Increase in multiple antibiotic resistance in nontyphoidal salmonellas from humans in England and Wales: a comparison of data for 1994 and 1996. Microb. Drug Res. 3:263-266. [DOI] [PubMed] [Google Scholar]
- 46.Threlfall, E. J., J. A. Frost, L. R. Ward, and B. Rowe. 1996. Increasing spectrum of resistance in multiresistant Salmonella typhimurium. Lancet 347:1053-1054. [DOI] [PubMed] [Google Scholar]
- 47.Verdet, C., G. Arlet, G. Barnaud, P. H. Lagrange, and A. Phillipon. 2000. A novel integron in Salmonella enterica serovar Enteritidis carrying the bla (DHA-1) gene and its regulator gene ampR, originated from Morganella morganii. Antimicrob. Agents Chemother. 44:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villar, R. G., M. D. Macek, S. Simons, P. S. Hayes, M. J. Goldoft, J. H. Lewis, L. L. Rowan, D. Hursh, M. Patnode, and P. S. Mead. 1999. Investigation of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in Washington State. JAMA 281:1811-1816. [DOI] [PubMed] [Google Scholar]
- 49.Wall, P. G., D. Morgan, K. Lamden, M. Griffin, E. J. Threlfall, L. R. Ward, and B. Rowe. 1995. Transmission of multiresistant strains of Salmonella typhimurium from cattle to man. Vet. Rec. 136:591-592. [DOI] [PubMed] [Google Scholar]
- 50.White, P. A., C. J. McIvor, and W. D. Rawlinson. 2001. Integrons and gene cassettes in Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]